Abstract
Increasing evidence indicates that inflammation can cause thrombosis by a von Willebrand factor (VWF)-mediated mechanism that includes endothelial activation, secretion of VWF, assembly of hyperadhesive VWF strings and fibers, cleavage by ADAMTS13, and adhesion and deposition of VWF-platelet thrombi in the vasculature. This mechanism appears to contribute to thrombosis not only in small vessels, but also in large vessels. Inflammation and VWF contribute to atherogenesis and may contribute to arterial and venous thrombosis as well as stroke. Elucidation of the mechanism will hopefully identify new targets and suggest new approaches for prevention and intervention.
Introduction
von Willebrand factor (VWF) is a large multimeric glycoprotein in plasma that plays a critical role in hemostasis and thrombosis by mediating platelet adhesion to injured and activated vessels. Deficiency or dysfunction of VWF can lead to either bleeding or thrombosis. VWF was named after Erik von Willebrand, who identified a new form of inherited bleeding diathesis in a kindred in the Åland Islands of Finland. The disorder was named von Willebrand disease and was corrected by infusion of a fraction of normal human plasma.1 This component in plasma was subsequently named von Willebrand factor (VWF). The complementary DNA (cDNA) for VWF was cloned by several groups in 1985,2-4 and the gene was cloned and characterized in 1989.5 Since then, many mutations in the VWF gene that give rise to functional deficiencies in VWF have been identified.6 The role of VWF in thrombosis was revealed in thrombotic thrombocytopenic purpura (TTP), a devastating disease characterized by the extensive deposition of VWF and platelet-rich thrombi in the microvasculature that causes vessel occlusion, tissue ischemia, organ failure, and death if untreated.7,8 TTP is caused by a deficiency of a VWF-specific metalloprotease, ADAMTS139 that normally cleaves at the Tyr1605-Met1606 bond in the A2 domain of the VWF subunit, which is exposed by shear stress–induced unfolding of the VWF molecule. Mutations in the ADAMTS13 gene or presence of autoantibodies to ADAMTS13 lead to unrestricted formation and accumulation of VWF and platelet-rich thrombi in the microvasculature in TTP. Studies on the pathogenesis of TTP highlight the inherent propensity of normal VWF to bind platelets under shear stress; dysregulation of this process is the main cause of TTP, an extreme form of thrombotic microangiopathy. There is increasing evidence that VWF also plays important roles in arterial10 and venous thrombosis,11-13 atherosclerosis,14-16 and thrombotic complications accompanying malaria,17 sepsis,18 sickle cell disease,19 and other thrombotic microangiopathies.20 In the majority of these pathological conditions, ADAMTS13 is present in normal or near normal levels. Therefore, ADAMTS13 cleavage is not the only mechanism that can regulate the adhesive function of VWF; other yet-to-be elucidated mechanisms must also exist to account for the spectrum of VWF-related thrombotic complications. These complications are often provoked and exacerbated by inflammation. In this review, we will examine the relationship between inflammation, VWF, and ADAMTS13, focusing on how inflammation, through modulating the biosynthesis, secretion, and reactivities of VWF, may promote thrombosis.
Transcription of VWF and ADAMTS13
VWF is synthesized only in megakaryocytes and endothelial cells. Although a portion of VWF is constitutively secreted, a major portion is packaged in storage compartments: the α-granules of platelets and the Weibel-Palade bodies (WPBs) of endothelial cells. Synthesis in endothelial cells accounts for the majority of VWF in plasma. Although restricted to endothelial cells, there may be subtle differences in VWF synthesis in various vascular beds. In general, endothelial cells of small vessels in the lung and brain express higher levels of VWF than vessels of similar size in the liver and kidney.21 Transcription of the VWF gene in endothelial cells is regulated by endothelial-specific cis-acting promoter elements located in the 5′ untranslated regions and trans-acting transcription factors GATA, Ets, H1, and NFAT5.22-25 Transcription is downregulated by microRNA-24 that interacts with the 3′ untranslated region.26 There is no evidence that transcription of the VWF gene is regulated by inflammatory cytokines and the JAK-STAT signaling pathway as in the hepatic synthesis of many positive acute-phase proteins.27 VWF transcription is upregulated in megakaryocytes and lung microvascular endothelial cells under hypoxic conditions.26,28,29
ADAMTS13 is primarily synthesized in the liver, although alternatively spliced and incomplete messenger RNAs (mRNAs) have been detected by northern blot in skeletal muscle, heart, and placenta; partial cDNAs that encode truncated or inactive forms of ADAMTS13 have been isolated from the brain and prostate.30 In situ hybridization of preserved human liver autopsy and biopsy samples showed an abundant ADAMTS13 transcript in a subpopulation of hepatic stellate cells, and no detectable transcript in hepatic sinusoidal endothelial cells or Kupffer cells.31 Factors leading to differential expression in subpopulations of hepatic stellate cells are not known. Acute liver injury or liver failure was associated with severely decreased circulating levels of ADAMTS13.32 Pediatric patients with advanced biliary cirrhosis, who often had TTP-like microangiopathy in the liver and low circulating levels of ADAMTS13, improved with successful liver transplantation.33 These correlations are consistent with the liver as the major organ of ADAMTS13 synthesis. There is also evidence of extrahepatic synthesis: mRNA and protein for ADAMTS13 can be detected in human umbilical vein endothelial cells, human umbilical artery endothelial cells,34 and kidney podocytes,35 although the contribution from these cells to the total level in circulation is not known. mRNA for ADAMTS13 has been detected in hepatocarcinoma Hep3B and HepG2 cells as well as in the prostate adenocarcinoma LNCaP cells by reverse transcription–polymerase chain reaction.20 In these studies, a fragment of the mRNA corresponding to exons 10-14 was amplified and the amplimers could have derived from alternatively spliced or incomplete mRNA in these cells, suggesting that the mRNA might not encode full-length or functional ADAMTS13. Transcription of ADAMTS13 is unaffected by proinflammatory stimuli, immune-suppressive agents, steroids, or doxycycline. There is no evidence that transcription plays any role in regulating the level of ADAMTS13 in clinical conditions.20
Translation and processing of VWF
The nascent VWF translation product comprises 2813 aa, which include a signal peptide, a 741-aa propeptide, and the mature VWF subunit of 2050 aa. Prepro-VWF undergoes extensive posttranslational modification in the endoplasmic reticulum (ER) and Golgi apparatus. After initial removal of the signal (pre-) sequence in the rough ER, pro-VWF monomers dimerize to form protomers that are linked by C-terminal disulfide bonds.36 Protomers are subsequently assembled into a collection of large multimers in the Golgi by the formation of additional disulfide bonds between the N termini of protomers, a process facilitated by the propeptide of VWF that functions as a VWF-specific disulfide isomerase.37,38 Propeptides are removed from the VWF multimers by a furin-like enzyme in the ER and Golgi. Although some of the assembled VWF multimers are constitutively secreted into the circulation, a major portion is stored in the WPBs of endothelial cells and the α-granules of platelets and megakaryocytes. VWF in storage compartments contains a spectrum of multimer sizes, including significant quantities of extremely large multimers (ultra-large VWF [ULVWF]), which are more adhesive than the smaller multimers in the circulation.39 Upon secretion, ULVWF, having a lower threshold of shear-induced unfolding, can spontaneously bind platelets. The added mass of bound platelets in the ULVWF-platelet complex would further enhance shear-induced unfolding of the ULVWF, facilitating its cleavage by ADAMTS1340 and its conversion to smaller VWF multimers, thus eliminating the hyperadhesive ULVWF from the circulation.
Formation of WPBs in endothelial cells depends on the expression of VWF. In Vwf knockout mice, lack of VWF synthesis results in agenesis of WPBs and simultaneous reduction of P-selectin exposure on the surface when the endothelial cells are activated; consequently, recruitment of leukocytes to the activated endothelium is reduced.41 This is an indirect way in which VWF synthesis and secretion can feedback and regulate the inflammatory response.
Inflammation and WPB secretion
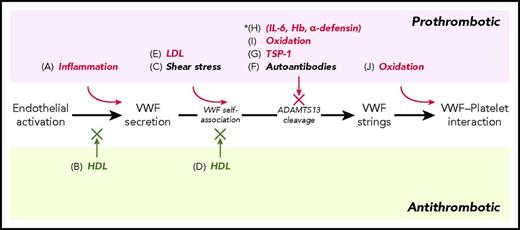
In response to agonist stimulation, endothelial cells become activated and release the content of WPBs, including the major component, VWF. Association of elevated VWF levels with inflammation and endothelial damage observed in glomerulonephritis,42 arteritis, diabetes, and sepsis43 led to early characterization of VWF as an acute-phase reactant.44 Inflammation is a complex biological response to harmful insult or irritants. To eliminate the insult and initiate repair, the inflammatory process begins with activation of resident immune cells, primarily tissue macrophages, and release of inflammatory mediators that include interleukin 1 (IL-1) and tumor necrosis factor α.45 These cytokines bind receptors on and potently activate endothelial cells, which release the content of WPBs and simultaneously expose P-selectin on the activated endothelial surface. WPB secretion elevates the level of VWF in circulation, making the circulating VWF level a marker of endothelial activation and the onset of vascular inflammation (Figure 1A). Thus, elevated plasma VWF levels have been shown to associate with acute respiratory distress syndrome46 and sepsis,18 and correlate independently with mortality. Because endothelial cells also respond to agonists besides those released by the activated tissue macrophages, elevated VWF levels are not exclusive biomarkers of inflammation.
Modulation of VWF-platelet thrombus formation by inflammation-mediated processes. (A-J) represent processes that affect VWF secretion, self-association, cleavage, and reactivity. Inflammation-mediated processes are shown in color: prothrombotic processes in red; antithrombotic processes in green. *In vitro only. Hb, hemoglobin.
Modulation of VWF-platelet thrombus formation by inflammation-mediated processes. (A-J) represent processes that affect VWF secretion, self-association, cleavage, and reactivity. Inflammation-mediated processes are shown in color: prothrombotic processes in red; antithrombotic processes in green. *In vitro only. Hb, hemoglobin.
High-density lipoprotein (HDL) possesses antithrombotic properties. It binds scavenger receptor BI on endothelial cells, activates endothelial nitric oxide (NO) synthase, and increases NO production.47 NO nitrosylates the N-ethylmaleimide–sensitive factor in the SNARE complex, resulting in decreased fusion of WPBs with the plasma membrane and VWF secretion.48 The level of HDL is significantly reduced during inflammation,49 reducing the capacity of HDL to attenuate VWF secretion from the endothelium. Thus, reduction in HDL levels may be an indirect way in which inflammation could enhance VWF-mediated thrombotic complications (Figure 1B).
Inflammation and VWF self-association
Upon secretion from endothelial cells, a portion of the VWF enters the circulation, whereas a portion remains bound to the endothelial surface. Secreted VWF molecules, both endothelial-bound and the circulatory forms, are sensitive to shear stress, which induces unfolding to expose interaction sites not only for platelet binding, but also for self-association and cleavage by ADAMTS13. Shear-induced VWF self-association was first reported in 2 studies in 2002. In a study by Savage and coworkers,50 a deletion mutant of VWF lacking the ability to bind collagen (deleted A3) functionally complemented another deletion mutant of VWF lacking the ability to bind platelets (deleted A1), restoring platelet adhesion to a collagen surface under flow. These studies inferred that the 2 types of mutant VWF multimers must have bound each other under shear stress to reconstitute a population of mixed mutant VWF multimers replete with the ability to simultaneously bind collagen and platelets, similar to that of normal VWF. In a separate study,51 Dong and coworkers observed that when VWF multimers were secreted from stimulated endothelial cells, a portion of the VWF multimers remained anchored to the endothelial surface by binding to P-selectin52 or integrin αvβ3.53 Under flow, these VWF molecules self-associated into VWF strings that exceeded 100 µm in length in the direction of flow. These VWF strings bound platelets under venous and arterial shear-stress levels to produce macroscopic structures with a beads-on-strings appearance. Perfusion of ADAMTS13 over the platelet-decorated VWF strings rapidly removed these structures from the endothelial surface. The ULVWF multimers released from the WPBs, with a lower threshold of shear-induced unfolding, may be the initiating molecules for the self-assembly process, leading to formation of the hyperadhesive strings that capture platelets in the absence of ADAMTS13. These studies suggest that the propensity of secreted VWF multimers to self-associate on the endothelium may play an important role in promoting the unrestrained accumulation of VWF-platelet thrombi in the microvasculature, which leads to occlusion of the vessels, tissue ischemia, and organ failure, the defining characteristics of TTP.7,8 Since these initial reports, VWF self-association has been observed on glass,54 collagen,55 and platelet surfaces,55 and appears to be reinforced by intermolecular disulfide bonds.56 In synthetic endothelialized microvessels, VWF secreted from stimulated endothelial cells can self-associate into strands of up to 5 cm in length; these strands can span the lumen of vessels up to 300 µm in diameter.57 When purified plasma VWF multimers, devoid of ULVWF, were perfused under arterial shear stress in a cell-free microfluidic device with a “post-in-channel” design58 that simulated a stenotic vessel, the VWF multimers readily self-associated into long and thick fibers that were captured on the post in the microchannel. Perfusion for 5 minutes led to accumulation of bundles of VWF fibers that occupied about half of the width of the 60-µm microchannel.59 These studies show that normal VWF multimers in the circulation, in the absence of ULVWF, endothelial cells, or platelets, are able to self-associate under shear stress into massive hyperadhesive structures (Figure 1C).
We recently observed that VWF was rapidly lost from polyethylene tubes by adsorption onto the tube surface followed by self-association to the adsorbed VWF under shear stress. We found that surface adsorption and self-association could be prevented by lipid-free apolipoprotein A-I (ApoA-I) or its common form in the circulation, the HDL particles.60 Addition of HDL to 50% above the normal plasma level prevented shear-induced VWF self-association, accompanied by formation of VWF-HDL complexes in vitro.60 In flow chambers and synthetic microvessels, HDL reduced secretion of VWF from endothelial cells, decreased the length and thickness of VWF strands formed on the activated endothelial surface, and reduced the incorporation of soluble VWF multimers to the preformed immobilized VWF strands. Platelet adhesion to the strands was decreased in proportion to the reduction in strand formation. The effect of HDL on VWF self-association was also shown in an animal model of thrombotic microangiopathy. When Adamts13 knockout mice were challenged by injection of high doses of purified human VWF, coadministration of HDL markedly attenuated the transient thrombocytopenia induced in these mice, recapitulating the antithrombotic properties of HDL on VWF self-association and platelet binding in vitro. Because the level of HDL is markedly reduced in acute and chronic inflammation,49 lower HDL levels, accompanied by a diminished capacity to attenuate VWF self-association, could promote microvascular thrombosis. Additionally, inflammation is also known to modify the composition of HDL particles, primarily by increasing the expression of the acute-phase protein serum amyloid A, which is incorporated into HDL in place of ApoA-I,61 and thus producing HDL particles with markedly reduced ApoA-I content. Because ApoA-I is the active component in HDL that attenuates VWF self-association, a reduction in its content in HDL during inflammation might also be prothrombotic by enhancing VWF self-association (Figure 1D).
During inflammation, the level of low-density lipoprotein (LDL) particles is increased.62 In preliminary studies,59 we observed that LDL had the opposite effect of HDL on VWF self-association: LDL enhanced VWF self-association under shear (Figure 1E). This observation suggests that the ratio of HDL to LDL, especially a reduction in this ratio during inflammation, may be an important factor in promoting microvascular thrombosis. Furthermore, dense LDL particles are more sensitive to lipid peroxidation.63 Whether lipid peroxidation affects the ability of HDL and LDL to modulate VWF self-association is not known at present.
Enhanced platelet binding to VWF during inflammation
The platelet-binding conformation of VWF is preferentially recognized by a llama-derived antibody fragment AU/VWFa-11, which has been used to identify “activated” VWF, the hyperadhesive fraction that preferentially binds platelets in the plasma of TTP and type 2B von Willebrand disease patients.64 Elevated activated VWF was also observed in pathological conditions with significant thrombotic complications, including the hemolysis, elevated liver enzymes, low platelet count (HELLP) syndrome, malaria, the antiphospholipid syndrome,65 sickle cell disease,19 and sepsis.60 Inflammation is also a common feature accompanying these disease conditions,66-69 although the mechanism for increased activation of VWF is not known.
Inflammation and cleavage of VWF by ADAMTS13
The majority of acquired TTP patients have circulating autoimmune antibodies directed to ADAMTS1370 that either inhibit the activity of ADAMTS13 or bind ADAMTS13 and mediate immune clearance. Persistent synthesis of ADAMTS13-specific inhibitory antibodies in TTP patients is associated with resistance to treatment71 (Figure 1F). Although no naturally occurring endogenous inhibitor for ADAMTS13 comparable to the serpins for serine proteases or the tissue inhibitors of metalloproteinases for metalloproteases has been identified, several proteins and peptides have been shown to inhibit ADAMTS13 cleavage of VWF multimers. In every case, the inhibitory agent apparently binds to VWF multimers, preventing the multimers from being cleaved instead of binding to ADAMTS13. Thrombospondin-1 (TSP-1), an abundant protein in the α granules of platelets that is released into the circulation upon platelet activation, binds the A2-A3 region of VWF, competitively blocking cleavage by ADAMTS13 in vitro.72 Consistent with its ability to inhibit VWF cleavage in vitro, platelet-derived TSP-1 has been shown to inhibit cleavage of VWF strings by ADAMTS13 in animal models, and TSP-1 deficiency caused thrombus embolization and delayed vessel occlusion in injured blood vessels.73 Similarly, TSP-1 has been shown to enhance arterial thrombosis only in the presence of VWF, and TSP-1 deficiency delays thrombus growth in mouse models of arterial thrombosis.74 Thus, platelet adhesion, activation, and degranulation may be a feedback mechanism that stabilizes the VWF-platelet thrombi by downregulating their removal (Figure 1G). Cell-free hemoglobin can bind VWF strings on the endothelial surface under flow, and competitively blocked cleavage by ADAMTS13 in vitro.75 However, free hemoglobin is rapidly sequestered by binding to haptoglobin and cleared from the circulation in vivo. Whether cell-free hemoglobin resulting from hemolysis can reach levels that would inhibit VWF cleavage in vivo is not known. High concentrations of IL-6 (50 and 100 ng/mL) have been shown to inhibit the cleavage of ULVWF strings on the endothelial surface by ADAMTS13 under flow in vitro,76 suggesting an indirect way in which inflammation may partially inhibit VWF cleavage. However, the circulation levels of IL-6 during acute inflammation are much lower than the inhibitory levels and therefore the inhibitory effect of IL-6 on VWF cleavage is uncertain. The human α-defensins, either released from neutrophils or their synthetic counterparts, have been shown to bind the A2 domain of VWF and competitively block ADAMTS13-mediated cleavage of VWF in vitro.77 A sevenfold increase in human defensins in the circulation of acquired TTP patients suggests that inflammation is present in these patients, and release of defensins and their binding to the VWF-platelet thrombi may have contributed to the onset of microvascular thrombosis in TTP by inhibiting VWF cleavage (Figure 1H).
Oxidative modification of VWF and ADAMTS13
In response to cytokines released by the activated tissue macrophages at the site of inflammation, gradients of chemokine are formed that attract and recruit neutrophils in the circulation to the site of inflammation. P-selectin exposed on activated endothelial cells also contributes to capturing neutrophils onto the activated endothelium. These neutrophils, in turn, become activated, and through the NADPH oxidase system, release reactive oxygen species, which include the superoxide radical and hydrogen peroxide. Activated neutrophils also release myeloperoxidase, which generates hypochlorous acid (HOCl) from hydrogen peroxide and chloride ions. HOCl oxidizes the side-chains of exposed cysteines and methionines in proteins, converting cysteines to cysteine sulfinic or sulfonic acids, methionines to methionine sulfoxides, and to lesser extents tyrosines to chlorotyrosines. Shear stress induces unfolding of VWF multimers and enhances the oxidation of exposed methionine residues in the A1A2A3 region of the VWF subunit, including Met1606 at the cleavage site of ADAMTS13.78 HOCl-oxidized VWF is uncleavable by ADAMTS13 and is stabilized in a hyperadhesive conformation that binds more avidly to the nanobody AU/VWFa-11 and agglutinates platelets in the presence of a lower threshold of ristocetin78,79 (Figure 1I-J). In a preliminary analysis of the extent of Met1606 oxidation and VWF cleavage in the plasma of patients with sickle cell disease, cleavage at the Tyr1605-Met1606 bond decreased by 40% compared with that of normal plasma, and decreased cleavage negatively correlated with Met1606 oxidation.80 Although the increase in Met1606 oxidation was small (∼3%), these were exactly the residues that were exposed by shear stress, became preferentially oxidized by HOCl, and prevented cleavage by ADAMTS13. Moreover, HOCl generated by activated neutrophils can also modify methionine residues in ADAMTS13, including Met249 in the “Met-turn” of the active center of ADAMTS13, and inhibit its proteolytic activity.81 These effects of oxidation on VWF and ADAMTS13 suggest that inflammation, through oxidative modification, could indirectly regulate VWF reactivity, and contribute to a prothrombotic state.
Atherosclerosis and VWF
Although the role of platelets in late stages of atherosclerosis, plaque rupture, coagulation, and myocardial infarction has been well established, recent studies have revealed that chronic inflammation, VWF, and platelets also play an important role in atherogenesis. Evidence from in vivo ultrasound molecular imaging shows increased VWF-mediated platelet adhesion to the endothelium at an early stage of atherosclerosis prior to plaque development in a murine model.14,82 These observations give rise to the concept that atherosclerotic risk factors, such as hypertension, elevated LDL cholesterol, reduced HDL, obesity, diabetes, and oxidative stress can provoke an inflammatory vascular response that initiates atherosclerotic plaque formation and growth. This vascular response includes endothelial activation, release of VWF, VWF self-association, and VWF-mediated platelet adhesion to the endothelium under high shear stress at atherosclerosis-prone sites in large arteries, such as at vessel bifurcations and locations of disturbed flow. In these studies, VWF-dependent platelet adhesion to atherosclerosis-prone sites is eliminated by infusion of ADAMTS13.14,82 Subsequent to adhesion to these sites, platelets become activated and further amplify platelet recruitment and inflammation in several ways. Activated platelets release α and dense granule contents and expose P-selectin on the surface, recruiting additional neutrophils and monocytes to the site. Activated platelets also activate the NOX-1 and NOX-2 system, and generate and release reactive oxygen species83 that sustain activation of the endothelium. This concept is consistent with studies in animal models of atherosclerosis, which show reduced plaque burden and plaque inflammation when platelet activation is inhibited.84,85 Similarly, deficiency of VWF also reduces the size of atherosclerotic lesions in a murine model of atherosclerosis.15 Deficiency of ADAMTS13 in ApoE knockout mice accelerates atherosclerosis and increases macrophage infiltration into atherosclerotic lesions.86,87 These findings extend the basic features of microvascular thrombosis, which include inflammation, endothelial activation, VWF release, and platelet adhesion, to thrombosis in large vessels.
Conclusion
As discussed in this article and summarized in Figure 1, inflammation may regulate VWF-platelet thrombus formation in large arteries and small vessels by elevating the level of VWF, enhancing the reactivity of VWF, and modulating the levels and activities of regulatory molecules in the circulation. Elucidation of the mechanism that links inflammation to thrombosis may identify new targets for intervention. For example, attenuating VWF self-association by HDL, ApoA-I, or agents with similar activities in addition to regulating platelet adhesion to VWF fibers may be a more effective approach than regulating platelet adhesion alone. Likewise, control of dyslipidemia and oxidative stress may also have antithrombotic effects through the VWF-platelet axis. In addition, elucidation of the mechanism serves as a starting point to examine how other processes besides inflammation, such as hypoxia, cancer, and autoimmune disorders, can provoke thrombosis through endothelial activation and VWF-mediated reactions.
The evidence that inflammation may be linked to thrombosis through VWF-mediated reactivities does not detract from the well-described mechanism that links activated neutrophils, tissue factor-mediated thrombin generation, fibrin deposition, and thrombosis in the vasculature. Similar to the complementary roles of VWF-platelet interactions and tissue factor–initiated fibrin formation in hemostasis, these 2 processes may, to varying extents, also contribute to the wide spectrum of thrombotic disorders. Although shear-dependent VWF-platelet thrombi deposition was important in microvascular thrombosis such as TTP, its role in venous thrombosis and stroke has also emerged in recent studies in animal models. In Vwf knockout mice, platelet adhesion to injured veins was significantly reduced and vessel occlusion was delayed.88 VWF-deficient mice were also protected from deep vein thrombosis induced by restricted blood flow in large veins. The protective effect is attributed to reduced recruitment of platelets and leukocytes to the location of restricted flow or stasis in large vessels.11 In Vwf knockout mice, the cerebral infarct volume induced by focal ischemia was significantly reduced, whereas in Adamts13 knockout mice, the infarct volume increased instead.89 Although the mechanism for these effects and their link to inflammation need further elucidation, these studies implicate VWF and platelets in the initial stages of deep vein thrombosis and stroke. Thus, inflammation, through modulation of VWF function and platelet adhesion, may affect not only thrombosis of small vessels but also large vessels. Accordingly, integrating measures of controlling the adhesive function of VWF with current antithrombotic regimens may improve prevention and treatment of a wide spectrum of thrombotic disorders.
Acknowledgments
This work was supported in part by grants HL137991, HL112633, HL117639, and HL129526 from the National Heart, Lung, and Blood Institute, National Institutes of Health, and institutional funds from the Bloodworks Research Institute, Seattle, WA.
Authorship
Contribution: D.W.C. and J.C. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dominic W. Chung, Bloodworks Research Institute, 1551 Eastlake Ave E, Suite 100, Seattle, WA 98102; e-mail: chung@bloodworksnw.org.