Key Points
Lenalidomide plus rituximab as induction and maintenance therapy for MCL can achieve durable MRD-negative complete remissions.
Chronic therapy–associated adverse events are generally nonaccumulative and remain manageable.
Abstract
We report 5-year follow-up of a multicenter phase 2 study of lenalidomide plus rituximab (LR) as initial treatment of mantle cell lymphoma (MCL). The regimen includes induction and maintenance with the LR doublet. Treatment was continuous until progression, with optional discontinuation after 3 years. The median age of the 38 participants was 65 years, with MCL international prognostic index scores balanced among low, intermediate, and high risk (34%, 34%, and 32%, respectively). Twenty-seven (75%) of the 36 evaluable patients completed ≥3 years of study treatment. At a median follow-up of 64 months (range, 21-78), the 3-year progression-free survival (PFS) and overall survival (OS) were 80% and 90%, respectively, with 5-year estimated PFS and OS of 64% and 77%, respectively. During maintenance, hematologic adverse events (AEs) included asymptomatic grade 3 or 4 cytopenias (42% neutropenia, 5% thrombocytopenia, 3% anemia) and mostly grade 1 or 2 infections managed in the outpatient setting (45% upper respiratory infection, 21% urinary tract infection, 13% sinusitis, 11% cellulitis, 8% pneumonia). Nonhematologic AEs, such as constitutional and inflammatory symptoms, occurred at reduced frequency and intensity compared with induction. A peripheral blood minimal residual disease (MRD) assay (clonoSEQ) showed MRD-negative complete remission in 8 of 10 subjects who had completed ≥3 years of treatment and with available samples for analysis. With longer follow-up, LR continues to demonstrate durable responses and manageable safety as initial induction and maintenance therapy for MCL (ClinicalTrials.gov NCT01472562).
Introduction
Mantle cell lymphoma (MCL) represents 5% to 8% of non-Hodgkin lymphomas and is generally incurable, with a reported median survival of ∼5 years in population studies.1-3 Initial treatment of MCL is variable, but historically includes chemoimmunotherapy4-6 and often involves intensive hospital-based approaches with high-dose chemotherapy and hematopoietic cell transplantation, despite lack of evidence of a cure.7-9 Treatment selection is influenced by lymphoma characteristics, such as disease burden, proliferation, and mutational profile, as well as patient factors, such as age, comorbidities, and individual preferences. Optimal management that balances efficacy and accessibility with safety and quality-of-life (QoL) remains a clinical challenge for most MCL patients in the community setting,10 many of whom are elderly with comorbidities.
Lenalidomide is a second-generation immunomodulatory compound that targets tumor cells, as well as the tumor microenvironment.11 Through interaction with its intracellular effector cereblon in diverse cell types, lenalidomide stimulates T-cell and NK cell expansion, inhibits tumor-associated angiogenesis and lymphangiogenesis, and induces lymphoma cell apoptosis by CDK inhibition and cyclin D1 downregulation.12-14 When combined with rituximab in vitro, lenalidomide augments NK cell–mediated antibody-dependent cell-mediated cytotoxicity15 and overcomes rituximab resistance in lymphoma patients.16 Lenalidomide has shown clinical efficacy in patients with recurrent MCL, either as a single agent (overall response rate [ORR], 28%-40%; complete response [CR], 5%-8%; median duration of response [DOR], 16 months)17,18 or in combination with rituximab (ORR, 57%; CR, 36%; median DOR, 18.9 months).19
A survival benefit with rituximab maintenance (MR) after initial therapy for MCL in the frontline setting has been demonstrated in 2 prospective randomized studies in MCL. In the European Elderly MCL Trial for patients ineligible for stem cell transplant, MR is associated with superior progression-free survival (PFS) and overall survival (OS) compared with interferon-α (IFN-α) maintenance (5-year PFS, 51% vs 22%; P < .0001 and 5-year OS, 79% vs 59%, P = .0026) following R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) induction.20,21 In the LYSA study for young and fit MCL patients, MR improves survival compared with observation (4-year PFS, 83% vs 64%; P < .001 and 4-year OS, 89% vs 80%; P = .004) following consolidative autologous stem cell transplant (ASCT).22
We initiated the first chemotherapy-free multicenter phase 2 study in 2011 to evaluate the efficacy and safety of the combination of lenalidomide plus rituximab (LR) as induction and maintenance treatment of patients with previously untreated MCL. Previous interim analysis at a median follow-up of 30 months showed that the LR regimen was effective (ORR 92%, CR 64%) and well tolerated, with improvement in QoL in response to therapy.23 Here, we report the 5-year follow-up of the efficacy and safety of the LR regimen, as well as studies of exploratory biomarkers, including minimal residual disease (MRD) and cytokine/chemokine profiles, to gain additional insights into the biologic actions of the combination.
Methods
Patient eligibility
Details about patient eligibility have been reported previously.23 Key eligibility criteria included histologically confirmed untreated MCL with measurable disease; low to intermediate MCL international prognostic index (MIPI) or high MIPI with contraindications to chemotherapy; Eastern Cooperative Oncology Group performance status ≤ 2; and creatinine clearance ≥ 30 mL/min. Key exclusions included central nervous system lymphoma, known HIV, active hepatitis B or C, and invasive malignancies within 5 years.
Study design
Details of the study design have been published previously.23 Briefly, this multicenter open-label single-arm study consisted of induction and maintenance. Lenalidomide was administered at 20 mg daily on days 1 to 21 of a 28-day cycle for 12 cycles during induction, followed by dose reduction to 15 mg during maintenance. Standard-dose rituximab was administered at 375 mg/m2 weekly for 4 weeks during cycle 1 and then once every other cycle. Treatment was continuous until disease progression, development of unacceptable toxicity, or study withdrawal, with an option to stop therapy after 3 years if otherwise in clinical remission based on routine surveillance computed tomography (CT) scans. The starting dose of lenalidomide was 10 mg daily for induction and 5 mg daily for maintenance for patients with reduced creatinine clearance of 30 to 60 mL/min. Patients received thromboprophylaxis with aspirin or low molecular weight heparin, unless requiring treatment of known thrombosis. Asymptomatic hepatitis B carriers received antiviral therapy. The study was conducted in accordance with the Declaration of Helsinki and good clinical practice guidelines. Institutional Review Boards approved the study protocol at the respective sites, and all participants provided written informed consent. An independent Data and Safety Monitoring Board at Weill Cornell conducted biannual safety reviews.
Efficacy and safety assessments
Response criteria were those reported by Cheson et al.24 CT scans were performed at study entry, every 3 months for 2 years, and every 6 months until progression. A positron emission tomography–CT scan was optional at baseline. Bone marrow biopsy and positron emission tomography-CT scans were done to confirm CR. Adverse event (AE) monitoring was continuous throughout induction and maintenance. Toxicities were graded according to National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0. Data from all participants receiving any therapy were included in the safety analyses.
MRD measurement by clonoSEQ assays
MRD was assessed using the clonoSEQ method25,26 (Adaptive Biotechnologies, Seattle, WA) for a subset of patients in remission treated at Weill Cornell Medicine and Moffitt Cancer Center who had available archival formalin-fixed paraffin-embedded tumor tissues. Genomic DNA was amplified using locus-specific primer sets for IGH-VDJH, IGH-DJH, and IGK. The amplified product was subjected to sequencing, and the sequences and frequencies of the different clonotypes in the sample were obtained. The tumor clonotype(s) were defined as the read(s) present at a frequency > 5%. The final MRD measurement was calculated as the number of tumor-derived clonal immunoglobulin molecules per 1 million input genome equivalents of DNA. In cases in which ≥2 tumor clones existed, the clone with the highest MRD value was reported. All MRD assays were performed using follow-up peripheral blood (PB) mononuclear samples paired with archival formalin-fixed paraffin-embedded tissues.
Cytokine/chemokine analysis
Blood samples were prospectively collected from the 36 evaluable patients at pretreatment baseline, cycle 2 day 1 (C2D1), and cycle 4 day 1 (C4D1). The concentrations of cytokines (IFNγ, IL1β, IL2, IL4, IL6, IL8, IL10, IL12, TNFα) and chemokines (MCP1, MCP4, Eotaxin, IP10, MDC, Eotaxin3, TARC, MIP1α, and MIP1β) in patient plasma were determined using V-PLEX immunoassays (Meso Scale Discovery, Rockville, MD) in triplicates, according to the manufacturer’s instructions.27 Concentration was reported as picogram per milliliter.
Statistical analysis
Details of the sample size determination have been published previously.23 Briefly, the primary end point was investigator-assessed ORR (CR + partial response [PR]). Sample size was determined according to Simon’s 2-stage minimax design.28 With final accrual of 38 patients (36 patients eligible for response assessment), a 90% confidence interval (CI) for an expected response rate of 60% could be constructed to be within ±14.0% of the response rate, and a 95% CI could be constructed to be within ±16.7% of the response rate. Secondary end points included PFS and OS assessed by Kaplan-Meier survival analysis. For biomarker studies, descriptive statistics (median/interquartile range/range, count, and percent) are reported for key variables. Wilcoxon signed-rank tests were performed for pairwise comparisons of the medians and interquartile ranges of cytokine/chemokine levels among the 3 time points (pretreatment baseline, C2D1, and C4D1). Wilcoxon rank-sum tests were done to correlate cytokine/chemokine medians with treatment-emergent rash symptom during induction. All P values were 2-sided, with statistical significance evaluated at the 0.05 α level. Ninety-five percent CIs were calculated to assess estimate precision. All analyses were performed using SAS 9.4 (SAS Institute, Cary, NC) or Stata 13 (StataCorp, College Station, TX).
Data sharing statement
Individual participant data will not be shared.
Results
Patient characteristics and disposition
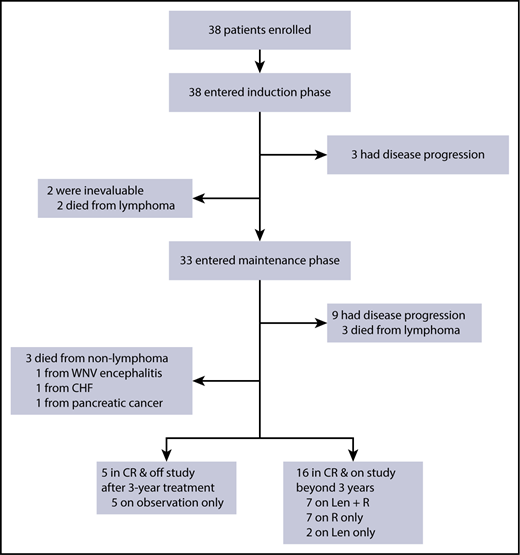
A total of 38 subjects with previously untreated MCL requiring therapy was enrolled at 4 centers from July of 2011 to April of 2014, as reported previously.23 They had disease features typical for the general MCL patient population, including median age of 65 years (range, 42-86), male prevalence (71%), advanced stage III and IV disease (100%), and evenly distributed MIPI scores among 3 risk groups. Eight-seven percent of subjects had evaluable Ki67; 21% had Ki67 > 30%. None had pleomorphic or blastoid histology. Two patients, with intermediate- and high-risk MIPI, were not evaluable due to intolerance of tumor flare in cycle 1 and withdrawal prior to response assessment. Both died from lymphoma after subsequent multiple regimens. Of the 36 evaluable patients, 33 completed induction and entered maintenance (Figure 1). Twelve patients had progression: 3 during induction with primary refractory disease and 9 during maintenance following initial responses (4 CRs with PFS of 18, 38, 39, and 49 months and 5 PRs with PFS at 14, 25, 28, 43, and 44 months). Six evaluable patients died, 3 patients from lymphoma progression (including an 83-year-old subject who relapsed 17 months after completion of 3-year study treatment) and 3 patients from unrelated comorbidities (1 patient each from congestive heart failure, West Nile viral encephalitis, and pancreatic cancer). As of February of 2018, at a median follow-up of 64 months (range, 21-78), 21 (58%) evaluable patients remain in durable remission beyond 3 years, including 5 patients who opted for treatment discontinuation after 3 years. Of the 16 patients on study treatment, 7 patients are maintained on LR, 2 patients are on lenalidomide alone, and 7 patients are on rituximab alone.
Consort diagram of patient treatment and disposition. The induction treatment consisted of lenalidomide administered at 20 mg daily on days 1 to 21 of a 28-day cycle for 12 cycles and rituximab weekly for 4 weeks during cycle 1 and then every other cycle. Of the 38 patients enrolled, 33 completed induction and entered maintenance, whereas lenalidomide was reduced to 15 mg, and rituximab was continued every other cycle. Treatment was continuous until disease progression, unacceptable toxicity, or study withdrawal, with an option to stop therapy after 3 years. CHF, congestive heart failure; Len, lenalidomide, R, rituximab; WNV, West Nile virus.
Consort diagram of patient treatment and disposition. The induction treatment consisted of lenalidomide administered at 20 mg daily on days 1 to 21 of a 28-day cycle for 12 cycles and rituximab weekly for 4 weeks during cycle 1 and then every other cycle. Of the 38 patients enrolled, 33 completed induction and entered maintenance, whereas lenalidomide was reduced to 15 mg, and rituximab was continued every other cycle. Treatment was continuous until disease progression, unacceptable toxicity, or study withdrawal, with an option to stop therapy after 3 years. CHF, congestive heart failure; Len, lenalidomide, R, rituximab; WNV, West Nile virus.
Efficacy
5-year survival summary
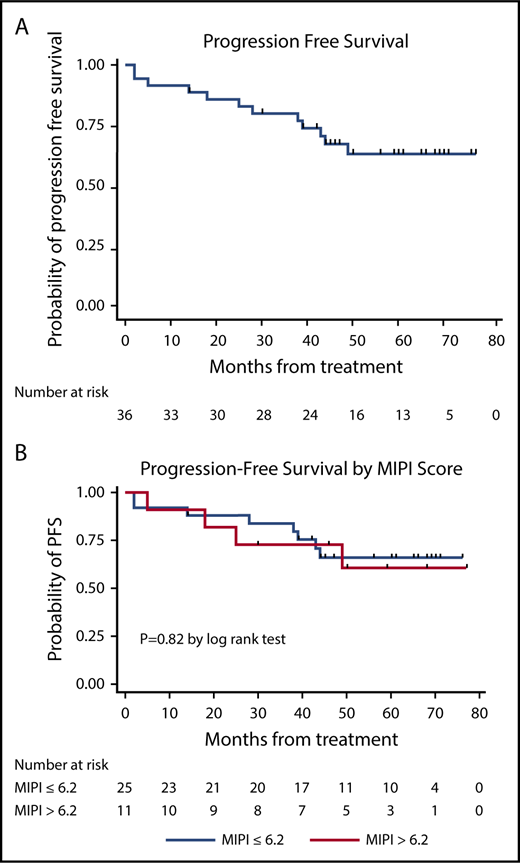
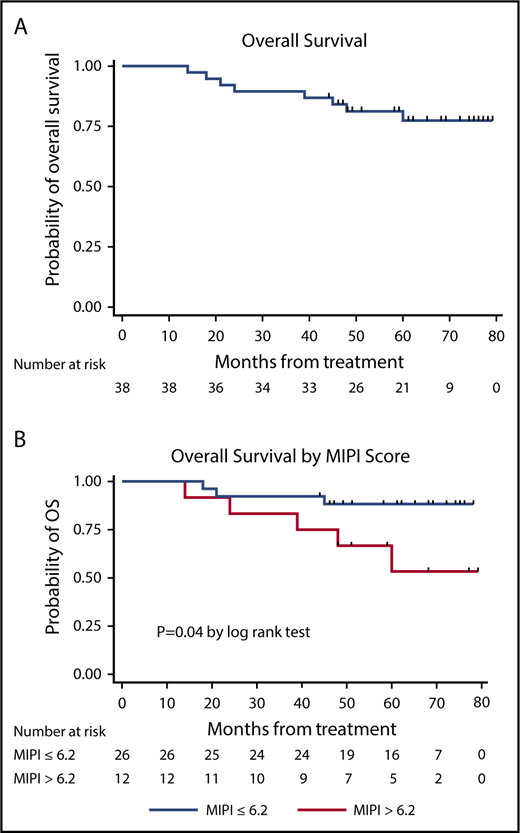
LR produced high response rates (23 CRs and 10 PRs in 36 evaluable patients), as reported previously.23 The median PFS and DOR have not been reached at a median follow-up of 64 months (Figure 2), with 21 of 33 (64%) of the responses ongoing, including 2 patients beyond 3 years, 3 patients beyond 4 years, 10 patients beyond 5 years, and 6 patients beyond 6 years. The 3-year and 5-year PFS rates were estimated at 80.3% (95% CI, 63.0-90.1) and 63.9% (95% CI, 44.8-77.9), respectively, with 3-year and 5-year OS rates at 89.5% (95% CI, 74.3-95.9) and 77.4% (95% CI, 59.4-88.1), respectively (Figures 3 and 4). The MIPI scores were not associated with response or PFS (Figure 3), whereas high-risk MIPI correlated with unfavorable OS (P = .04) (Figure 4). There was no difference in PFS (P = .72) or OS (P = .54) based on Ki67 proliferation index using a cutoff of 30%.
Swimmer's plot of response duration. Thirty-three patients had PR or CR on study treatment. Bar length indicates DOR. The gray shading indicates the induction phase, which consisted of 12 cycles of treatment.
Swimmer's plot of response duration. Thirty-three patients had PR or CR on study treatment. Bar length indicates DOR. The gray shading indicates the induction phase, which consisted of 12 cycles of treatment.
Kaplan-Meier PFS curves. PFS curve (A) and PFS stratification based on MIPI (B).
Kaplan-Meier PFS curves. PFS curve (A) and PFS stratification based on MIPI (B).
Kaplan-Meier OS curves. OS curve (A) and OS stratification based on MIPI (B).
MRD result
The tumor clonotypes were identified in 10 of 11 patients with available diagnostic tissue who are in remission during maintenance (Table 1). The tumor clonotype identification failed in 1 patient because of low DNA input. One-time MRD levels were analyzed in the follow-up PB samples obtained at a median of 46 months (range, 42-62). At the time of PB sampling, 3 of the 10 patients remain on LR maintenance, 5 patients remain on MR alone, and 2 patients were off all treatment at 3 and 10 months. Eight of the 9 CR patients, including the 2 patients off therapy, had MRD levels below the detection threshold of 10−6, whereas the remaining 2 patients had detectable MRD levels of 1.2 × 10−5 and 3 × 10−6.
PB clonoSEQ MRD results
| Subject . | Age at diagnosis, y . | MIPI risk . | Best response . | PFS, mo . | MRD assay time point . | |||
|---|---|---|---|---|---|---|---|---|
| Time on study, mo . | Maintenance regimen . | MRD result . | MRD interpretation . | |||||
| 1 | 51 | Low | CR | 69+ | 51 | LR | <10−6 | Undetectable |
| 2 | 55 | Intermediate | CR | 65+ | 49 | LR | <10−6 | Undetectable |
| 3 | 53 | Low | CR | 65+ | 51 | LR | <10−6 | Undetectable |
| 4 | 65 | Intermediate | CR | 62+ | 42 | None* | <10−6 | Undetectable |
| 5 | 66 | High | CR | 62+ | 62 | R | 3.0 × 10−6 | Detectable |
| 6 | 73 | High | PR | 61+ | 46 | R | 1.2 × 10−5 | Detectable |
| 7 | 56 | Intermediate | CR | 56+ | 43 | None* | <10−6 | Undetectable |
| 8 | 80 | High | CR | 53+ | 35 | R | <10−6 | Undetectable |
| 9 | 60 | Low | CR | 45+ | 42 | R | <10−6 | Undetectable |
| 10 | 82 | High | CR | 46+ | 46 | R | <10−6 | Undetectable |
| Subject . | Age at diagnosis, y . | MIPI risk . | Best response . | PFS, mo . | MRD assay time point . | |||
|---|---|---|---|---|---|---|---|---|
| Time on study, mo . | Maintenance regimen . | MRD result . | MRD interpretation . | |||||
| 1 | 51 | Low | CR | 69+ | 51 | LR | <10−6 | Undetectable |
| 2 | 55 | Intermediate | CR | 65+ | 49 | LR | <10−6 | Undetectable |
| 3 | 53 | Low | CR | 65+ | 51 | LR | <10−6 | Undetectable |
| 4 | 65 | Intermediate | CR | 62+ | 42 | None* | <10−6 | Undetectable |
| 5 | 66 | High | CR | 62+ | 62 | R | 3.0 × 10−6 | Detectable |
| 6 | 73 | High | PR | 61+ | 46 | R | 1.2 × 10−5 | Detectable |
| 7 | 56 | Intermediate | CR | 56+ | 43 | None* | <10−6 | Undetectable |
| 8 | 80 | High | CR | 53+ | 35 | R | <10−6 | Undetectable |
| 9 | 60 | Low | CR | 45+ | 42 | R | <10−6 | Undetectable |
| 10 | 82 | High | CR | 46+ | 46 | R | <10−6 | Undetectable |
R, rituximab.
Two subjects opted to discontinue study treatment after 3 years.
Toxicities
Summary of AEs during maintenance
Treatment was associated with previously reported toxicities (Tables 2 and 3),23 with patients on chronic therapy monitored closely during maintenance. Hematologic toxicities were less frequent or intense compared with those during induction and included asymptomatic grade 3 or 4 cytopenias (42% neutropenia, 5% thrombocytopenia, 3% anemia). Two patients developed febrile neutropenia (5%): 1 patient in the setting of gallstone cholecystitis and the other patient with urosepsis; both resolved with granulocyte colony-stimulating factor and systemic antibiotics. Infections, including upper respiratory infection (45%), urinary tract infection (UTI; 21%), sinusitis (13%), and cellulitis (11%), were primarily grade 1 and 2 and were managed in outpatient settings. Seven patients had grade 3 infections and required brief hospitalization for intravenous antibiotics: in addition to the 2 (5%) aforementioned patients with neutropenic fever, 3 patients (8%) had pneumonia, 1 patient (3%) had recurrent UTI, and 1 patient had West Nile viral encephalitis. Nonhematologic toxicities included primarily grade 1 and 2 diarrhea, hyperglycemia, cough, constipation, hyponatremia, edema, arthralgia, headache, nausea, anorexia, dyspnea, and elevated alanine aminotransferase/aspartate aminotransferase (Figure 5). Grade 1 and 2 constitutional and inflammatory symptoms, such as fatigue (39%), rash (16%), and fever (11%), occurred less frequently during maintenance compared with induction. In comparison, more patients (21%) developed grade 1 and 2 neuropathy during maintenance with prolonged therapy compared with those (8%) who developed it during induction.
LR AEs
| Toxicities* . | Induction, n (%) . | Maintenance (n, %) . | ||
|---|---|---|---|---|
| Any grade . | Grade ≥3 . | Any grade . | Grade ≥3 . | |
| Hematologic | ||||
| Neutropenia | 26 (68) | 16 (42) | 25 (66) | 16 (42) |
| Anemia | 18 (47) | 3 (8) | 12 (32) | 1 (3) |
| Thrombocytopenia | 11 (29) | 4 (11) | 14 (37) | 2 (5) |
| Febrile neutropenia | 1 (3) | 1 (3) | 2 (5) | 2 (5) |
| Infectious | ||||
| URI | 9 (24) | 0 (0) | 17 (45) | 0 (0) |
| UTI | 4 (11) | 0 (0) | 8 (21) | 2 (5) |
| Sinusitis | 2 (5) | 0 (0) | 5 (13) | 0 (0) |
| Cellulitis | 2 (5) | 0 (0) | 4 (11) | 1 (3) |
| Pneumonia | 1 (3) | 1 (3) | 3 (8) | 3 (8) |
| Zoster reactivation | 0 (0) | 0 (0) | 3 (8) | 0 (0) |
| Other | ||||
| Fatigue | 29 (76) | 4 (11) | 15 (39) | 1 (3) |
| Rash | 26 (68) | 11 (29) | 6 (16) | 0 (0) |
| Fever | 22 (58) | 0 (0) | 4 (11) | 0 (0) |
| Cough | 20 (53) | 0 (0) | 9 (24) | 0 (0) |
| Diarrhea | 20 (53) | 0 (0) | 21 (55) | 0 (0) |
| Hyperglycemia | 13 (34) | 2 (5) | 16 (42) | 0 (0) |
| Constipation | 17 (45) | 0 (0) | 7 (18) | 0 (0) |
| Edema | 15 (39) | 0 (0) | 5 (13) | 0 (0) |
| Tumor flare | 14 (37) | 4 (11) | 0 (0) | 0 (0) |
| Infusion reaction | 13 (34) | 1 (3) | 0 (0) | 0 (0) |
| Nausea | 12 (32) | 0 (0) | 2 (5) | 0 (0) |
| Anorexia | 10 (26) | 0 (0) | 3 (8) | 0 (0) |
| Dyspnea | 10 (26) | 1 (3) | 2 (5) | 0 (0) |
| Hyponatremia | 9 (24) | 0 (0) | 7 (18) | 0 (0) |
| Elevated ALT | 9 (24) | 1 (3) | 6 (16) | 1 (3) |
| Elevated AST | 8 (21) | 1 (3) | 5 (13) | 1 (3) |
| Arthralgia | 8 (21) | 1 (3) | 5 (13) | 0 (0) |
| Elevated alkaline phosphatase | 8 (21) | 1 (3) | 6 (16) | 0 (0) |
| Headache | 7 (18) | 0 (0) | 5 (13) | 0 (0) |
| Dizziness | 7 (18) | 0 (0) | 3 (8) | 0 (0) |
| Hypothyroidism | 6 (16) | 0 (0) | 1 (3) | 0 (0) |
| Myalgia | 6 (16) | 1 (3) | 4 (11) | 0 (0) |
| Neuropathy | 3 (8) | 0 (0) | 8 (21) | 0 (0) |
| HGG | 1 (3) | 0 (0) | 3 (8) | 0 (0) |
| Toxicities* . | Induction, n (%) . | Maintenance (n, %) . | ||
|---|---|---|---|---|
| Any grade . | Grade ≥3 . | Any grade . | Grade ≥3 . | |
| Hematologic | ||||
| Neutropenia | 26 (68) | 16 (42) | 25 (66) | 16 (42) |
| Anemia | 18 (47) | 3 (8) | 12 (32) | 1 (3) |
| Thrombocytopenia | 11 (29) | 4 (11) | 14 (37) | 2 (5) |
| Febrile neutropenia | 1 (3) | 1 (3) | 2 (5) | 2 (5) |
| Infectious | ||||
| URI | 9 (24) | 0 (0) | 17 (45) | 0 (0) |
| UTI | 4 (11) | 0 (0) | 8 (21) | 2 (5) |
| Sinusitis | 2 (5) | 0 (0) | 5 (13) | 0 (0) |
| Cellulitis | 2 (5) | 0 (0) | 4 (11) | 1 (3) |
| Pneumonia | 1 (3) | 1 (3) | 3 (8) | 3 (8) |
| Zoster reactivation | 0 (0) | 0 (0) | 3 (8) | 0 (0) |
| Other | ||||
| Fatigue | 29 (76) | 4 (11) | 15 (39) | 1 (3) |
| Rash | 26 (68) | 11 (29) | 6 (16) | 0 (0) |
| Fever | 22 (58) | 0 (0) | 4 (11) | 0 (0) |
| Cough | 20 (53) | 0 (0) | 9 (24) | 0 (0) |
| Diarrhea | 20 (53) | 0 (0) | 21 (55) | 0 (0) |
| Hyperglycemia | 13 (34) | 2 (5) | 16 (42) | 0 (0) |
| Constipation | 17 (45) | 0 (0) | 7 (18) | 0 (0) |
| Edema | 15 (39) | 0 (0) | 5 (13) | 0 (0) |
| Tumor flare | 14 (37) | 4 (11) | 0 (0) | 0 (0) |
| Infusion reaction | 13 (34) | 1 (3) | 0 (0) | 0 (0) |
| Nausea | 12 (32) | 0 (0) | 2 (5) | 0 (0) |
| Anorexia | 10 (26) | 0 (0) | 3 (8) | 0 (0) |
| Dyspnea | 10 (26) | 1 (3) | 2 (5) | 0 (0) |
| Hyponatremia | 9 (24) | 0 (0) | 7 (18) | 0 (0) |
| Elevated ALT | 9 (24) | 1 (3) | 6 (16) | 1 (3) |
| Elevated AST | 8 (21) | 1 (3) | 5 (13) | 1 (3) |
| Arthralgia | 8 (21) | 1 (3) | 5 (13) | 0 (0) |
| Elevated alkaline phosphatase | 8 (21) | 1 (3) | 6 (16) | 0 (0) |
| Headache | 7 (18) | 0 (0) | 5 (13) | 0 (0) |
| Dizziness | 7 (18) | 0 (0) | 3 (8) | 0 (0) |
| Hypothyroidism | 6 (16) | 0 (0) | 1 (3) | 0 (0) |
| Myalgia | 6 (16) | 1 (3) | 4 (11) | 0 (0) |
| Neuropathy | 3 (8) | 0 (0) | 8 (21) | 0 (0) |
| HGG | 1 (3) | 0 (0) | 3 (8) | 0 (0) |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; HGG, hypogammaglobulinemia; URI, upper respiratory infection.
Nonhematologic AEs occurring in >10% of 38 patients.
Severe AEs
| Severe AE . | Grade 3 or 4, n (%) . | Phase . |
|---|---|---|
| Pneumonia | 4 (11) | Induction and maintenance |
| Neutropenic fever | 3 (8) | Induction and maintenance |
| Tumor flare | 4 (11) | Induction |
| Abdominal pain | 2 (5) | Induction |
| Serum sickness | 2 (5) | Induction |
| Syncope | 2 (5) | Maintenance |
| Cholecystitis | 2 (5) | Maintenance |
| UTI | 2 (5) | Maintenance |
| Atrial fibrillation | 1 (3) | Induction |
| Cholangitis | 1 (3) | Induction |
| Dyspnea | 1 (3) | Induction |
| Vertigo | 1 (3) | Induction |
| Ventricular fibrillation | 1 (3) | Induction |
| Rash | 1 (3) | Induction |
| Infusion reaction | 1 (3) | Induction |
| Escherichia coli urosepsis | 1 (3) | Maintenance |
| Hand cellulitis | 1 (3) | Maintenance |
| West Nile viral encephalitis | 1 (3) | Maintenance |
| Car accident | 1 (3) | Maintenance |
| Left femoral neck fracture | 1 (3) | Maintenance |
| SPMs* | ||
| Nonmelanoma skin cancers | 3 (8) | Induction and maintenance |
| Melanoma in situ | 2 (5) | Induction and maintenance |
| Merkel cell carcinoma | 1 (3) | Maintenance |
| Pancreatic cancer | 1 (3) | Maintenance |
| Severe AE . | Grade 3 or 4, n (%) . | Phase . |
|---|---|---|
| Pneumonia | 4 (11) | Induction and maintenance |
| Neutropenic fever | 3 (8) | Induction and maintenance |
| Tumor flare | 4 (11) | Induction |
| Abdominal pain | 2 (5) | Induction |
| Serum sickness | 2 (5) | Induction |
| Syncope | 2 (5) | Maintenance |
| Cholecystitis | 2 (5) | Maintenance |
| UTI | 2 (5) | Maintenance |
| Atrial fibrillation | 1 (3) | Induction |
| Cholangitis | 1 (3) | Induction |
| Dyspnea | 1 (3) | Induction |
| Vertigo | 1 (3) | Induction |
| Ventricular fibrillation | 1 (3) | Induction |
| Rash | 1 (3) | Induction |
| Infusion reaction | 1 (3) | Induction |
| Escherichia coli urosepsis | 1 (3) | Maintenance |
| Hand cellulitis | 1 (3) | Maintenance |
| West Nile viral encephalitis | 1 (3) | Maintenance |
| Car accident | 1 (3) | Maintenance |
| Left femoral neck fracture | 1 (3) | Maintenance |
| SPMs* | ||
| Nonmelanoma skin cancers | 3 (8) | Induction and maintenance |
| Melanoma in situ | 2 (5) | Induction and maintenance |
| Merkel cell carcinoma | 1 (3) | Maintenance |
| Pancreatic cancer | 1 (3) | Maintenance |
Severe AEs are listed, regardless of attribution.
SPMs were reported in 6 subjects, including 2 patients with invasive SPMs. One subject had both Merkel cell carcinoma and melanoma in situ.
Treatment-emergent AEs (≥15% of patients), regardless of attribution. Orange and red bars denote induction AEs. Lavender and blue bars denote maintenance AEs.
Treatment-emergent AEs (≥15% of patients), regardless of attribution. Orange and red bars denote induction AEs. Lavender and blue bars denote maintenance AEs.
Secondary primary malignancies
A total of 6 subjects (16%) reported secondary primary malignancies (SPMs): 5 patients with noninvasive skin cancers requiring local therapy without the need for study interruption, including nonmelanomatous lesions in 3 subjects and melanoma in situ in 2 subjects. Two cases (5%) of invasive systemic malignancies were observed: an 86-year-old subject with Merkel cell carcinoma after 20 months of therapy, who also developed melanoma in situ, and a 68-year-old patient who developed pancreatic cancer after 12 months of therapy (Table 3); both succumbed to the SPMs.
Treatment modifications
During induction, the median dose of lenalidomide was 20 mg in patients with normal renal function; 36% tolerated dose escalation from 20 mg to 25 mg, whereas 42% required dose reduction from 20 mg to ≤15 mg. Of the 33 patients who completed induction, 3 patients permanently discontinued lenalidomide while entering maintenance: 1 patient due to pancreatic cancer detected at month 14, 1 patient due to an incidence of reversible grade 4 ventricular arrhythmia requiring placement of an automated implantable cardioverter defibrillator at month 12, and a third patient per the investigator’s discretion. Of the 30 patients receiving lenalidomide maintenance, 12 discontinued lenalidomide while in remission: 1 patient after development of an asymptomatic grade 4 liver function abnormality, 3 patients after ≥2 years of maintenance lenalidomide per the investigators’ discretion, and 8 patients after ≥3 years of maintenance lenalidomide allowed by the protocol. The median dose of lenalidomide was 10 mg, with 21 of the 30 patients (70%) needing dose reduction from 15 mg to ≤10 mg during the first 3 years of maintenance lenalidomide. In comparison, 13 patients discontinued rituximab while in remission, including 2 patients during induction for serum sickness. Of the 11 who discontinued MR, 1 patient had West Nile viral encephalitis, 1 patient was diagnosed with SPM of pancreatic cancer, 2 patients had frequent infections due to hypogammaglobinemia, and 7 patients completed ≥3 years of MR required by the protocol.
Exploratory treatment-associated chemokine and cytokine profiles
Plasma samples that were prospectively collected at baseline and during treatment on C2D1 and C4D1 were subjected to multiplex assays of cytokines (including IFN-γ, IL-10, IL-12p70, IL-13, IL-2, IL-4, IL-6, IL-8, and TNF-α) and chemokines (including MCP1, MCP4, Eotaxin, IP10, MDC, Eotaxin3, TARC, MIP1α, and MIP1β) (Table 4). Compared with baseline, chemokine levels at C2D1 and C4D1 showed uniform reduction in MCP1 (P = .001 and P = .009), MDC (P = .001 and P = .001), MIP1α (P = .063 and P = .009), and MIP1β (P = .001 and P = .001), suggesting a Th2 to Th1 switch following ≥1 cycle of therapy. Cytokine levels at C2D1 and C4D1 showed a significant decrease in TNF-α (P = .001 and P = .001) and IL-10 (P = .036 and P = .063), as well as a significant increase in IFN-γ (P = .036 and P = .063), illustrating dynamic Th1/Th2 modulation within the assay timeframe. Treatment-associated rash was significantly associated with elevated IL-2 levels at C2D1 (P = .03) and C4D1 (P = .01), reflecting potential immune-based etiology.
Cytokine and chemokine profiles during early induction
| Assays . | C1D1 median, pg/mL . | C2D1 median, pg/mL . | C4D1 median, pg/mL . | P (C1 vs C2) . | P (C1 vs C2)* . | P (C1 vs C4) . | P (C1 vs C4)* . |
|---|---|---|---|---|---|---|---|
| Cytokines | |||||||
| IFN-γ | 6.185 | 6.398 | 8.253 | .004 | .036 | .007 | .063 |
| TNF-α | 21.500 | 5.840 | 4.433 | <.0001 | .001 | <.0001 | .001 |
| IL-2 | 0.275 | 0.117 | 0.139 | .046 | .414 | .032 | .288 |
| IL-4 | 0.000 | 0.008 | 0.001 | .004 | .036 | .017 | .153 |
| IL-6 | 0.974 | 0.815 | 0.768 | .065 | .585 | .122 | 1.000 |
| IL-8 | 4.955 | 4.718 | 5.615 | .864 | 1.000 | .210 | 1.000 |
| IL-10 | 1.575 | 0.936 | 0.780 | .004 | .036 | .007 | .063 |
| IL-12p70 | 0.041 | 0.058 | 0.047 | .202 | 1.000 | .982 | 1.000 |
| IL-13 | .471 | 0.396 | 0.347 | .869 | 1.000 | .841 | 1.000 |
| Chemokines | |||||||
| MCP1 | 34.850 | 22.725 | 22.575 | <.0001 | .001 | .001 | .009 |
| MCP4 | 19.700 | 25.050 | 20.025 | .032 | .288 | .255 | 1.000 |
| Eotaxin | 72.550 | 67.850 | 63.125 | .053 | .477 | .034 | .306 |
| Eotaxin3 | 21.800 | 20.050 | 16.013 | .452 | 1.000 | .007 | .063 |
| IP-10 | 193.500 | 179.000 | 185.500 | .032 | .288 | .624 | 1.000 |
| MDC | 686.500 | 325.000 | 291.500 | <.0001 | .001 | <.0001 | .001 |
| TARC | 30.400 | 31.725 | 19.925 | .339 | 1.000 | .265 | 1.000 |
| MIP1α | 20.300 | 13.600 | 9.828 | .007 | .063 | .001 | .009 |
| MIP1β | 75.750 | 35.175 | 34.525 | <.0001 | .001 | <.0001 | .001 |
| Assays . | C1D1 median, pg/mL . | C2D1 median, pg/mL . | C4D1 median, pg/mL . | P (C1 vs C2) . | P (C1 vs C2)* . | P (C1 vs C4) . | P (C1 vs C4)* . |
|---|---|---|---|---|---|---|---|
| Cytokines | |||||||
| IFN-γ | 6.185 | 6.398 | 8.253 | .004 | .036 | .007 | .063 |
| TNF-α | 21.500 | 5.840 | 4.433 | <.0001 | .001 | <.0001 | .001 |
| IL-2 | 0.275 | 0.117 | 0.139 | .046 | .414 | .032 | .288 |
| IL-4 | 0.000 | 0.008 | 0.001 | .004 | .036 | .017 | .153 |
| IL-6 | 0.974 | 0.815 | 0.768 | .065 | .585 | .122 | 1.000 |
| IL-8 | 4.955 | 4.718 | 5.615 | .864 | 1.000 | .210 | 1.000 |
| IL-10 | 1.575 | 0.936 | 0.780 | .004 | .036 | .007 | .063 |
| IL-12p70 | 0.041 | 0.058 | 0.047 | .202 | 1.000 | .982 | 1.000 |
| IL-13 | .471 | 0.396 | 0.347 | .869 | 1.000 | .841 | 1.000 |
| Chemokines | |||||||
| MCP1 | 34.850 | 22.725 | 22.575 | <.0001 | .001 | .001 | .009 |
| MCP4 | 19.700 | 25.050 | 20.025 | .032 | .288 | .255 | 1.000 |
| Eotaxin | 72.550 | 67.850 | 63.125 | .053 | .477 | .034 | .306 |
| Eotaxin3 | 21.800 | 20.050 | 16.013 | .452 | 1.000 | .007 | .063 |
| IP-10 | 193.500 | 179.000 | 185.500 | .032 | .288 | .624 | 1.000 |
| MDC | 686.500 | 325.000 | 291.500 | <.0001 | .001 | <.0001 | .001 |
| TARC | 30.400 | 31.725 | 19.925 | .339 | 1.000 | .265 | 1.000 |
| MIP1α | 20.300 | 13.600 | 9.828 | .007 | .063 | .001 | .009 |
| MIP1β | 75.750 | 35.175 | 34.525 | <.0001 | .001 | <.0001 | .001 |
P values were derived from the Wilcoxon signed-rank test that compares medians.
C1, cycle 1; C1D1, cycle 1 day 1; C2, cycle 2; C4, cycle 4.
P values were derived from Bonferroni-corrected Wilcoxon signed-rank tests that compare medians.
Discussion
The combination of lenalidomide plus rituximab (LR) as initial treatment for MCL produced high rates of responses (ORR of 92% and CR of 64%) and durable remissions (5-year PFS of 64% and 5-year OS of 77%). Our long-term data provide proof of concept that an outpatient-based induction and maintenance strategy free of conventional chemotherapy is effective, safe, and feasible as first-line therapy for MCL. The efficacy and survival outcome observed in our study compared favorably with those reported with lenalidomide as a single agent17,18 or in combination with rituximab19 in relapsed and refractory settings, lending support for prioritizing novel agents, such as lenalidomide, early in the treatment sequence, in comparison with conventional chemotherapy-based approach.
Remission duration and outcome in MCL have been shown to correlate with MRD status in chemotherapy-based clinical trials, including intensive treatment protocols incorporating high-dose cytarabine and consolidative ASCT.29-33 As consistently demonstrated in the Nordic MCL 2 and MCL 3 studies, the European MCL Network MCL Younger and MCL Elderly trials, and the United States cooperative CALGB 59909 study, molecular remission after induction treatment was highly predictive of response duration and disease progression.30,31,33 In addition, sustained molecular remission was predictive of outcome in MCL Younger after ASCT, MCL Elderly during maintenance,30 and following rituximab pre-emptive treatment after molecular relapses in Nordic MCL studies.33 Although MRD sampling and assay were not prospectively designed in our current study, a 1-time MRD assay with clonoSEQ high-throughput sequencing during maintenance after ≥3 years of therapy showed a high rate of MRD negativity in 8 of 9 CR patients in remission, highlighting the potential to achieve MRD-negative remission with the LR combination. The kinetics of achieving MRD negativity with LR induction, the predictive value of MRD status on response duration, and the outcome both on and off LR maintenance therapy, as well as the molecular remission rate of LR relative to other lenalidomide-based novel regimens,34-36 remain to be determined in future studies. A number of ongoing frontline MCL studies with prospective and serial monitoring of MRD may provide clues to some of the questions, including the phase 2 randomized United States intergroup E1411 with induction of bendamustine and rituximab (BR), with or without bortezomib, followed by maintenance with LR vs R alone (NCT01415752) and the phase 3 randomized European MCL Network LR Elderly study evaluating induction with R-CHOP + R-HAD (rituximab, high dose cytarabine, and dexamethasone) vs R-CHOP alone, followed by maintenance with LR vs R alone.
Treatment-related side effects with LR were as expected23 and similar to those reported in the chemo-free LR arm of the RELEVANCE study,37 which were distinct from the toxicity profile of conventional R-chemotherapy. Primary hematologic toxicity was asymptomatic myelosuppression, which was managed with dose modifications. Neutropenic fever was rare, and there were no treatment-induced toxic deaths. Nonhematologic adverse effects included an inflammatory syndrome with cutaneous rash and tumor flares at the initiation of induction therapy, which resolved with supportive care. Serial plasma sampling during the first 3 cycles of induction therapy showed dynamic Th1/Th2 modulation in cytokine levels and a mostly Th2-to-Th1 transition in the chemokine profile, providing a potential immune-based mechanism underpinning the inflammatory clinical symptoms. Compared with induction, the majority of nonhematologic toxicities during maintenance were nonaccumulative and less frequent with continuous treatment, allowing general preservation of functionality and QoL. A notable exception is the more frequent grade 1 and 2 neuropathy (21%) with prolonged therapy, a known side effect of lenalidomide that was generally reversible with stopping lenalidomide. The incidence of SPM in our study mostly involved skin cancer (5 of 6 subjects), which was managed with local excision while subjects continued their treatment. Invasive SPMs in the current study were reported in 2 patients (5%, no therapy-related acute myeloid leukemia or myelodysplastic syndrome), at a rate comparable to prior studies with single-agent lenalidomide in relapsed/refractory MCL.17,18 Data from the randomized RELEVANCE study comparing LR with R-chemotherapy have shown similar rates of invasive SPMs (5%) in both arms. In comparison, the single-arm LENA-BERIT study reported a higher rate of invasive SPMs (14%),34 which could be attributed to concurrent BR chemotherapy with lenalidomide and/or more advanced patient age.
Over the past decade, 4 nonchemotherapy options (bortezomib, lenalidomide, ibrutinib, and acalabrutinib) have been approved by the US Food and Drug Administration for treatment of MCL.17,38-40 The introduction of novel agents is poised to transform MCL management by making accessible effective and potentially less toxic “chemo-free” treatment in the relapsed/refractory setting, as well as challenging the conventional chemotherapy-based treatment paradigm in the frontline setting. Our LR frontline study is the first chemotherapy-free combination with the longest follow-up that provides induction and maintenance therapy for untreated MCL. The 5-year PFS of 64% is particularly notable relative to historical data reported for patients receiving outpatient-based chemotherapy regimens, such as R-CHOP or BR,23 whereas emerging data on other novel agents, such as Bruton’s tyrosine kinase inhibitors, in the frontline setting remain to mature. The apparent activity of the LR regimen warrants further evaluation in larger and randomized studies. Efforts are also needed to further delineate maintenance duration and intensity by incorporating a real-time MRD-adapted strategy to guide treatment de-escalation vs intensification based on molecular remission status. Future studies with a prospective design to correlate disease biology, including genetic mutations of prognostic significance, with treatment outcome will help to define patient subsets who could potentially benefit the most from targeted therapy, including patients ineligible for, or with high-risk mutations that are resistant to, intensive chemotherapy.36,41,42
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Kseniya Gololobova, Dana Benyas, Emily Kaplan, Louisa Drake, and Paige Wolstencroft for assistance with patient data collection and Lidia Roman-Gonzalez for assistance with biomarker assays.
This work was supported, in part, by Celgene Corporation, Clinical Translational Science Center grant 1-UL1-TR002384-01 (P.C.), and the Lymphoma Foundation (J.P.L.).
Authorship
Contribution: J.R. designed the study, wrote the manuscript, performed data analysis, and participated in patient care; J.P.L. critically contributed to study design, patient care, coordination of the logistics, interpretation of the data, and writing of the manuscript; P.M., B.S., S.J.S., and S.M.S. contributed to the logistic support of the study, patient care, and critically reviewed the manuscript; P.C. performed biostatistical analysis of patient and biomarker data; R.R.F., J.S., M.C., and A.R. contributed to patient care and critical review of the manuscript; L.C., W.T., and M.N.C.-V. performed biomarker analysis; and D.H. assisted in patient data collection and analysis.
Conflict-of-interest disclosure: J.R. has received research support, acted as a consultant for, and served on the advisory board of Celgene. P.M. has received research support and served as a consultant for Celgene. R.R.F. has received research support from Celgene. J.P.L. has received research support and/or served as a consultant for Genentech and Celgene. S.M.S. has served as a consultant for Genentech and Celgene, as well as participated on a Data Safety Monitoring Board for Genentech. J.S. has received research support from Celgene. The remaining authors declare no competing financial interest.
Correspondence: Jia Ruan, Meyer Cancer Center, Weill Cornell Medicine, 1305 York Ave, 7th Floor, New York, NY 10021; e-mail: jruan@med.cornell.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal