Key Points
Daratumumab is effective against T-ALL in human xenograft models.
CD38 is a novel target with broad potential in the treatment of T-ALL.
Abstract
As a consequence of acquired or intrinsic disease resistance, the prognosis for patients with relapsed or refractory T-cell acute lymphoblastic leukemia (T-ALL) is dismal. Novel, less toxic drugs are clearly needed. One of the most promising emerging therapeutic strategies for cancer treatment is targeted immunotherapy. Immune therapies have improved outcomes for patients with other hematologic malignancies including B-cell ALL; however no immune therapy has been successfully developed for T-ALL. We hypothesize targeting CD38 will be effective against T-ALL. We demonstrate that blasts from patients with T-ALL have robust surface CD38 surface expression and that this expression remains stable after exposure to multiagent chemotherapy. CD38 is expressed at very low levels on normal lymphoid and myeloid cells and on a few tissues of nonhematopoietic origin, suggesting that CD38 may be an ideal target. Daratumumab is a human immunoglobulin G1κ monoclonal antibody that binds CD38, and has been demonstrated to be safe and effective in patients with refractory multiple myeloma. We tested daratumumab in a large panel of T-ALL patient-derived xenografts (PDX) and found striking efficacy in 14 of 15 different PDX. These data suggest that daratumumab is a promising novel therapy for pediatric T-ALL patients.
Introduction
Patients with relapsed T-cell acute lymphoblastic leukemia (T-ALL) have dismal outcomes with 3-year event-free survival <15%, as a consequence of chemotherapy-refractory disease.1,2 It is difficult to prevent relapse in de novo T-ALL because of high rates of treatment-related morbidity and mortality with current dose-intensified chemotherapy regimens. Furthermore, significant biologic and genetic heterogeneity exists in T-ALL blasts, challenging the development of a broadly applicable targeted therapy.
A number of targeted immunotherapies have been successfully used in B-cell ALL (B-ALL) patients. Unfortunately, the same is not true for T-ALL. One potential target in T-ALL is CD38, a type II-transmembrane glycoprotein that has been implicated in the regulation of cytoplasmic calcium flux and that mediates signal transduction in immune cells.3 CD38 is expressed on thymocytes, activated T cells, and terminally differentiated B cells, but expressed at very low levels on normal lymphoid and myeloid cells and in some tissues of nonhematopoietic origin. Some hematologic malignancies express CD38.3 Daratumumab is an US Food and Drug Administration–approved human immunoglobulin G1κ monoclonal antibody that binds to a specific epitope of CD38 and is well tolerated and effective in relapsed multiple myeloma (MM).3-7
We hypothesized targeting CD38 would be effective against T-ALL. We demonstrate blasts from patients with T-ALL have robust surface expression of CD38 and that this expression remains stable after exposure to 1 month of multiagent chemotherapy. Further, we demonstrate daratumumab is an effective, potent immunotherapy in vivo, using preclinical models of human T-ALL.
Study design
Patient samples
Blasts were collected from children and young adults with de novo T-ALL under institutional research board–approved protocols in accordance with the Declaration of Helsinki.
CD38 surface staining
Blasts collected from 21 children with T-ALL enrolled on the Combination Chemotherapy With or Without Bortezomib in Treating Younger Patients With Newly Diagnosed T-Cell Acute Lymphoblastic Leukemia or Stage II-IV T-Cell Lymphoblastic Lymphoma clinical trial (AALL1231; clinicaltrials.gov: NCT02112916) were stained for CD38 at diagnosis and after 1 month of chemotherapy by flow cytometry, using published techniques.8 Blasts collected from 10 children with T-ALL enrolled on the Combination Chemotherapy in Treating Young Patients With Newly Diagnosed T-Cell Acute Lymphoblastic Leukemia or T-cell Lymphoblastic Lymphoma clinical trial (AALL0434; clinicaltrials.gov: NCT00408005) who subsequently relapsed were also stained for CD38 expression at diagnosis and relapse.
In vivo xenograft experiments
Patient-derived xenograft (PDX) models using nonobese diabetic/severe combined immunodeficiency (NOD/SCID/Il2rgtm1wjl/SzJ) mice were developed from 15 patients with T-ALL as previously described.9,10 Mice (5 per treatment arm, 10 per sample) were randomized to receive daratumumab (8 mg/kg intraperitoneally [IP] weekly) vs isotype control (8 mg/kg IP) following the detection of at least 1% peripheral blood (PB) blasts. A second cohort termed “low disease burden” began treatment with daratumumab within 1 week of initial adoptive transfer of ALL cells. Disease burden was assessed weekly by flow cytometric measurement of PB. See the supplemental Data, available on the Blood Web site, for additional methods and statistical analyses.
Results and discussion
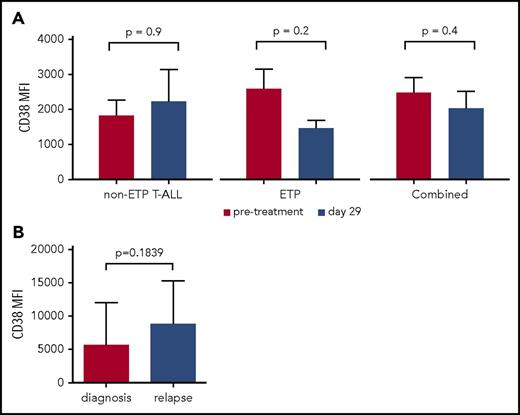
To demonstrate CD38 is a relevant and stable target, we measured CD38 expression from a panel of patients with T-ALL at diagnosis and following induction therapy. This panel included 10 patients with early T-cell precursor (ETP) ALL, a subtype characterized by a distinct immunophenotype, poor initial response to chemotherapy, and inferior outcome.9,11-14 We observed robust CD38 expression at baseline in 21 of 21 patients tested (11 non-ETP T-ALL and 10 ETP T-ALL) (Figure 1A). We demonstrated persistent CD38 expression in matched samples after 1 month of therapy, which included dexamethasone, vincristine, daunorubicin, and pegaspargase, with or without bortezomib. Further, paired T-ALL samples at diagnosis and at relapse similarly demonstrate persistent CD38 expression (Figure 1B). In contrast, we saw downregulation of CD38 in B-ALL samples following induction chemotherapy (supplemental Figure 1). Baseline demographic data including sex, age, and presenting white blood cell count are shown in supplemental Table 1. These data suggest CD38 expression is not affected by conventional cytotoxic chemotherapy in T-ALL, making it an attractive therapeutic target. We also measured CD38 in a panel of 15 PDX samples (7 ETP and 8 non-ETP), of which only 1 had low expression, TALL20 (Figure 2; supplemental Figure 2).
CD38 is expressed on T-ALL and ETP T-ALL blasts with stable expression following induction chemotherapy and at relapse. (A) Averages of MFI measured by flow cytometry of 21 patients with T-ALL enrolled on the Combination Chemotherapy With or Without Bortezomib in Treating Younger Patients With Newly Diagnosed T-Cell Acute Lymphoblastic Leukemia or Stage II-IV T-Cell Lymphoblastic Lymphoma (AALL1231) study. The gating strategy is described in the supplemental Methods. Gating is on the blast population and therefore represents patients with residual, detectable ALL. (B) MFI of CD38 from 10 T-ALL patients previously enrolled on the Combination Chemotherapy in Treating Young Patients With Newly Diagnosed T-Cell Acute Lymphoblastic Leukemia or T-cell Lymphoblastic Lymphoma (AALL0434) study from initial diagnosis and at first relapse. MFI, median fluorescence intensity.
CD38 is expressed on T-ALL and ETP T-ALL blasts with stable expression following induction chemotherapy and at relapse. (A) Averages of MFI measured by flow cytometry of 21 patients with T-ALL enrolled on the Combination Chemotherapy With or Without Bortezomib in Treating Younger Patients With Newly Diagnosed T-Cell Acute Lymphoblastic Leukemia or Stage II-IV T-Cell Lymphoblastic Lymphoma (AALL1231) study. The gating strategy is described in the supplemental Methods. Gating is on the blast population and therefore represents patients with residual, detectable ALL. (B) MFI of CD38 from 10 T-ALL patients previously enrolled on the Combination Chemotherapy in Treating Young Patients With Newly Diagnosed T-Cell Acute Lymphoblastic Leukemia or T-cell Lymphoblastic Lymphoma (AALL0434) study from initial diagnosis and at first relapse. MFI, median fluorescence intensity.
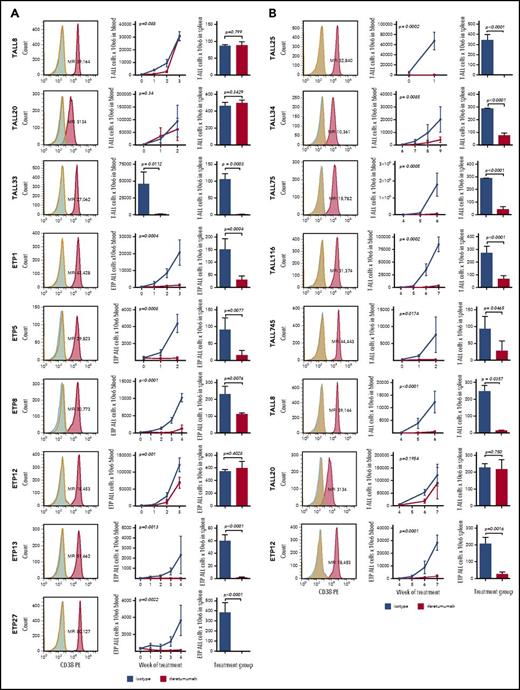
In vivo efficacy of daratumumab in ETP T-ALL and non-ETP T-ALL. PDX models were treated with isotype control or daratumumab weekly for 3 to 8 weeks depending on the rate of leukemia progression in control animals. Experiments were also varied based on daratumumab treatment at “low” vs “high” disease burden. The mice were treated an average of 4 doses at the high disease burden and 7 at low disease burden. The duration of treatment was determined by the need to euthanize control animals because of overwhelming disease burden. Each experiment represents n = 5 mice per treatment group. (A) Trials of mice treated at high disease burden that did not experience toxicity are shown for T-ALL and ETP T-ALL xenografts. (Left) Histogram of CD38-PE (red) with the corresponding MFI compared with an unstained (blue) and isotype control (green) to demonstrate positivity above background. Graphed are means and standard deviations of absolute blast count on the vertical axis and weeks of treatment on the horizontal axis. Treatment groups (red) were compared with mice treated with isotype antibody control (blue) for each PDX model using a nonparametric Mann-Whitney U test. P values are shown. ID-TALL33 was only bled once at the time of harvest because of the aggressiveness of disease progression. Mice engrafted from this sample do not tolerate serial bleeds; therefore, blood and spleen data are shown only at the time euthanization. (B) Experiments performed at low disease burden of samples that experienced toxicity when tested at high disease burden. Two samples, ID-TALL8 and ID-ETP12, represent repeated trials of daratumumab tested at low disease state; they did not respond at the high disease state.
In vivo efficacy of daratumumab in ETP T-ALL and non-ETP T-ALL. PDX models were treated with isotype control or daratumumab weekly for 3 to 8 weeks depending on the rate of leukemia progression in control animals. Experiments were also varied based on daratumumab treatment at “low” vs “high” disease burden. The mice were treated an average of 4 doses at the high disease burden and 7 at low disease burden. The duration of treatment was determined by the need to euthanize control animals because of overwhelming disease burden. Each experiment represents n = 5 mice per treatment group. (A) Trials of mice treated at high disease burden that did not experience toxicity are shown for T-ALL and ETP T-ALL xenografts. (Left) Histogram of CD38-PE (red) with the corresponding MFI compared with an unstained (blue) and isotype control (green) to demonstrate positivity above background. Graphed are means and standard deviations of absolute blast count on the vertical axis and weeks of treatment on the horizontal axis. Treatment groups (red) were compared with mice treated with isotype antibody control (blue) for each PDX model using a nonparametric Mann-Whitney U test. P values are shown. ID-TALL33 was only bled once at the time of harvest because of the aggressiveness of disease progression. Mice engrafted from this sample do not tolerate serial bleeds; therefore, blood and spleen data are shown only at the time euthanization. (B) Experiments performed at low disease burden of samples that experienced toxicity when tested at high disease burden. Two samples, ID-TALL8 and ID-ETP12, represent repeated trials of daratumumab tested at low disease state; they did not respond at the high disease state.
We investigated whether daratumumab would be effective in decreasing leukemia burden in vivo using T-ALL PDX models. We tested a total of 15 unique T-ALL samples (7 ETP and 8 non-ETP); Figure 2). Daratumumab was effective in 6 of 7 ETP samples, demonstrating significant reduction of leukemia burden in blood and spleen as compared with controls without evidence of toxicity (Figure 2A). Of the 8 T-ALL PDX tested with daratumumab, mice engrafted from 5 of the samples became moribund after initial injection of daratumumab (range, 20-60 minutes). This reproducible and sample-dependent toxicity occurred independently of mode of drug administration (IV vs IP) or with isotype control. Autopsy performed of moribund mice compared with isotype-treated animals (n = 20; 10 isotype, 10 daratumumab) did not identify a distinguishing etiology (data not shown) and eliminated tumor aggregates with embolism as a possible cause of death. We hypothesize the mice died of massive tumor lysis syndrome (supplemental Data; supplemental Figure 4). These samples were considered inevaluable for efficacy when treated with this standard approach. Three T-ALL samples did not experience the toxicity (TALL8, TALL20, TALL33) and daratumumab was effective in 1, TALL33 (Figure 2A).
To obviate the toxicity, we adopted an alternative approach termed low disease burden. This method introduced daratumumab within 1 week of initial transfer of ALL cells, akin to a minimal residual disease positive state (eg, undetectable disease in the PB but likely blasts in the bone marrow or spleen). With this approach, we found daratumumab was well tolerated and effective in 5 of 5 of the T-ALL samples that experienced toxicity when treated at high disease burden (Figure 2B). Repeat testing of the 2 samples that did not show a response at high disease burden responded at low disease burden. The only nonresponder was the sample with low to negative CD38 expression (TALL20). We also demonstrated significantly prolonged survival among 3 samples: TALL116, ETP1, and TALL33 (supplemental Figure 3). In summary, 7 of 10 evaluable PDX responded to daratumumab when treated at high disease burden and 14 of 15 PDX responded to daratumumab using either approach.
Preclinical studies of daratumumab in MM suggest the level of CD38 expression correlates with sensitivity to drug.15-17 We measured CD38 expression pre- and postdaratumumab to evaluate its potential pressure on CD38 expression using multiple techniques (supplemental Data; supplemental Figure 6). Our data suggest CD38 is not downregulated following exposure to daratumumab.
We have extensive experience treating ALL xenografts with cytotoxic chemotherapy and newer targeted agents.9,10,18-20 The degree of efficacy seen with daratumumab was more pronounced than the majority of drugs we have tested in B-ALL or T-ALL PDX models. Daratumumab is purported to mediate its antitumor effects via antibody-dependent cytotoxicity and complement-dependent cytotoxicity.3 Surprisingly, we demonstrated efficacy of daratumumab in an immunodeficient mouse without exogenous immune cells to mediate antibody-dependent cytotoxicity or complement-dependent cytotoxicity. This may indicate that other mechanisms including apoptosis after CD38 crosslinking or modulation of enzymatic activation play key roles in the mechanism of daratumumab-killing. The pronounced efficacy of daratumumab in the absence of a normal immune system is compelling, suggesting it may be even more potent in immunocompetent patients, although the underlying cellular mechanisms and the contribution of each mechanism to the overall antitumor effect in vivo needs to be elucidated (supplemental Data; supplemental Figure 5). These data suggest other modalities to target CD38, including chimeric antigen receptor T cells, are worth exploring for T-ALL.
In summary, we present data of a potent targeted immunotherapy approach for T-ALL. We found daratumumab was highly effective in a large number of T-ALL samples, suggesting broad antileukemic efficacy. Based on preclinical data and the emerging evidence that daratumumab can be combined with conventional cytotoxics with minimal toxicity in adults with MM, we are planning to open a multi-institutional early-phase trial for children and young adults with relapsed and refractory T-ALL. We anticipate we will successfully translate daratumumab into the clinic and hopefully improve cure rates for T-ALL patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all participants and their families.
This study was supported by grant funding from the Alex’s Lemonade Stand Foundation for Childhood Cancer (K.L.B.), Leukemia and Lymphoma Society (D.T.T., W.L.C., S.A.G., S.P.H., and E.A.R.), the National Institutes of Health National Cancer Institute (R01CA193776) (D.T.T., M.L.H., T.M.H., and B.L.W.), a Stand Up 2 Cancer-St. Baldricks’ Pediatric Dream Team translational research grant (SU2C-AACR-DT1113) (D.T.T., R.A., S.A.G., and S.L.M.), and a Research Scholar Grant from the American Cancer Society (RSG-13-022-01-CDD) (D.T.T.). S.P.H is the Jeffrey E. Perelman Distinguished Chair in the Department of Pediatrics, Children’s Hospital of Philadelphia.
The funding sources had no role in data collection, analysis, or interpretation of the data, the writing of the report, or the decision to submit for publication.
Authorship
Contribution: K.L.B., T.L.V., D.T.T., and B.L.W. designed the study, analyzed data, and wrote the manuscript; K.L.B., B.L.W., S.-Y.I., Y.D., M.D., and T.F. contributed to data acquisition and manuscript editing; and R.A., D.M.B., W.L.C., R.C., K.P.D., T.G.-A., S.A.G., T.M.H., S.P.H., M.L.L., S.L.M., E.A.R., S.S.W., and M.L.H. contributed to study design and manuscript editing.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David T. Teachey, 3008 Colket Translational Research Building, 3501 Civic Center Blvd, Philadelphia, PA 19104; e-mail: teacheyd@email.chop.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal