Key Points
The combination of romidepsin and pralatrexate is safe and well tolerated in patients with relapsed/refractory lymphoma.
The combination led to an overall response rate of 71% (10/14, with 4/14 complete responses) in patients with relapsed/refractory T-cell lymphoma.
Abstract
Peripheral T-cell lymphomas (PTCL) are a group of rare malignancies characterized by chemotherapy resistance and poor prognosis. Romidepsin and pralatrexate were approved by the US Food and Drug Administration for patients with relapsed/refractory PTCL, exhibiting response rates of 25% and 29% respectively. Based on synergy in preclinical models of PTCL, we initiated a phase 1 study of pralatrexate plus romidepsin in patients with relapsed/refractory lymphoma. This was a single institution dose-escalation study of pralatrexate plus romidepsin designed to determine the dose-limiting toxicities (DLTs), maximum tolerated dose, pharmacokinetic profile, and response rates. Patients were treated with pralatrexate (10 to 25 mg/m2) and romidepsin (12 to 14 mg/m2) on 1 of 3 schedules: every week × 3 every 28 days, every week × 2 every 21 days, and every other week every 28 days. Treatment continued until progression, withdrawal of consent, or medical necessity. Twenty-nine patients were enrolled and evaluable for toxicity. Coadministration of pralatrexate and romidepsin was safe, well tolerated, with 3 DLTs across all schedules (grade 3 oral mucositis × 2; grade 4 sepsis × 1). The recommended phase 2 dose was defined as pralatrexate 25 mg/m2 and romidepsin 12 mg/m2 every other week. Twenty-three patients were evaluable for response. The overall response rate was 57% (13/23) across all patients and 71% (10/14) in PTCL. The phase 1 study of pralatrexate plus romidepsin resulted in a high response rate in patients with previously treated PTCL. A phase 2 study in PTCL will determine the efficacy of the combination. This trial was registered at www.clinicaltrials.gov as #NCT01947140.
Introduction
Peripheral T-cell lymphomas (PTCL) are a group of rare heterogeneous malignancies with an aggressive course, characterized by relative resistance to conventional chemotherapy and an inferior prognosis compared with their B-cell counterparts.1,2 Front-line therapy has been extrapolated from experiences treating B-cell lymphoma and is predicated on a CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone)–based backbone.3 Modest attempts to improve outcome have been made by adding agents like etoposide and/or by consolidating responses with autologous stem cell transplantation.4,5 The lack of randomized studies for these approaches makes it difficult to precisely quantitate the clinical benefit, although most believe the effect on survival is marginal.
Over the past 8 years, 3 new classes of drugs have been approved for the group of diseases recognized as PTCL. The novel antifolate pralatrexate was the first drug approved for patients with relapsed or refractory PTCL in 2009.6 Four histone deacetylase (HDAC) inhibitors have been approved, including vorinostat, romidepsin, belinostat, and chidamide (approved in China).7-13 The antibody drug conjugate brentuximab vedotin was approved in one subtype of PTCL, anaplastic large T-cell lymphoma.14 The HDAC inhibitors and pralatrexate exhibit near–lineage-specific activity with limited to no activity in B-cell lymphomas. As single agents in the relapsed setting, romidepsin and pralatrexate exhibit response rates of 25% to 38% and 29% to 54%, respectively, across published phase 1 and 2 studies.7-10,15 Although these studies are not identical in their patient composition, they included patients who are heavily pretreated from a diversity of PTCL subtypes. A recent case-control analysis demonstrated that patients treated with pralatrexate on PROPEL achieved a statistically significant survival advantage when compared with a matched historical population.16 In addition, subanalysis of patients treated on PROPEL revealed that response and time-to-event metrics (duration of response [DOR] and progression-free survival [PFS]) with pralatrexate improved as the therapy was used earlier in their treatment course, with a complete response (CR), PFS, and DOR of 17%, 8 months, and not reached (at 2 years) as second-line treatment.17 Patients achieving a response to romidepsin also exhibited a prolonged DOR of 28 months, with the median DOR not being reached in patients achieving CR.18
Rather than merely adding new agents to CHOP,19-22 our group pioneered the concept of creating novel platforms according to the following principles: (1) translating drugs uniquely approved in PTCL found to be synergistic in preclinical models of TCL; (2) exploring the merits of integrating drugs targeting the molecular derangements seen in PTCL; and (3) integrating complementary agents based on our evolving understanding of the mechanism of synergy.23-26 One example vetted in preclinical models was the combination of pralatrexate and romidepsin.24 These data established that the 2 drugs demonstrated potent synergy at dose levels 50% of their maximum tolerated dose (MTD). We translated these findings into a phase 1 clinical study of the combination of pralatrexate and romidepsin in patients with relapsed or refractory lymphoma. Herein, we report these findings.
Methods
Study design and patients
This was a single-institution, open-label, 3+3 dose-escalation phase 1 study aimed at assessing the safety, tolerability, and early activity of response for the combination of pralatrexate and romidepsin. In addition, the trial was designed to explore schedule and pharmacokinetic (PK) profile. Patients were enrolled at the Center for Lymphoid Malignancies at Columbia University Medical Center (New York, NY) under an institutional review board–approved protocol. The study was conducted according to the provisions of the Declaration of Helsinki and the International Conference on Harmonization Guidelines for Good Clinical Practice and was registered at www.clinicaltrials.gov as #NCT01947140. All patients provided written informed consent. Eligible patients were required to have histologically confirmed relapsed or refractory lymphoma of any subtype or myeloma. There was no upper limit for the number of prior therapies. Patients who relapsed after autologous or allogeneic stem cell transplant were eligible. Inclusion criteria were as follows: evaluable disease, age ≥18 years, Eastern Cooperative Group performance status ≤2, negative pregnancy test result for females of childbearing potential, adequate contraception, and adequate organ and marrow function. Patients were ineligible if they had central nervous system disease or lymphomatous meningitis; took concomitant CYP3A4 inhibitors, had a history of any severe cardiac abnormalities, were HIV positive, or had active hepatitis A, B, or C. Patients were eligible if they had previously received romidepsin or pralatrexate.
Procedures
Patients were treated with pralatrexate (Spectrum Pharmaceuticals) and romidepsin (Celgene Corporation) administered IV on 1 of 3 treatment schedules: cohort 1 (days 1, 8, and 15 on a 28-day schedule [every week × 3 every 28 days]), schedule A (days 1 and 8 on a 21-day cycle [every week × 2 every 21 days]), and schedule B (days 1 and 15 on a 28-day treatment cycle [every other week every 28 days) (Figure 1A). All patients received 1 mg folic acid orally daily starting 7 days prior to initiation of study drugs and 1000 μg of vitamin B12 intramuscularly once every 8 to 10 weeks per US Food and Drug Administration label. Cohorts of 3 patients were enrolled at pralatrexate doses starting at 10 mg/m2 incrementally escalated to 25 mg/m2 and romidepsin 12 mg/m2 escalated to 14 mg/m2. Dose escalations commenced for each schedule if less than 33% of patients experienced a dose-limiting toxicity (DLT).
Schematic of study design, patient disposition, and thrombocytopenia as a function of schedule dose. (A) Screening and enrollment data for all patients. (B-D) Platelet trend over time. Platelet-retreatment parameter is 50 000/μL. (B) Cohort 1 patients were treated with pralatrexate 10 mg/m2 and romidepsin 12 mg/m2 on days 1, 8, and 15 every 28 days. (C) Cohorts treated on schedule A received pralatrexate 15 to 20 mg/m2 and romidepsin 12 to 14 mg/m2 on days 1 and 8 every 21 days. (D) Cohorts treated on schedule B received pralatrexate 15 to 25 mg/m2 and romidepsin 12 to 14 mg/m2 days 1 and 15 every 28 days. C, cycle; D, day; SCR, screening.
Schematic of study design, patient disposition, and thrombocytopenia as a function of schedule dose. (A) Screening and enrollment data for all patients. (B-D) Platelet trend over time. Platelet-retreatment parameter is 50 000/μL. (B) Cohort 1 patients were treated with pralatrexate 10 mg/m2 and romidepsin 12 mg/m2 on days 1, 8, and 15 every 28 days. (C) Cohorts treated on schedule A received pralatrexate 15 to 20 mg/m2 and romidepsin 12 to 14 mg/m2 on days 1 and 8 every 21 days. (D) Cohorts treated on schedule B received pralatrexate 15 to 25 mg/m2 and romidepsin 12 to 14 mg/m2 days 1 and 15 every 28 days. C, cycle; D, day; SCR, screening.
Treatment continued until disease progression or voluntary withdrawal of consent or because of medical necessity. Once a DLT was identified, 3 additional patients were recruited to that cohort. If a second DLT was observed, this cohort was determined to be the maximum administrable dose, and the escalation was halted. No intrapatient dose escalations were allowed. The MTD was defined as the dose level at which one-third or fewer patients experienced a DLT. Standard supportive treatment was allowed, including antiemetics, antidiarrheals, antipyretics, antihistamines, analgesics, antibiotics, and blood products. Leucovorin (15 mg orally twice a day on days 3-6) was permitted following cycle 1.
Blood samples for safety and PK analyses were taken on days 1, 8, 15, and 22 during cycle 1 and on days of study drug administration in subsequent cycles. The last study visit was 4 weeks after the last dose of study drug administration. All adverse events were evaluated according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0. Patients receiving 1 dose of drug were considered evaluable for toxicity and DLT determination. DLTs were determined in cycle 1 only. DLTs were defined as any missed dose within cycle 1 and/or toxicity that is possibly related to drug, occurring up to 7 days after completion of cycle 1 that results in a delay of initiating cycle 2; grade 4 neutropenia that does not resolve to grade ≤2 within ≤7 days; grade 3 febrile neutropenia (absolute neutrophil count <1000/mm3 with a single temperature of >38.3°C or sustained temperature of ≥38°C for >1 hour); grade ≥3 thrombocytopenia associated with clinically important bleeding or lasting ≥7 days or grade 4 thrombocytopenia that necessitates a platelet transfusion or does not resolve within 7 days; grade 5 (death) hematologic toxicity; any grade ≥3 nonhematologic toxicity, with the specific exception of nausea, vomiting, diarrhea, or dehydration lasting >48 hours in the setting of inadequate compliance with supportive care measures; acidosis or alkalosis that responds to medical intervention and returns to grade ≤2 within 48 hours; elevation of liver function test results or amylase without clinical symptoms lasting ≤5 days; hypocalcemia, hypokalemia, hypomagnesemia, hyponatremia, or hypophosphatemia that responds to medical intervention; and grade 3 hypercholesterolemia, hypertriglyceridemia, constipation, and fatigue. All adverse events, both drug related and non–drug related, were monitored for 4 weeks after discontinuation of study treatment. Staging with computed tomography or positron emission tomography-computed tomography were performed as well as the modified severity-weighted assessment tool for patients with cutaneous involvement.27 Response assessments were performed every 2 cycles through the first 6 cycles and then at the treating physician’s discretion, but no more than at 6-month intervals until progression. All patients were monitored after discontinuation of study treatment for both survival and subsequent lines of therapy, where possible.
Statistical analysis
The study employed a 3+3 dose-escalation design to assess safety, and tolerability of pralatrexate plus romidepsin. All patients were included in the safety analysis. The primary objective was to the determine MTD and DLT of the combination in patients with relapsed or refractory lymphoma and multiple myeloma. Secondary objectives included describing overall response rate (CR plus partial response [PR]), PFS and DOR. Response was determined using clinical parameters, computed tomography or positron emission tomography-computed tomography, bone marrow biopsy, and modified severity-weighted assessment tool as defined by the guidelines of the International Harmonization Project Group 2014 Revised Response Criteria.28 Patients considered evaluable for response were required to have received at least 2 cycles of therapy.
Descriptive statistics were used to summarize patient’s demographic, baseline characteristics, prior therapies, and safety and efficacy measures. Summary statistics for continuous variables included mean ± standard deviation and/or median (interquartile range); categorical variables were reported as frequency counts and percentages. Time-to-event end points such as overall survival (OS) and PFS were estimated using Kaplan-Meier method and group comparison were assessed using 2-sided log-rank test and Cox regression for estimating the hazard ratio (95% confidence interval [CI]). OS was defined as time from first treatment to death or last date of contact. PFS was measured from time of first treatment to progression/death or to the date of transitioning treatment. DOR was measured from time of first response to progression/death and summarized as medians (interquartile range). All the analyses were performed in SAS (version 9.4; SAS, Cary, NC) using a type I error of 0.05.
PK analysis
To define the PK profile of pralatrexate and romidepsin, plasma samples were collected during cycle 1 at the start of infusion and end of infusion and then at 0.5, 1, 2, 24, and 48 hours. Noncompartmental analysis was performed using Phoenix Winnonlin software (Certara, Princeton, NJ) to define the maximum plasma concentration (Cmax), the time to maximum plasma concentration (Tmax), the terminal half-life (t1/2), the area under the plasma concentration time curve from t = 0 to the last data point (AUClast) and infinity (AUCinf), and the clearance. Paired t tests were calculated using GraphPad Prism version 5.04 (GraphPad Software, San Diego, CA).
For analytical pharmacology, romidepsin and pralatrexate were purchased from Selleck Chemicals (Houston, TX) and romidepsin-d7 from Clearsynth (Mumbai, India). All solvents and chemicals were liquid chromatography-mass spectrometry grade. Romidepsin and pralatrexate were extracted from blood plasma (EDTA) by mixing 50 μL plasma with 500 μL acetonitrile/methanol. Liquid chromatography-tandem mass spectrometry analysis was done using Agilent 6410 triple quad mass spectrometer connected to Agilent 1290 Infinity UHPLC (Santa Clara, CA). Data acquisition and peak integration was done using MassHunter software version 3.1. Quantitative measurements were done in Multiple Selected Reaction Monitoring mode using positive electrospray ionization. The assay performance was validated according to US Food and Drug Administration guidelines.29
Results
Patients and treatment
As of 1 March 2017, 29 patients were enrolled on the phase 1 study and all were evaluable for toxicity (Figure 1A). Table 1 details the demographic characteristics of the patients enrolled in the study. The median age was 54 years (range, 23-73 years), and 18 (62%) were male. The median number of prior systemic therapies was 3 (range, 1-16). Histologies included Hodgkin lymphoma (n = 3), diffuse large B-cell lymphoma (n = 1), Burkitt lymphoma (n = 1), indolent B-cell lymphoma (n = 5), blastic plasmacytoid dendritic cell neoplasm (n = 1), and T-cell lymphoma (n = 18).
Demographic features of study populations
| Demographic feature . | n . |
|---|---|
| Age (y), median (range) | 54 (23-73) |
| Sex (%) | |
| Male | 18 (62) |
| Female | 11 (38) |
| Race (%) | |
| Black | 8 (29) |
| White | 17 (59) |
| Asian | 3 (10) |
| Other | 1 (3) |
| Ethnicity (%) | |
| Hispanic | 7 (24) |
| Non-Hispanic | 22 (76) |
| Disease type (%) | |
| B-cell lymphomas | 7 (24) |
| Burkitt | 1 (3) |
| DLBCL | 1 (3) |
| Follicular | 5 (17) |
| T-cell lymphomas | 18 (62) |
| ATLL | 6 (21) |
| ALCL ALK(−) | 3 (10) |
| Sezary syndrome | 2 (7) |
| CTCL | 1 (3) |
| CD4+ T cell | 1 (3) |
| Hepatosplenic T cell | 1 (3) |
| Intestinal T cell | 1 (3) |
| NK T cell | 1 (3) |
| PTCL | 1 (3) |
| SPTL-AB | 1 (3) |
| Other | 4 (14) |
| Blastic plasmacytoid dendritic cell neoplasm | 1 (3) |
| Hodgkin lymphoma | 3 (10) |
| Prior therapies, median (range) | 3 (1-16) |
| CHOP/RCHOP/CHOEP/EPOCH/hyper-CVAD | 24 (83) |
| Experimental therapies: clinical trials | 11 (38) |
| Gemcitabine based: GEM/GemiFOX/GemOX/GVD | 9 (31) |
| HDAC inhibitors | 9 (31) |
| Alkylator based: Benda/CTX/CVP | 8 (28) |
| Platinum based: RICE/ICE/DHAP/ESHAP | 7 (24) |
| Radiation | 7 (24) |
| Biologics: bexarotene/ublituximab/Rituxan | 7 (24) |
| Autologous stem cell transplant | 6 (21) |
| MTX/SMILE | 5 (17) |
| ABVD/ABV-COPP/MOPP | 4 (14) |
| Brentuximab vedotin | 4 (14) |
| Lenalidomide based | 4 (14) |
| Phototherapy: light/PUVA | 3 (10) |
| Pralatrexate | 2 (7) |
| Allogeneic transplant | 1 (3) |
| Demographic feature . | n . |
|---|---|
| Age (y), median (range) | 54 (23-73) |
| Sex (%) | |
| Male | 18 (62) |
| Female | 11 (38) |
| Race (%) | |
| Black | 8 (29) |
| White | 17 (59) |
| Asian | 3 (10) |
| Other | 1 (3) |
| Ethnicity (%) | |
| Hispanic | 7 (24) |
| Non-Hispanic | 22 (76) |
| Disease type (%) | |
| B-cell lymphomas | 7 (24) |
| Burkitt | 1 (3) |
| DLBCL | 1 (3) |
| Follicular | 5 (17) |
| T-cell lymphomas | 18 (62) |
| ATLL | 6 (21) |
| ALCL ALK(−) | 3 (10) |
| Sezary syndrome | 2 (7) |
| CTCL | 1 (3) |
| CD4+ T cell | 1 (3) |
| Hepatosplenic T cell | 1 (3) |
| Intestinal T cell | 1 (3) |
| NK T cell | 1 (3) |
| PTCL | 1 (3) |
| SPTL-AB | 1 (3) |
| Other | 4 (14) |
| Blastic plasmacytoid dendritic cell neoplasm | 1 (3) |
| Hodgkin lymphoma | 3 (10) |
| Prior therapies, median (range) | 3 (1-16) |
| CHOP/RCHOP/CHOEP/EPOCH/hyper-CVAD | 24 (83) |
| Experimental therapies: clinical trials | 11 (38) |
| Gemcitabine based: GEM/GemiFOX/GemOX/GVD | 9 (31) |
| HDAC inhibitors | 9 (31) |
| Alkylator based: Benda/CTX/CVP | 8 (28) |
| Platinum based: RICE/ICE/DHAP/ESHAP | 7 (24) |
| Radiation | 7 (24) |
| Biologics: bexarotene/ublituximab/Rituxan | 7 (24) |
| Autologous stem cell transplant | 6 (21) |
| MTX/SMILE | 5 (17) |
| ABVD/ABV-COPP/MOPP | 4 (14) |
| Brentuximab vedotin | 4 (14) |
| Lenalidomide based | 4 (14) |
| Phototherapy: light/PUVA | 3 (10) |
| Pralatrexate | 2 (7) |
| Allogeneic transplant | 1 (3) |
ABV-COPP, adriamycin, bleomycin, vinblastin, cyclophosphamide, vincristine, procarbazine, prednisone; ABVD, adriamycin, bleomycin, vinblastine, dacarbazine; ALCL, anaplastic large cell lymphoma; ALK, anaplastic lymphoma kinase; ATLL, adult T-cell leukemia/lymphoma; CHOEP, cyclophosphamide, doxorubicin, vincristine, etoposide, and prednisone; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; CTCL, cutaneous T-cell lymphoma; CTX, cyclophosphamide; CVAD, cyclophosphamide, vincristine, doxorubicin, and dexamethasone; CVP, cyclophosphamide, vincristine, prednisone; DHAP, dexamethasone, high dose cytarabine, cisplatin; DLBCL, diffuse large B-cell lymphoma; EPOCH, etoposide, prednisone, vincristine, cyclophosphamide, adriamycin; ESHAP, etoposide, solumedrol, high dose cytarabine, cisplatin; GEM, gemcitabine; GemiFOX, gemcitabine, ifosfamide, oxaliplatin; GemOX, gemcitabine, oxaliplatin; GVD, gemcitabine, vinorelbine, doxil; ICE, ifosfamide, carboplatin, etoposide; MOPP, mustargen, vincristine, procarbazine, prednisone; MTX, methotrexate; NK, natural killer; PUVA, psoralen and UV A; RCHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone; RICE, rituxan, ifosfamide, carboplatin, etoposide; SPTL-AB, subpanniculitis-like T-cell lymphoma alpha/beta.
Initially, the protocol was designed to administer both drugs on days 1, 8, and 15 on a 28-day schedule (n = 3; see Figure 1). Although there were no DLTs in the initial cohort, 2 of the 3 patients enrolled did not meet the platelet threshold for treatment on cycle 1 day 15, and therefore, their treatment was held that day (Figure 1B). The protocol was subsequently revised to explore dosing on 1 of 2 schedules (A or B) through alternate assignment. On schedule A, patients were treated on days 1 and 8 on a 21-day cycle (every week × 2 every 21 days; n = 11), and on schedule B, patients were treated on day 1 and day 15 on a 28-day treatment cycle (every other week every 28 days; n = 15). For patients treated on schedule A, at all dose levels, thrombocytopenia continued to result in held treatment doses (Figure 1C). This was not observed for patients treated on schedule B, with the exception of cohort 3B, when the romidepsin dose was increased to 14 mg/m2 (Figure 1D).
Safety
Most adverse events were grade 1 or 2. The most common grade 1 or 2 toxicities included nausea (66%), fatigue (52%), anorexia (24%), diarrhea (24%), and fever (24%). The most common grade 3 toxicities included anemia (29%), oral mucositis (14%), thrombocytopenia (14%), and neutropenia (10%). Five grade 4 toxicities were observed, including thrombocytopenia (14%), neutropenia (10%), sepsis (7%), fever (3%), and pneumonia (3%) (Table 2). Growth factor support was allowed beyond cycle 1, but no patients required or received growth factor support, and there was no recurrence of grade 3 or 4 neutropenia in any patient treated beyond cycle 1. There were no effects seen on electrocardiogram and there were no treatment-related deaths. All patients recovered from adverse events within 1 or 2 weeks of study drug administration. Dose reductions occurred in 5 patients in cohorts 3A and 4A. There were 2 patients in cohort 3A who required dose reductions of romidepsin from 14 mg/m2 to 12 mg/m2 (1 for neutropenia and 1 for thrombocytopenia). There were 3 patients in cohort 4A who required dose reductions of pralatrexate from 20 mg/m2 to 15 mg/m2 (2 patients experienced grade 3 mucositis, and 1 patient did not experience any toxicity but was dose-reduced per protocol, as cohort 4A was determined to represent the maximum administered dose. As a result, this last patient required a dose reduction to the MTD to continue on study). The median number of cycles completed was 4 (range, 1-12).
Toxicities occurring in more than 5% of the study population
| Adverse event . | Grade 1-2, n (%) . | Grade 3, n (%) . | Grade 4, n (%) . |
|---|---|---|---|
| Abdominal pain | 5 (19) | 1 (3) | 0 |
| Allergic rhinitis | 3 (10) | 0 | 0 |
| Anemia | 0 | 7 (29) | 0 |
| Anorexia | 7 (24) | 0 | 0 |
| Anxiety | 3 (10) | 0 | 0 |
| Back pain | 3 (10) | 0 | 0 |
| Constipation | 5 (19) | 0 | 0 |
| Cough | 6 (21) | 0 | 0 |
| Dehydration | 2 (7) | 1 (3) | 0 |
| Diarrhea | 7 (24) | 1 (3) | 0 |
| Dizziness | 2 (7) | 0 | 0 |
| Dysgeusia | 2 (7) | 0 | 0 |
| Dyspnea | 2 (7) | 0 | 0 |
| Edema | 4 (14) | 0 | 0 |
| Epistaxis | 3 (10) | 0 | 0 |
| Fatigue | 15 (52) | 0 | 0 |
| Febrile neutropenia | 0 | 2 (7) | 2 (7) |
| Fever | 7 (24) | 0 | 1 (3) |
| Gastroesophageal reflux disease | 2 (7) | 0 | 0 |
| Gastrointestinal disorders | 1 (3) | 1 (3) | 0 |
| Headache | 3 (10) | 0 | 0 |
| Hyponatremia | 0 | 2 (7) | 0 |
| Laryngitis | 2 (7) | 0 | 0 |
| Mucositis oral | 5 (19) | 4 (14) | 0 |
| Nasal congestion | 3 (10) | 0 | 0 |
| Nausea | 19 (66) | 0 | 0 |
| Neutropenia | 0 | 1 (3) | 1 (3) |
| Pain | 6 (21) | 0 | 0 |
| Pain in extremity | 2 (7) | 0 | 0 |
| Pneumonia | 0 | 1 (3) | 1 (3) |
| Pruritus | 2 (7) | 0 | 0 |
| Rash maculopapular | 2 (7) | 0 | 0 |
| Sepsis | 0 | 0 | 2 (7) |
| Sore throat | 2 (7) | 0 | 0 |
| Stomach pain | 2 (7) | 0 | 0 |
| Thrombocytopenia | 2 (7) | 4 (14) | 4 (14) |
| Upper respiratory infection | 2 (7) | 0 | 0 |
| Urinary tract infection | 2 (7) | 0 | 0 |
| Vomiting | 6 (21) | 1 (3) | 0 |
| Adverse event . | Grade 1-2, n (%) . | Grade 3, n (%) . | Grade 4, n (%) . |
|---|---|---|---|
| Abdominal pain | 5 (19) | 1 (3) | 0 |
| Allergic rhinitis | 3 (10) | 0 | 0 |
| Anemia | 0 | 7 (29) | 0 |
| Anorexia | 7 (24) | 0 | 0 |
| Anxiety | 3 (10) | 0 | 0 |
| Back pain | 3 (10) | 0 | 0 |
| Constipation | 5 (19) | 0 | 0 |
| Cough | 6 (21) | 0 | 0 |
| Dehydration | 2 (7) | 1 (3) | 0 |
| Diarrhea | 7 (24) | 1 (3) | 0 |
| Dizziness | 2 (7) | 0 | 0 |
| Dysgeusia | 2 (7) | 0 | 0 |
| Dyspnea | 2 (7) | 0 | 0 |
| Edema | 4 (14) | 0 | 0 |
| Epistaxis | 3 (10) | 0 | 0 |
| Fatigue | 15 (52) | 0 | 0 |
| Febrile neutropenia | 0 | 2 (7) | 2 (7) |
| Fever | 7 (24) | 0 | 1 (3) |
| Gastroesophageal reflux disease | 2 (7) | 0 | 0 |
| Gastrointestinal disorders | 1 (3) | 1 (3) | 0 |
| Headache | 3 (10) | 0 | 0 |
| Hyponatremia | 0 | 2 (7) | 0 |
| Laryngitis | 2 (7) | 0 | 0 |
| Mucositis oral | 5 (19) | 4 (14) | 0 |
| Nasal congestion | 3 (10) | 0 | 0 |
| Nausea | 19 (66) | 0 | 0 |
| Neutropenia | 0 | 1 (3) | 1 (3) |
| Pain | 6 (21) | 0 | 0 |
| Pain in extremity | 2 (7) | 0 | 0 |
| Pneumonia | 0 | 1 (3) | 1 (3) |
| Pruritus | 2 (7) | 0 | 0 |
| Rash maculopapular | 2 (7) | 0 | 0 |
| Sepsis | 0 | 0 | 2 (7) |
| Sore throat | 2 (7) | 0 | 0 |
| Stomach pain | 2 (7) | 0 | 0 |
| Thrombocytopenia | 2 (7) | 4 (14) | 4 (14) |
| Upper respiratory infection | 2 (7) | 0 | 0 |
| Urinary tract infection | 2 (7) | 0 | 0 |
| Vomiting | 6 (21) | 1 (3) | 0 |
Table 3 presents the DLTs per dose cohort and disease subtype. There were 5 DLTs in total in cohort 3 on both schedule A and schedule B (pralatrexate 15 mg/m2 and romidepsin 14 mg/m2), consisting of 3 cases of grade 4 thrombocytopenia, one case of grade 4 pancytopenia, and one case of grade 4 neutropenia attributed to romidepsin. Based on the cytopenias attributed to romidepsin, the romidepsin dose was reduced to 12 mg/m2 for all cohorts while the pralatrexate dose was escalated per protocol, which eliminated the recurrence of thrombocytopenia. There were 3 DLTs in cohort 4A (pralatrexate 20mg/m2 and romidepsin 12 mg/m2, every week × 2 every 21 days) consisting of 2 cases of grade 3 oral mucositis and 1 case of grade 4 sepsis. Schedule A was closed to enrollment, and the MTD on this schedule was determined to be pralatrexate 15 mg/m2 and romidepsin 12 mg/m2 every week × 2 every 21 days. Schedule B continued with dose escalation of pralatrexate with no DLTs in cohorts 4B and 5B. The MTD for schedule B was not reached, and the recommended phase 2 dose was determined to be pralatrexate 25 mg/m2 and romidepsin 12 mg/m2 every other week every 28 days.
Patient characteristics, toxicity, and outcomes as a function of cohort
| Cohort . | Patient . | Disease subtype . | Prior lines of therapies/past romidepsin or pralatrexate . | Toxicities . | Best response . |
|---|---|---|---|---|---|
| 1: 10 mg/m2 pralatrexate and 12 mg/m2 romidepsin, days 1, 8, and 15 (Q28) | 1 | ALCL ALK(−), multiple myeloma, MF | 6 (ASCT) | No DLT | CR |
| 2 | Hodgkin lymphoma | 14 (ASCT) | No DLT | SD | |
| 3 | Intestinal T-cell lymphoma | 1/romidepsin | No DLT | PR | |
| 2A: 15 mg/m2 pralatrexate and 12 mg/m2 romidepsin, days 1 and 8 (Q21) | 1 | T-cell lymphoma | 2 | No DLT | PR |
| 2 | ATLL | 2 | No DLT | CR | |
| 3 | Follicular lymphoma | 4/pralatrexate | No DLT | PR | |
| 2B: 15 mg/2 pralatrexate and 12 mg/m2 romidepsin, days 1 and 15 (Q28) | 1 | CD4+ T-cell lymphoma | 1 | No DLT | PR |
| 2 | Follicular lymphoma | 9 | No DLT | NE | |
| 3 | Follicular lymphoma | 3 | No DLT | PR | |
| 3A: 15 mg/m2 pralatrexate and 14 mg/m2 romidepsin, days 1 and 8 (Q21) | 1 | SPTL-AB | 2 | DLT (pancytopenia, Plts = 4) | PR (PET neg) |
| 2 | Burkitt lymphoma | 3 | DLT (neutropenia, ANC = 0.244) | POD | |
| 3 | Follicular lymphoma | 5 | DLT (thrombocytopenia, Plts = 17) | PR | |
| 3B: 15 mg/m2 pralatrexate and 14 mg/m2 romidepsin, days 1 and 15 (Q28) | 1 | PTCL | 2 | No DLT | CR |
| 2 | DLBCL, CML | 3 | DLT (thrombocytopenia, Plts = 10) | NE | |
| 3 | ALCL ALK(−) | 2 | DLT (thrombocytopenia, Plts = 3) | NE | |
| 4A: 20 mg/m2 pralatrexate and 12 mg/m2 romidepsin, days 1 and 8 (Q21) | 1 | Hodgkin lymphoma | 16 (ASCT)/romidepsin and pralatrexate | No DLT | POD |
| 2 | Sezary syndrome | 5/romidepsin | DLT (grade 3 oral mucositis) | POD | |
| 3 | ATLL | 3 | DLT (grade 4 sepsis) | NE | |
| 4 | ATLL | 3 | DLT (grade 3 oral mucositis) | PR | |
| 5 | CD30+ ALK(−) ALCL | 2 (ASCT) | No DLT | CR | |
| 4B: 20 mg/m2 pralatrexate and 12 mg/m2 romidepsin, days 1 and 15 (Q28) | 1 | Hodgkin lymphoma | 12 (ASCT and allo)/ romidepsin | No DLT | POD |
| 2 | BPDCN | 1 | No DLT | SD | |
| 3 | ATLL | 3 (ASCT) | No DLT | POD | |
| 5B: 25 mg/m2 pralatrexate and 12 mg/m2 romidepsin, days 1 and 15 (Q28) | 1 | ATLL | 2 | No DLT | PR |
| 2 | Follicular lymphoma | 2/romidepsin | No DLT | POD | |
| 3 | CTCL | 2/romidepsin | No DLT | SD | |
| 5B: safety expansion | 4 | NK T cell | 2 | No DLT | NE |
| 5 | Sezary syndrome | 5 | No DLT | NE | |
| 6 | ATLL | 1 | No DLT | SD |
| Cohort . | Patient . | Disease subtype . | Prior lines of therapies/past romidepsin or pralatrexate . | Toxicities . | Best response . |
|---|---|---|---|---|---|
| 1: 10 mg/m2 pralatrexate and 12 mg/m2 romidepsin, days 1, 8, and 15 (Q28) | 1 | ALCL ALK(−), multiple myeloma, MF | 6 (ASCT) | No DLT | CR |
| 2 | Hodgkin lymphoma | 14 (ASCT) | No DLT | SD | |
| 3 | Intestinal T-cell lymphoma | 1/romidepsin | No DLT | PR | |
| 2A: 15 mg/m2 pralatrexate and 12 mg/m2 romidepsin, days 1 and 8 (Q21) | 1 | T-cell lymphoma | 2 | No DLT | PR |
| 2 | ATLL | 2 | No DLT | CR | |
| 3 | Follicular lymphoma | 4/pralatrexate | No DLT | PR | |
| 2B: 15 mg/2 pralatrexate and 12 mg/m2 romidepsin, days 1 and 15 (Q28) | 1 | CD4+ T-cell lymphoma | 1 | No DLT | PR |
| 2 | Follicular lymphoma | 9 | No DLT | NE | |
| 3 | Follicular lymphoma | 3 | No DLT | PR | |
| 3A: 15 mg/m2 pralatrexate and 14 mg/m2 romidepsin, days 1 and 8 (Q21) | 1 | SPTL-AB | 2 | DLT (pancytopenia, Plts = 4) | PR (PET neg) |
| 2 | Burkitt lymphoma | 3 | DLT (neutropenia, ANC = 0.244) | POD | |
| 3 | Follicular lymphoma | 5 | DLT (thrombocytopenia, Plts = 17) | PR | |
| 3B: 15 mg/m2 pralatrexate and 14 mg/m2 romidepsin, days 1 and 15 (Q28) | 1 | PTCL | 2 | No DLT | CR |
| 2 | DLBCL, CML | 3 | DLT (thrombocytopenia, Plts = 10) | NE | |
| 3 | ALCL ALK(−) | 2 | DLT (thrombocytopenia, Plts = 3) | NE | |
| 4A: 20 mg/m2 pralatrexate and 12 mg/m2 romidepsin, days 1 and 8 (Q21) | 1 | Hodgkin lymphoma | 16 (ASCT)/romidepsin and pralatrexate | No DLT | POD |
| 2 | Sezary syndrome | 5/romidepsin | DLT (grade 3 oral mucositis) | POD | |
| 3 | ATLL | 3 | DLT (grade 4 sepsis) | NE | |
| 4 | ATLL | 3 | DLT (grade 3 oral mucositis) | PR | |
| 5 | CD30+ ALK(−) ALCL | 2 (ASCT) | No DLT | CR | |
| 4B: 20 mg/m2 pralatrexate and 12 mg/m2 romidepsin, days 1 and 15 (Q28) | 1 | Hodgkin lymphoma | 12 (ASCT and allo)/ romidepsin | No DLT | POD |
| 2 | BPDCN | 1 | No DLT | SD | |
| 3 | ATLL | 3 (ASCT) | No DLT | POD | |
| 5B: 25 mg/m2 pralatrexate and 12 mg/m2 romidepsin, days 1 and 15 (Q28) | 1 | ATLL | 2 | No DLT | PR |
| 2 | Follicular lymphoma | 2/romidepsin | No DLT | POD | |
| 3 | CTCL | 2/romidepsin | No DLT | SD | |
| 5B: safety expansion | 4 | NK T cell | 2 | No DLT | NE |
| 5 | Sezary syndrome | 5 | No DLT | NE | |
| 6 | ATLL | 1 | No DLT | SD |
ALCL, anaplastic large cell lymphoma; ALK, anaplastic lymphoma kinase; allo, allogeneic stem cell transplant; ANC, absolute neutrophil count; ASCT, autologous or stem cell transplant; ATLL, adult T-cell leukemia/lymphoma; CTCL, cutaneous T-cell lymphoma; DLBCL, diffuse large B-cell lymphoma; MF, mycosis fungoides; NE, not evaluable; NK, natural killer; PET neg, positron emission tomography negative; Plts, platelets; POD, progression of disease; Q, every; SD, stable disease.
Efficacy
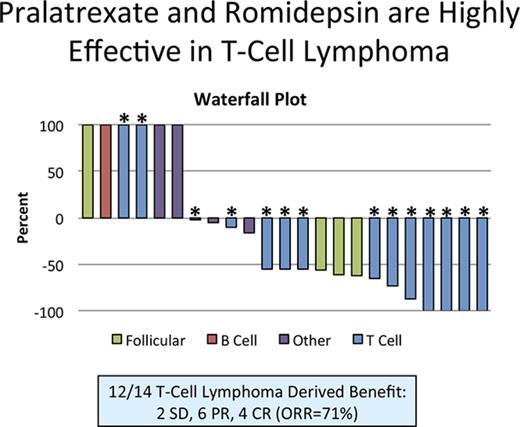
Twenty-three patients were evaluable for response (Figure 2B). Four patients achieved a CR (17%, all with PTCL), 7 patients achieved a PR (30%), 4 had stable disease (17%), and 6 had progression of disease (26%). Among the T-cell lymphoma patients, 10 of 14 (71%) achieved a response, with 4 of the 14 achieving a CR (29%). An additional 2 patients with T-cell lymphoma exhibited stabilization of their disease. Figure 2B depicts the waterfall plot for all patients on study. Three of 4 follicular lymphoma patients achieved a response (all PR). The median time to response was 2 cycles or 1.6 months, and responses were observed across all treatment schedules (Table 3).
Summary of response rates across study population for patients treated with romidepsin and pralatrexate. (A) Response rates by disease subtype. (B) Waterfall plot representing the percentage change in tumor growth following treatment depicted by disease subtype. ORR, overall response rate; TCL, T-cell lymphoma.
Summary of response rates across study population for patients treated with romidepsin and pralatrexate. (A) Response rates by disease subtype. (B) Waterfall plot representing the percentage change in tumor growth following treatment depicted by disease subtype. ORR, overall response rate; TCL, T-cell lymphoma.
The PFS, OS, and DOR were calculated for all patients enrolled onto the study and further analyzed as a function of the histologic subtype (Figure 3). The median PFS for the entire population was 3.7 months (1.4-10.8), while the PFS for patients with non–T-cell and T-cell lymphoma was 1.8 (95% CI, 3.5 to N/A) and 4.4 months (95% CI, 1.2 to N/A), respectively. The median OS for the T-cell lymphoma and non–T-cell lymphoma patients was 12.4 months (95% CI, 8.1 to N/A) and 34.0 months (95% CI, 9.7 to N/A), respectively. Figure 3D depicts the duration of treatment, DOR, and time to first response for all evaluable T-cell lymphoma patients. The median DOR was 4.29 months (interquartile range, 2.97-6.98). Five of the 14 (36%) patients had a durable response lasting ≥6 months.
PFS and OS as a function of treatment in study population. Curves on the left represent all patients who received study drug (N = 29) and CIs. Curves on the right are subdivided between non–T-cell (n = 11) and T-cell patients (n = 18). (A) Median PFS is 3.7 months for all patients (95% CI, 1.4, 10.8) and 4.4 months for T-cell lymphoma patients (95% CI, 3.5) and for non–T-cell lymphoma is 1.8 months (95% CI, 1.2). (B) Median OS for all patients is 13.8 months (95% CI, 8.8 to not achieved [N/A]); for TCL patients is 12.4 months (95% CI, 8.1) and non–T-cell lymphoma 34 months (95% CI, 9.7). (C) Swimmer’s plot detailing the PFS of all T-cell lymphoma patients enrolled in the study. Start time denotes the first dose of study drugs. Stop time denotes progression of disease (POD), change in treatment (including transplant), or death.
PFS and OS as a function of treatment in study population. Curves on the left represent all patients who received study drug (N = 29) and CIs. Curves on the right are subdivided between non–T-cell (n = 11) and T-cell patients (n = 18). (A) Median PFS is 3.7 months for all patients (95% CI, 1.4, 10.8) and 4.4 months for T-cell lymphoma patients (95% CI, 3.5) and for non–T-cell lymphoma is 1.8 months (95% CI, 1.2). (B) Median OS for all patients is 13.8 months (95% CI, 8.8 to not achieved [N/A]); for TCL patients is 12.4 months (95% CI, 8.1) and non–T-cell lymphoma 34 months (95% CI, 9.7). (C) Swimmer’s plot detailing the PFS of all T-cell lymphoma patients enrolled in the study. Start time denotes the first dose of study drugs. Stop time denotes progression of disease (POD), change in treatment (including transplant), or death.
PK analysis
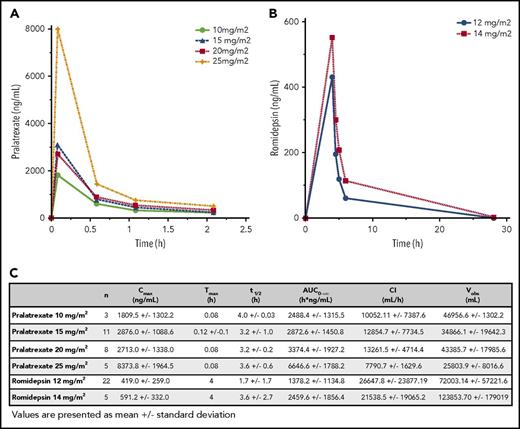
First-dose PK analysis for pralatrexate and romidepsin was evaluated in 27 patients. Figure 4 summarizes the PK parameters and average serum concentrations for pralatrexate and romidepsin. The mean Cmax for patients receiving pralatrexate 25 mg/m2 was 8373.8 ng/mL or 17.5 µM (50% inhibitory concentration in lymphoma cell lines, 2.0-23 nM).30 Romidepsin 14 mg/m2 demonstrated a mean Cmax of 591.2 ng/mL or 1.1 µM (50% inhibitory concentration in T-cell lymphoma cell lines, 1.2-1.6 nM).31
PK parameters for pralatrexate and romidepsin in the study population. (A-B) Concentration over time for each dose cohort of pralatrexate (A) and romidepsin (B). (C) Cmax, Tmax, t1/2, AUC, CI, and Vobs (volume observed) for pralatrexate and romidepsin at each dose cohort.
PK parameters for pralatrexate and romidepsin in the study population. (A-B) Concentration over time for each dose cohort of pralatrexate (A) and romidepsin (B). (C) Cmax, Tmax, t1/2, AUC, CI, and Vobs (volume observed) for pralatrexate and romidepsin at each dose cohort.
PK profiles from patients who received pralatrexate 15 mg/m2 in conjunction with romidepsin at 12 mg/m2 were compared with those treated with romidepsin 14 mg/m2. No difference was detected when the effects of romidepsin were tested against pralatrexate. When comparing the influence of pralatrexate on romidepsin, PK parameters were compared among patients who received romidepsin 12 mg/m2 with varying doses of pralatrexate. Notably, a statistically significant difference was noted in Cmax, t1/2 and plasma levels of romidepsin at 4 hours between patients receiving pralatrexate 10 mg/m2 (n = 3) vs pralatrexate 25 mg/m2 (n = 5), with patients receiving pralatrexate 25 mg/m2 having slightly higher concentration of romidepsin (P = .045, 0.044, and 0.045, respectively). Additionally, AUC0→∞ and Cmax of pralatrexate and romidepsin were compared with historical PK data from single-agent studies of each drug. In this study, patients who received pralatrexate 25 mg/m2 had a mean Cmax and AUC0→∞ of 8373.8 ng/mL and 6646.6 ng × h/mL, whereas patients who were exposed to single-agent pralatrexate at 30 mg/m2 had values of 5815 ng/mL and 4464.2 ng × h/mL, respectively.6 Comparing the same PK parameters for romidepsin 14 mg/m2 cohort to single-agent romidepsin PK data available from the National Cancer Institute 1312 study, romidepsin values were higher in our population (Cmax and AUC0→∞ 591.2 ng/mL and 2459.6 ng × h/mL vs 427.0 ng/mL and 1899 ng × h/mL).9 It is possible that the coadministration leads to an increase in the relative exposure of each drug compared with what has been seen by the single agents, explaining in part the benefit seen at lower doses.
Discussion
The prospect of creating novel platforms to treat T-cell lymphoma predicated on principles we outlined above offers promise in developing strategies that are not CHOP predicated. The challenge lies in identifying the doses and schedules of drugs that do not exacerbate the toxicities of the single agent while retaining the synergy demonstrated in preclinical models. Both pralatrexate and romidepsin produce thrombocytopenia, which creates pause in thinking about how these drugs should be combined. Fortunately, the thrombocytopenia seen with these agents is short-lived and reversible, owing to the fact they are likely not toxic to megakaryocytes as is seen with conventional chemotherapy drugs. Patients who completed therapy and required additional treatment were able to do so without any lasting sequelae from the combination. Interestingly, thrombocytopenia emerged with only a relatively small increase in romidepsin from 12 mg/m2 to 14 mg/m2. Grade 1 to 3 mucositis was appreciated on the weekly schedule, albeit at levels that appeared substantially lower compared with the PROPEL study.6 A modest schedule adjustment from weekly to every other week abrogated mucositis as a DLT. Schedule A (every week × 2 every 21 days) was associated with DLTs of mucositis and sepsis, but no DLTs were observed for schedule B (every other week every 28 days) when the romidepsin dose was maintained at ≤12 m/m2. The MTD was not reached on schedule B, and the recommended phase 2 dose was determined to be pralatrexate 25 mg/m2 and romidepsin 12 mg/m2 on every other week every 28 days (administration instructions may be found in the supplemental Data, available on the Blood Web site). This dose and schedule was very well tolerated.
As predicted by the preclinical data, the combination of these 2 agents produced a high level of activity in patients with T-cell lymphoma. Despite a very heavily treated patient population, which included patients who had previously been treated with these agents and many who were treated at early dose cohorts, the combination exhibited an overall response rate of 71% (10/14), compared with an overall response rate of 33% (3/9) among the non–T-cell lymphoma patients. Six of the 14 evaluable PTCL patients had received either an autologous (n = 6) or allogeneic (n = 1) stem cell transplantation. The median time to response was rapid; the median DOR, PFS, and OS were 4.29, 4.4, and 12.4 months, respectively, with one of these patients being successfully bridged to an allogeneic transplant. Interestingly, responses were observed across all dose levels, perhaps underscoring the synergistic activity of romidepsin in combination with pralatrexate.
The PK data provide important insights into the disposition of these drugs when given in combination in this population. These data suggest that the plasma concentrations achieved in the combination were slightly higher compared with historical single-agent exposure and warrant further investigation. Slight increases in doses correlated with increased toxicity, which was most pronounced when romidepsin was increased from 12 to 14 mg/m2, leading to thrombocytopenia. The conspicuous lack of mucositis especially on the every-other-week schedule raises interesting questions regarding mechanism. This study (beyond cycle 1) provided provisions for leucovorin on days 3 to 6 (15 mg orally twice daily), which appears to substantially reduce the risk of pralatrexate-associated mucositis with no impact on its efficacy.32
Albeit early, these data, coupled with compelling preclinical data, support the contention that novel combinations of drugs highly active in PTCL can be combined safely with a meaningful signal of activity. This combination is now being explored in a multicenter phase 2 study. The strategy of defining unique doublets active in PTCL and leveraging the recent promising advances in experimental drug development offers an opportunity to reconfigure the paradigm of care for patients in both the upfront and relapsed or refractory setting. Creating novel doublet platforms opens the prospect for the creation of novel triplet-based combinations, exploiting novel biological agents deemed active in T-cell lymphoma.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This investigator-initiated clinical trial was supported by the Celgene Corporation and Spectrum Pharmaceuticals, the Leukemia and Lymphoma Society Translational Research Program, the Lymphoma Research Fund at the Center for Lymphoid Malignancies, and the National Center for Advancing Translational Sciences, National Institutes of Health (grant UL1TR001873). The investigators were wholly responsible for study design, data collection and interpretation, and preparation of the manuscript; all authors had access to the data. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: J.E.A., A.S., and O.A.O. conceived and designed the study; J.E.A., O.A.O., R.L., J.L., E.L., K.K., L.A., H.A.K., S.C., C.D., M.A.F., L.S., L.F., E.M., M.K., A.R., and A.S. acquired the data; J.E.A., R.L., J.L., C.C., S.C., and O.A.O. analyzed and interpreted the data; J.E.A., R.L., J.L., and O.A.O. wrote the paper and C.C. performed the statistical analysis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jennifer E. Amengual, Center for Lymphoid Malignancies, Columbia University Medical Center, 51 West 51st St, Suite 200, New York, NY 10019; e-mail: jea2149@columbia.edu.



![Figure 3. PFS and OS as a function of treatment in study population. Curves on the left represent all patients who received study drug (N = 29) and CIs. Curves on the right are subdivided between non–T-cell (n = 11) and T-cell patients (n = 18). (A) Median PFS is 3.7 months for all patients (95% CI, 1.4, 10.8) and 4.4 months for T-cell lymphoma patients (95% CI, 3.5) and for non–T-cell lymphoma is 1.8 months (95% CI, 1.2). (B) Median OS for all patients is 13.8 months (95% CI, 8.8 to not achieved [N/A]); for TCL patients is 12.4 months (95% CI, 8.1) and non–T-cell lymphoma 34 months (95% CI, 9.7). (C) Swimmer’s plot detailing the PFS of all T-cell lymphoma patients enrolled in the study. Start time denotes the first dose of study drugs. Stop time denotes progression of disease (POD), change in treatment (including transplant), or death.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/131/4/10.1182_blood-2017-09-806737/4/m_blood806737f3.jpeg?Expires=1765889661&Signature=FYTxM6UYhs6fcx51M6-0Nwi4Q2QLNJQFJHJ~Mh0R97LzTWzDIPx7RT28iaVS6thM5HqtDtUV8V1hscjiWXWHxHd89UDLEvelkyeWj2UuEuclymq3Rww33HJNU1mdmCnYULYSx9~77v2sMQurqndepe1UC7pXB-GHw7LeQ6AZ9NS5NQRaEVmmPdqW42Mrlyy68jTPizLpZ0mzxpzTH7mlelA0kiYW6dLmCb7FYwQinBWO-Xu1mE-mYJZnOdcFers53iARQabydUrgUMrGS~JHpPubhODa0CamTOySzMEdQBuQHni4xqoFS29G141pDnbweNUeJgzWfj~Tn-ZTxvP7YQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal