Key Points
Vadastuximab talirine, a novel antibody-drug conjugate, consists of an anti-CD33 monoclonal antibody conjugated to pyrrolobenzodiazepine dimers.
In a phase 1 trial, vadastuximab talirine demonstrated single-agent activity and minimal nonhematologic toxicity in patients with AML.
Abstract
Vadastuximab talirine (SGN-CD33A, 33A) is an antibody-drug conjugate consisting of pyrrolobenzodiazepine dimers linked to a monoclonal antibody targeting CD33, which is expressed in the majority of acute myeloid leukemia (AML) patients. This phase 1 study evaluated the safety, pharmacokinetics, and preliminary activity of vadastuximab talirine and determined the recommended monotherapy dose in patients with relapsed or refractory AML. Additional expansion cohorts tested vadastuximab talirine in specific subpopulations of relapsed AML, and in a cohort of older, treatment-naive patients. Patients received vadastuximab talirine IV on day 1 (5-60 µg/kg) or on days 1 and 4 (20 µg/kg) of 21-day cycles. A total of 131 patients (median age, 73 years [range, 26-89 years]) had intermediate I-II (48%) or adverse (34%) risk by European LeukemiaNet classification; 50% of patients had underlying myelodysplasia. Two dose-limiting toxicities (grade 2 pulmonary embolism and grade 4 hypocellular marrow) occurred during dose finding. Most adverse events (AEs) were consistent with myelosuppression; nonhematologic AEs included fatigue, nausea, and diarrhea. The 30-day mortality was 8%. At the recommended monotherapy dose of 40 µg/kg, the complete remission + CRi rate was 28% (5 of 18 patients); 50% of patients who responded achieved minimal residual disease negativity. In patients across dose levels who achieved CR or CRi, the median time to full count recovery was 6.4 weeks for neutrophils (≥1000/µL) and 10.6 weeks for platelets (≥100 × 109/L). Vadastuximab talirine demonstrates activity and a tolerable safety profile as a single agent in patients with AML. The recommended monotherapy dose of vadastuximab talirine is 40 µg/kg. This trial was registered at www.clinicaltrials.gov as # NCT01902329.
Introduction
The overall survival (OS) of patients with acute myeloid leukemia (AML) has remained poor despite decades of clinical investigation. Standard-of-care therapy for patients with newly diagnosed and/or relapsed AML relies on intensive cytotoxic chemotherapy, hypomethylating agents (HMAs), or low-dose cytarabine. Although dose increases and reformulations of cytotoxic chemotherapy have led to modest improvements in OS, the vast majority of patients succumb to their disease.1
In most AML cases, leukemic myeloblasts express CD33, a member of the sialic acid–binding immunoglobulin-like lectin family.2,3 Over the past 20 years, efforts to target CD33 with antibody-based therapies using unconjugated antibodies, antibody-drug conjugates (ADCs), antibody-toxin conjugates, and radiolabeled antibodies have met with varying levels of success.4 Gemtuzumab ozogamicin (GO), a calicheamicin-based anti-CD33 ADC, received accelerated approval for use in the United States, but was voluntarily withdrawn from the market because of concerns of toxicity and failure to confirm clinical benefit.5 However, a recent meta-analysis of 5 randomized controlled trials reported that the addition of GO to intensive induction chemotherapy resulted in improved survival in patients with favorable-risk or intermediate-risk cytogenetics.6
Vadastuximab talirine (SGN-CD33A; 33A) is a highly potent ADC with synthetic pyrrolobenzodiazepine (PBD) dimers conjugated to engineered cysteine residues of an anti-CD33 monoclonal antibody via a highly stable, cleavable maleimidocaproyl-valinealanine dipeptide linker.7,8 This engineering allows precise, homogeneous loading of the PBD dimer to the cysteine residues. The PBD dimer is a crosslinking agent that binds DNA with high intrinsic affinity. Preclinical studies with vadastuximab talirine show potent activity against AML cell lines, primary AML cells in vitro, and xenotransplantation models of AML.7
Based on this encouraging preclinical activity, a phase 1, first-in-human dose-escalation study of vadastuximab talirine was conducted to assess the safety, tolerability, maximum tolerated dose (MTD), and activity in patients with CD33-positive AML, primarily in the relapsed setting. Several expansion cohorts explored the activity of vadastuximab talirine in specific subsets of AML. These included patients with relapsed nucleophosmin-1 (NPM1) mutated AML, relapse after allogeneic hematopoietic stem cell transplant (alloHSCT), and previously untreated AML (treatment-naive patients receiving vadastuximab talirine at the recommended monotherapy dose and treatment-naive patients receiving vadastuximab talirine in combination with HMAs). The results of the monotherapy cohorts of the study are presented here.
Methods
Patient eligibility
For dose escalation and expansion, eligible patients had CD33-positive AML (any level of CD33 expression as detected by local flow cytometric assessment) and had either newly diagnosed AML (declining intensive induction/consolidation chemotherapy) or AML relapsed after a minimum remission duration of 12 weeks after intensive induction/consolidation. Newly diagnosed patients who declined intensive therapy, but had an antecedent myeloid neoplasm, received no ≤2 nonintensive therapies before enrollment. Relapsed patients received ≤1 postrelapse, nonintensive regimen (eg, azacitidine, decitabine, low-dose cytarabine) before enrollment, and had a marrow blast percentage of ≥5%. Patients were ≥18 years of age with an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, with adequate baseline renal, hepatic, and pulmonary function. Patients with central nervous system leukemia were ineligible. Antileukemic treatment, excluding hydroxyurea, was prohibited within 14 days of starting study treatment.
Study design and treatment
The dose-escalation portion of this phase 1 study (#NCT01902329) was designed to evaluate the safety, tolerability, pharmacokinetics (PK), and antileukemic activity of vadastuximab talirine as monotherapy, with the primary objectives of determining the safety, tolerability, and MTD of vadastuximab talirine in patients with CD33-expressing AML. Fourteen centers in the United States recruited patients between July 2013 and February 2016 under approval of institutional review boards in accordance with the Declaration of Helsinki. All patients provided informed consent before administration of any study treatment.
End points included type, incidence, severity, seriousness, and relatedness of adverse events (AEs), laboratory abnormalities, OS, and relapse-free survival, as well as complete remission (CR) rate and remission duration. The study was monitored by a Safety Monitoring Committee (SMC) comprised of the study investigators and the study medical monitor.
Dose escalation and expansion
All patients were allowed a maximum of 2 cycles of vadastuximab talirine, administered via slow IV push, to achieve a response. Patients who achieved a CR or CRi during the first 2 cycles were eligible for extension treatment every 3 weeks at a dose of 5 or 10 µg/kg. Extension treatment was allowed in the absence of new or cumulative, clinically significant toxicity and in the absence of relapse.
A 3 + 3 study design was used during dose escalation. Dose levels in the dose-escalation phase ranged from 5 to 60 µg/kg (Table 1). Expansion cohorts of ≤12 patients at dose levels 20, 30, 40, and 50 µg/kg further evaluated the safety, PK, pharmacodynamics, and antitumor activity of vadastuximab talirine.
Dose escalation scheme
| Vadastuximab talirine dose cohorts on day 1 of 3-wk cycles, µg/kg . | |
|---|---|
| Planned . | Actual . |
| 5 | 5 |
| 10 | 10 |
| 20 | 20 |
| 40 | 30 |
| 80 | 40 |
| 120 | 50 |
| 180 | 60 |
| 250 | — |
| Vadastuximab talirine dose cohorts on day 1 of 3-wk cycles, µg/kg . | |
|---|---|
| Planned . | Actual . |
| 5 | 5 |
| 10 | 10 |
| 20 | 20 |
| 40 | 30 |
| 80 | 40 |
| 120 | 50 |
| 180 | 60 |
| 250 | — |
Patients were allowed a maximum of 2 cycles at each dose level of to achieve a response. Patients who achieved a CR or CRi during the first 2 cycles were eligible for extension treatment every 3 weeks at a dose of 5 or 10 µg/kg. Although formal MTD was never exceeded at the doses tested, planned dose levels between 80 μg/kg and 250 μg/kg were never tested.
The dose-limiting toxicity (DLT) evaluation period was the first cycle of treatment. A DLT was defined as any clinically significant, nonhematologic AE ≥ grade 3 (per the National Cancer Institute Common Toxicity Criteria for Adverse Events, Version 4.03) or hypocellular (≤5% cellularity) bone marrow (BM) without evidence of leukemia, myelodysplasia, or infectious organism that lasted >28 days from the first observation (ie, the first posttreatment BM examination showing ≤5% cellularity or aplasia). Febrile neutropenia that resolved with appropriate treatment or marrow recovery was not considered a DLT.
Additional monotherapy cohorts were activated at doses recommended by the SMC to explore dose levels in subgroups of patients, such as a fractionated dosing schedule (20 µg/kg per dose on days 1 and 4), relapsed patients with NPM1 mutation (in the absence of an fms-like tyrosine kinase 3 mutation [NPM1-mutated, FLT-3 wild type]; 40 µg/kg), treatment-naive patients with AML who had declined intensive therapy (40 µg/kg), and patients who had relapsed after alloHSCT (40-µg/kg and 20-µg/kg cohorts).
End-of-treatment assessments were conducted ∼30 days after receiving the final dose of study drug. Patients who discontinued treatment before disease progression were followed for response assessments. All patients were followed for survival.
Study assessments
Safety assessments
Safety assessments included the surveillance and recording of AEs, vital signs, clinical laboratory tests, ECOG performance status, and physical examination. Grade and term of AEs were reported by the treating physician. Complete blood counts and serum chemistries were obtained weekly during the first 2 cycles for all patients; laboratory surveillance was less frequent in extension treatment. Pulmonary function tests, including diffusing capacity of the lung for carbon monoxide (DLCO), were measured during the first cycle and then after every 2 cycles. Electrocardiograms were used to assess the effect of vadastuximab talirine monotherapy on cardiac repolarization.
Response/efficacy assessment
Antileukemic activity was assessed by routine laboratory tests, BM examinations, and flow cytometry evaluation of leukemic blasts. Response categorization was based on the 2003 revised recommendations of the International Working Group for AML.9 BM examinations were performed at baseline (within 28 days of starting treatment) and frequently throughout the study for response assessment and as surveillance of marrow recovery. Minimal residual disease (MRD) was assessed by 10-color multiparameter flow cytometry at a central laboratory as described previously.10
PK, pharmacodynamic, and immunogenicity assessments
Sensitive, qualified assays were used to measure concentrations of ADC (vadastuximab talirine), total antibody (TAb), and released-free drug, SGD-1882, in plasma and antitherapeutic antibodies in serum. The assays included enzyme-linked immunosorbent assays and liquid chromatography tandem mass spectrometry assays.
Statistical analysis
Study measures of safety and activity were summarized by descriptive statistics. The analysis set for all treated patients included those treated with any amount of vadastuximab talirine. The DLT-evaluable analysis set included all treated patients who either experienced a DLT or were followed for the full DLT evaluation period. The safety of each cohort of the study was summarized independently. The efficacy-evaluable analysis set included all treated patients who received study treatment and had a postbaseline BM examination and response determination.
Results
Patients
A total of 131 patients with AML were treated in the monotherapy portion of the study. The median age was 73 years (range, 26-89 years); most patients had intermediate I (27%), intermediate II (21%), or adverse (34%) risk by European LeukemiaNet (ELN) classification.11 Fifty percent of patients had underlying myelodysplasia. Fifty-eight (44%) patients had relapsed after a CR or partial response to their most recent therapy, and 44 (34%) patients were refractory. Additional demographic and disease characteristics are presented by study cohort in Table 2.
Demographics and disease characteristics
| . | Dose escalation/ expansion (N = 69) . | Fractionated dosing (N = 12) . | NPM1 (N = 13) . | Post-alloHSCT (N = 12) . | Treatment naive* (N= 27) . |
|---|---|---|---|---|---|
| Median age (range), y | 75 (27-86) | 72.5 (64-83) | 69 (41-80) | 56 (26-72) | 74 (67-89) |
| Sex, male, % | 61 | 67 | 62 | 58 | 48 |
| ECOG score, % | |||||
| 0 | 16 | 25 | 23 | 17 | 37 |
| 1 | 84 | 75 | 77 | 83 | 63 |
| De novo AML, % | 58 | 58 | 92 | 58 | 56 |
| Secondary AML, % | 42 | 42 | 8 | 42 | 44 |
| MRC cytogenetic risk group, % | |||||
| Favorable | 1 | 0 | 0 | 0 | 0 |
| Intermediate | 64 | 50 | 100 | 25 | 70 |
| Adverse | 33 | 50 | 0 | 75 | 26 |
| Underlying myelodysplasia, % | 57 | 58 | 8 | 42 | 48 |
| FLT3 mutation only, % | 4 | 17 | 0 | 17 | 13 |
| NPM1 mutation only, % | 12 | 0 | 92 | 0 | 0 |
| FLT3 and NPM1 mutations, % | 7 | 0 | 8 | 8 | 13 |
| Enrollment BM (range), % | 40 (4-93) | 47 (20-83) | 72.5 (6-99) | 44 (0-90) | 47 (20-96) |
| Baseline WBC (range), × 109/L | 2.4 (0.5-55.1) | 1.7 (0.3-14.4) | 4 (0.7-36.3) | 1.6 (0.5-15.6) | 2.7 (0.5-26.3) |
| . | Dose escalation/ expansion (N = 69) . | Fractionated dosing (N = 12) . | NPM1 (N = 13) . | Post-alloHSCT (N = 12) . | Treatment naive* (N= 27) . |
|---|---|---|---|---|---|
| Median age (range), y | 75 (27-86) | 72.5 (64-83) | 69 (41-80) | 56 (26-72) | 74 (67-89) |
| Sex, male, % | 61 | 67 | 62 | 58 | 48 |
| ECOG score, % | |||||
| 0 | 16 | 25 | 23 | 17 | 37 |
| 1 | 84 | 75 | 77 | 83 | 63 |
| De novo AML, % | 58 | 58 | 92 | 58 | 56 |
| Secondary AML, % | 42 | 42 | 8 | 42 | 44 |
| MRC cytogenetic risk group, % | |||||
| Favorable | 1 | 0 | 0 | 0 | 0 |
| Intermediate | 64 | 50 | 100 | 25 | 70 |
| Adverse | 33 | 50 | 0 | 75 | 26 |
| Underlying myelodysplasia, % | 57 | 58 | 8 | 42 | 48 |
| FLT3 mutation only, % | 4 | 17 | 0 | 17 | 13 |
| NPM1 mutation only, % | 12 | 0 | 92 | 0 | 0 |
| FLT3 and NPM1 mutations, % | 7 | 0 | 8 | 8 | 13 |
| Enrollment BM (range), % | 40 (4-93) | 47 (20-83) | 72.5 (6-99) | 44 (0-90) | 47 (20-96) |
| Baseline WBC (range), × 109/L | 2.4 (0.5-55.1) | 1.7 (0.3-14.4) | 4 (0.7-36.3) | 1.6 (0.5-15.6) | 2.7 (0.5-26.3) |
MRC, Medical Research Council; WBC, white blood cell.
Includes the treatment-naive cohort and 3 patients in the 40 µg/kg–dose-escalation cohort who were treatment naive.
PK
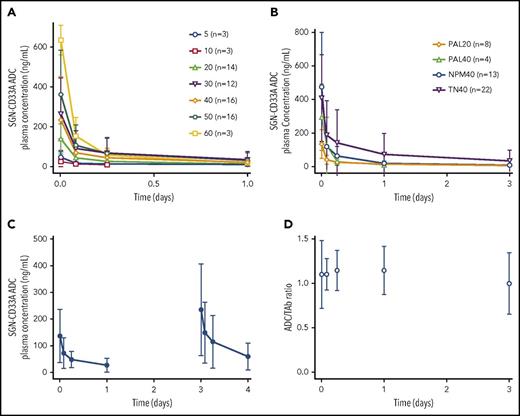
Concentration-time profiles for cycle 1 of vadastuximab talirine ADC are shown in Figure 1A-C. Based on preliminary analysis, 3 patients had detectable PBD concentrations (lower limit of quantification = 60 pg/mL), none >90 pg/mL. These PBD concentrations were detected during cycle 1 for the first patient (60 µg/kg), cycle 2 for the second patient (40 µg/kg), and cycles 10, 11, and 13 for the third patient (5 µg/kg). As shown in Figure 1A, concentration-time profiles of vadastuximab talirine demonstrated dose-dependent rapid elimination of ADC from the circulation, consistent with target-mediated disposition. In the highest-dose group (60 µg/kg, n = 3), ADC was not detectable in any patient past day 4. Increases in cycle 1 vadastuximab talirine doses generally resulted in an increase in exposure for doses ranging from 5 to 60 µg/kg. Concentration-time profiles for additional single-dose cohorts were consistent with the dose escalation and expansion cohorts. Figure 1C shows the mean concentration-time profile for subjects receiving a fractionated dose, 20 µg/kg on days 1 and 4 of cycle 1, demonstrating similar profiles to the other cohorts. Figure 1D demonstrates the ratio of ADC concentration to TAb concentration vs time for patients across all doses and cohorts in this analysis. The majority of the TAb concentration was circulating as ADC, represented by an ADC:TAb ratio of ∼1, consistent with a stable linker for vadastuximab talirine.
Mean concentration-time profiles and ADC to TAb ratio. (A) Cycle 1 arithmetic mean vadastuximab talirine ADC plasma concentration-time profiles after administration of the first dose of vadastuximab talirine at doses ranging from 5 to 60 µg/kg. (B) Cycle 1 arithmetic mean vadastuximab talirine ADC plasma concentration-time profiles after administration of the first dose of vadastuximab talirine at doses of 20 and 40 µg/kg. (C) Cycle 1 arithmetic mean vadastuximab talirine ADC plasma concentration-time profile after administration of 2 doses of vadastuximab talirine, both at 20 µg/kg. Each dose represents n = 12. (D) Ratio of ADC over TAb concentration vs time. n = x represents the total number of subjects in each cohort. NPM40, 40 µg/kg-dose level NPMI mutation cohort; PAL20, 20 µg/kg-dose level post-alloHSCT cohort; PAL40, 40-µg/kg-dose level post-alloHSCT cohort; TN40, 40-µg/kg dose level treatment-naive cohort.
Mean concentration-time profiles and ADC to TAb ratio. (A) Cycle 1 arithmetic mean vadastuximab talirine ADC plasma concentration-time profiles after administration of the first dose of vadastuximab talirine at doses ranging from 5 to 60 µg/kg. (B) Cycle 1 arithmetic mean vadastuximab talirine ADC plasma concentration-time profiles after administration of the first dose of vadastuximab talirine at doses of 20 and 40 µg/kg. (C) Cycle 1 arithmetic mean vadastuximab talirine ADC plasma concentration-time profile after administration of 2 doses of vadastuximab talirine, both at 20 µg/kg. Each dose represents n = 12. (D) Ratio of ADC over TAb concentration vs time. n = x represents the total number of subjects in each cohort. NPM40, 40 µg/kg-dose level NPMI mutation cohort; PAL20, 20 µg/kg-dose level post-alloHSCT cohort; PAL40, 40-µg/kg-dose level post-alloHSCT cohort; TN40, 40-µg/kg dose level treatment-naive cohort.
Safety: dose escalation/expansion
No DLTs were observed in the 5-µg/kg or 10-µg/kg dose levels. One DLT was observed at the 20-µg/kg dose level (grade 3 pulmonary embolism in the setting of previous deep venous thrombosis), whereas 13 other patients did not experience any DLTs at that dose level. No DLTs were observed at the 30-µg/kg dose level or in the first 3 patients treated at the 40-µg/kg dose level. Of the 3 patients treated at the 60-µg/kg dose level, 1 patient experienced a DLT (grade 4 hypocellular marrow); 1 patient died of disease-related complications and was not DLT evaluable. Given the increasing myelosuppression seen at higher doses, the 40-µg/kg cohort was expanded. One DLT (grade 4 hypocellular marrow) was observed in the 18 patients treated at the 40-µg/kg dose level in the dose-escalation/expansion phase, and no events of sepsis occurred. The first 3 patients treated at an intermediate dose level of 50 µg/kg had no DLTs, and the cohort was expanded. In the 16 total patients treated at the 50-µg/kg dose level, 2 DLTs (grade 3 mucositis and grade 5 sepsis) were observed, and 5 additional cases of sepsis were observed in this cohort (31%). Although formal MTD was not exceeded among the doses tested, 40 µg/kg was chosen as the recommended monotherapy dose for further study based on the optimal balance of safety and activity; this dose of 40 µg/kg was the starting dose tested in subsequent expansion cohorts exploring specific AML subpopulations.
Across all monotherapy cohorts (N = 131), the 30- and 60-day mortality rates were 8% and 27%, respectively. The majority of deaths on study were considered to be related to disease.
The majority of treatment-emergent AEs (TEAEs) observed in all treated patients were consistent with myelosuppression; no grade 3/4 nonhematologic TEAEs were observed in ≥20% of patients. The TEAEs observed in ≥20% of all treated patients are listed in Table 3.
TEAEs in ≥20% of all treated patients
| Preferred term . | 40-µg/kg dose (N = 18) . | All treated patients (N=131) . | ||
|---|---|---|---|---|
| All grades . | >Grade 3 . | All grades . | >Grade 3 . | |
| Any event | 100 | 100 | 100 | 96 |
| Febrile neutropenia | 72 | 72 | 57 | 57 |
| Fatigue | 56 | 11 | 49 | 10 |
| Thrombocytopenia | 50 | 25 | 44 | 40 |
| Anemia | 22 | 42 | 38 | 33 |
| Nausea | 39 | — | 34 | — |
| Diarrhea | 17 | — | 31 | 2 |
| Decreased appetite | 22 | 6 | 30 | 2 |
| Peripheral edema | 28 | — | 30 | 2 |
| Dyspnea | 44 | 6 | 29 | 7 |
| Constipation | 33 | — | 25 | — |
| Epistaxis | 22 | — | 24 | 2 |
| Hypokalemia | 11 | — | 24 | 5 |
| Cough | 28 | — | 22 | 1 |
| Chills | 33 | — | 21 | 1 |
| Contusion | 39 | — | 21 | — |
| Pyrexia | 17 | — | 20 | 1 |
| Neutropenia | 28 | 28 | 18 | 19 |
| Asthenia | 22 | 18 | 18 | — |
| Preferred term . | 40-µg/kg dose (N = 18) . | All treated patients (N=131) . | ||
|---|---|---|---|---|
| All grades . | >Grade 3 . | All grades . | >Grade 3 . | |
| Any event | 100 | 100 | 100 | 96 |
| Febrile neutropenia | 72 | 72 | 57 | 57 |
| Fatigue | 56 | 11 | 49 | 10 |
| Thrombocytopenia | 50 | 25 | 44 | 40 |
| Anemia | 22 | 42 | 38 | 33 |
| Nausea | 39 | — | 34 | — |
| Diarrhea | 17 | — | 31 | 2 |
| Decreased appetite | 22 | 6 | 30 | 2 |
| Peripheral edema | 28 | — | 30 | 2 |
| Dyspnea | 44 | 6 | 29 | 7 |
| Constipation | 33 | — | 25 | — |
| Epistaxis | 22 | — | 24 | 2 |
| Hypokalemia | 11 | — | 24 | 5 |
| Cough | 28 | — | 22 | 1 |
| Chills | 33 | — | 21 | 1 |
| Contusion | 39 | — | 21 | — |
| Pyrexia | 17 | — | 20 | 1 |
| Neutropenia | 28 | 28 | 18 | 19 |
| Asthenia | 22 | 18 | 18 | — |
Dashes indicate that toxicity did not occur at higher than Grade 3.
Although thrombocytopenia and associated minor bleeding was frequently observed, serious hemorrhage events were uncommon. In this study, 57 patients (44%) experienced the TEAE of thrombocytopenia, with 40% of all patients experiencing grade 3 or higher events. The most frequently reported bleeding events were epistaxis (24%), contusion (21%), and petechiae (15%). All bleeding events that were grade 3 or higher occurred in <10% of patients, including 5 cases of gastrointestinal bleeding and 3 cases of intracranial hemorrhage. Infection of any grade was observed in 70% of patients across cohorts. The most common forms of grade 3 or higher infection presented as lung infection (25%), sepsis (16%), and bacteremia (8%). Thirty-four patients (26%) discontinued treatment due to AEs, including thrombocytopenia (6 patients), sepsis (3 patients), and decreased DLCO (2 patients); no infusion-related reactions were observed.
The most frequent AEs observed at the recommended monotherapy dose (40 µg/kg; n = 18) are listed in Table 3. The recommended monotherapy dose had no associated cases of sepsis and a low early mortality rate (30-day mortality rate, 6%). At 40 µg/kg, a minority of doses were delayed (30%) due to AEs, including neutropenia, thrombocytopenia, and febrile neutropenia.
The median overall duration of treatment was 6 weeks (range, 2-50 weeks) in all patients. Twenty (15%) patients continued vadastuximab talirine in extension treatment, generally administered at a dose of 5 µg/kg.
Hepatic events
Hepatic AEs of any grade were rare across the study, including increased blood bilirubin (5%), increased aspartate aminotransferase (5%), increased alanine aminotransferase (2%), and hepatic failure (2%). No cases of sinusoidal obstruction syndrome or veno-occlusive disease (SOS/VOD) were observed in patients who did not receive alloHSCT. Eleven percent of patients (14 of 131) treated with vadastuximb talirine monotherapy went on to receive subsequent alloHSCT after completing study treatment. One patient (NPM1+ relapsed cohort) received 2 doses of vadastuximab talirine at 40 µg/kg without achieving remission and subsequently went on to receive further salvage therapy with mitoxantrone and etoposide before proceeding to alloHSCT. This patient developed fatal SOS/VOD after receiving a haploidentical BM transplant conditioned with fludarabine, cyclophosphamide, and total lymphocyte irradiation. No cases of SOS/VOD were observed in patients who went on to alloHSCT after vadastuximab talirine without intervening therapy.
Activity: dose escalation/expansion
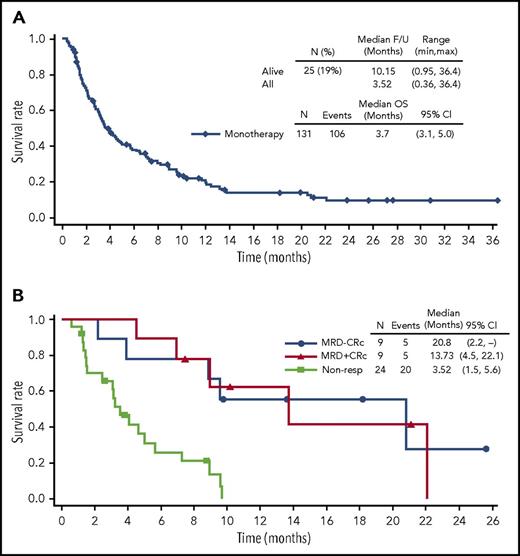
The composite complete remission (CRc) rate among the 69 patients in the dose-finding cohorts, defined as the sum of CR and CRi, was 19% (6% CR + 13% Cri; 95% confidence interval [CI], 10.4%-30.1%) with 41% blast clearance (CR + CRi + morphologic leukemia-free state [mLFS]). Response rates for the expansion cohorts are summarized in supplemental Table 1 (available on the Blood Web site). In aggregate in the dose-finding experience, of the 4 patients who achieved a best response of CR, 50% achieved an MRD-negative response, and 67% of the 9 patients who achieved a best response of CRi achieved an MRD-negative response. At the 40-µg/kg dose level (n = 18), the CRc rate was 28% (11% CR +17% Cri; 95% CI, 9.5%-53.5%) with 47% blast clearance (Table 4). The MRD-negative rates for the patients who responded at this dose level were 50% (CR) and 67% (CRi); OS by response status and MRD-status is shown in Figure 2.
Observed responses for patients treated at the 40-µg/kg dose level
| . | Dose escalation/expansion (40 µg/kg), n (%) . | Treatment naive* (40 µg/kg), n (%) . | ||
|---|---|---|---|---|
| All treated (N = 18) . | Efficacy evaluable† (N = 17) . | All treated (N = 27) . | Efficacy evaluable† (N = 26) . | |
| CR | 2 (11) | 2 (12) | 6 (22) | 6 (23) |
| CRi | 3 (17) | 3 (18) | 9 (33) | 9 (35) |
| mLFS | 3 (17) | 3 (18) | 4 (15) | 4 (15) |
| Resistant disease | 9 (50) | 9 (53) | 7 (26) | 7 (27) |
| CRc rate (CR + CRi) | 5 (28) | 5 (29) | 15 (56) | 15 (58) |
| Blast clearance (CR+ CRi + mLFS) | 8 (44) | 8 (47) | 19 (70) | 19 (73) |
| . | Dose escalation/expansion (40 µg/kg), n (%) . | Treatment naive* (40 µg/kg), n (%) . | ||
|---|---|---|---|---|
| All treated (N = 18) . | Efficacy evaluable† (N = 17) . | All treated (N = 27) . | Efficacy evaluable† (N = 26) . | |
| CR | 2 (11) | 2 (12) | 6 (22) | 6 (23) |
| CRi | 3 (17) | 3 (18) | 9 (33) | 9 (35) |
| mLFS | 3 (17) | 3 (18) | 4 (15) | 4 (15) |
| Resistant disease | 9 (50) | 9 (53) | 7 (26) | 7 (27) |
| CRc rate (CR + CRi) | 5 (28) | 5 (29) | 15 (56) | 15 (58) |
| Blast clearance (CR+ CRi + mLFS) | 8 (44) | 8 (47) | 19 (70) | 19 (73) |
Includes the treatment-naive cohort and 3 patients in the 40 µg/kg–dose-escalation cohort who were treatment naive.
Includes all treated patients who had a BM examination and response determination at least once after the first study treatment.
Summary of OS. (A and B) Monotherapy and summary of OS by best MRD status at the 40-µg/kg dose level.
Summary of OS. (A and B) Monotherapy and summary of OS by best MRD status at the 40-µg/kg dose level.
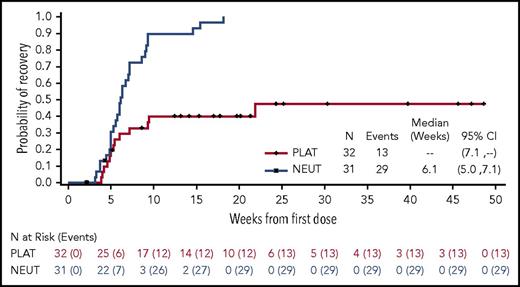
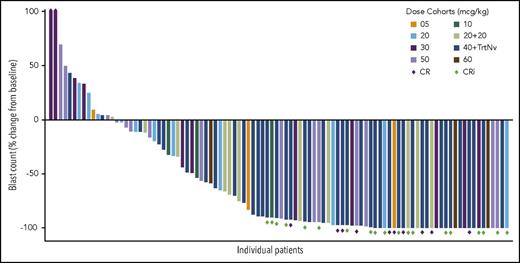
The median time to neutrophil count recovery was 6.4 weeks (range, 3-9 weeks), and the median time to platelet recovery was 10.6 weeks (range, 4-25 weeks) (Figure 3). A dose-response relationship was observed, with a trend toward increasing blast clearance with increasing dose level (Figure 4). Forty-nine percent of patients cleared their marrow blasts at higher dose levels (≥30 µg/kg) compared with a 32% blast clearance rate observed at lower dose levels.
Summary of count recovery. Time to platelet count ≥100 × 109 units/L and neutrophils ≥1000 units/uL for patients who achieved a CR or CRi.
Summary of count recovery. Time to platelet count ≥100 × 109 units/L and neutrophils ≥1000 units/uL for patients who achieved a CR or CRi.
Percent change in BM blasts from baseline. Antileukemic activity was observed across all dose cohorts.
Percent change in BM blasts from baseline. Antileukemic activity was observed across all dose cohorts.
Expansion cohorts
Fractionated dosing (20 + 20 µg/kg)
A fractionated-dosing schedule (40 µg/kg total administered as 20 µg/kg on days 1 and 4) was explored in a 12-patient cohort to determine whether the safety and activity profile was favorable compared with the single-dose schedule. One DLT (grade 3 mucositis) was observed in these 12 patients treated with fractionated dosing, as well as 2 cases of sepsis (17%). Thus, the SMC recommended the single dose rather than fractionated dose schedule on the basis of safety profile.
NPM1 mutation–positive relapsed AML
Given the observation that NPM1 mutational status correlates with increased CD33 expression in the BM,2,3 an expansion cohort was explored at 40 µg/kg to test the activity of vadastuximab talirine in patients with relapsed NPM1+/FLT3-wild type AML. Patients in this cohort (n = 13) were allowed ≤2 lines of salvage therapy before enrolling in this study. The median age for this cohort of relapsed patients was 69 years (range, 41-80 years), and 3 patients (23%) had an ECOG score of 0 and 10 patients (77%) had an ECOG score of 1. Patients in this cohort received a median of 1 cycle (range, 1-5 cycles). Four patients (31%) went on to receive alloHSCT after completion of vadastuximab talirine therapy. Although count recovery was difficult to achieve in this cohort’s more heavily pretreated patient population (all patients received ≥1 previous intensive chemotherapy regimen), blast clearance was readily achievable (69% mLFS rate, supplemental Table 1).
Older treatment-naive AML
Among the 27 treatment-naive patients who declined intensive therapy and who were treated with vadastuximab talirine at the 40-µg/kg dose level, the median age was 74 years (range 67-89 years); 10 patients (37%) had an ECOG score of 0, and 17 patients (63%) had an ECOG score of 1. Thirteen (48%) patients had underlying myelodysplasia. The median BM blast count at enrollment was 47%; a total of 6 patients (22%) harbored FLT3 mutations, and patients were classified per ELN risk class as favorable (4%), intermediate I (33%), intermediate II (30%), or adverse (26%). Two patients experienced DLTs (decreased DLCO, grade 3 [in the setting of congestive heart failure exacerbation; pulmonary function tests ultimately recovered to baseline after the end of treatment] and hematuria, grade 3). The most frequent AEs observed in this cohort were decreased appetite (52%), diarrhea, fatigue, peripheral edema, thrombocytopenia (48% each), anemia (44%), dizziness, and febrile neutropenia (41% each). No deaths occurred within 30 days of the first dose.
Post-alloHSCT relapsed AML
The median age for this cohort (n = 12) of relapsed patients post-alloHSCT was 56 years (range, 26-72 years), and the majority (83%) had an ECOG performance status score of 1. An initial cohort of 4 patients was treated at the 40-µg/kg dose level. One patient was not evaluable for DLT, 2 experienced DLTs (grade 4 hypocellular marrow and grade 4 hyperbilirubinemia in the setting of sepsis and persistent AML). Thus, a lower dose level of 20 µg/kg was explored in a total of 8 patients. Two DLTs were noted in this cohort: 1 patient experienced grade 4 acute kidney injury in the setting of prolonged septic shock and ongoing relapsed AML, and 1 patient experienced grade 3 hyperbilirubinemia, which resolved to grade 2 in 14 days.
Baseline CD33 expression and response
Baseline CD33 expression on blasts from samples of sufficient quality (BM, n = 90; peripheral blood [PB], n = 105) was measured by multicolor flow cytometry at a central laboratory (in log units of molecules of equivalent soluble fluorochrome).
In a univariate analysis of clinical response, excluding post-alloHSCT relapsed specimens, CD33 expression in the BM and PB correlated with the odds of achieving blast clearance (odds ratio [OR], 3.95; 95% CI, 1.31-13.4; P = .020 and OR, 3.69; 95% CI, 1.31-11.22; P = .016, respectively), but neither CRc nor MRD negativity. After accounting for treatment naivety, cytogenetic risk (ELN), baseline percent blasts in BM, and the administered dose of vadastuximab talirine on cycle 1, the effect of CD33 on blast clearance remained significant (multivariate OR PB CD33, 3.58; 95% CI, 1.10-12.6; P = .040; multivariate OR BM CD33, 4.50; 95% CI, 1.31-17.0; P = .021). However, including NPM1 mutation as a covariate decreased the significance of this relationship (multivariate OR PB CD33, 1.86; 95% CI, 0.54-6.80; P = .33; multivariate OR BM CD33, 2.38; 95% CI, 0.67-9.21; P = .19). The association between CD33 expression and CRc or MRD negativity remained insignificant in the multivariate analysis.
Together, these data suggest a possible increased potential for achieving blast clearance with higher levels of CD33 expression, but that this effect is closely linked to higher CD33 levels on blasts with NPM1 mutation, a known favorable prognostic marker. Furthermore, our current inability to observe a significant relationship between CD33 expression and CRc or MRD negativity rates suggests the potential for all patients with CD33-positive AML to respond to vadastuximab talirine.
Discussion
CD33 is an attractive target for antibody-based therapy because it is detectable on at least a subset of leukemic blasts in nearly all patients.2,3 This first-in-human trial of vadastuximab talirine supports the potential for CD33-directed therapy in AML.
Vadastuximab talirine was designed to address the limitations of other CD33-directed ADCs. The protease-cleavable linker is highly stable in the circulation and releases the cytotoxic payload only on internalization into CD33-expressing cells, mitigating the potential for off-target toxicity due to unconjugated drug. The clinical safety data observed in this phase 1 trial provide evidence supporting this molecular design; AEs were primarily related to on-target myelosuppression. Nonhematologic DLTs were primarily observed in the cohort of patients who had relapsed post-alloHSCT. In all other cohorts, single-agent vadastuximab talirine treatment was tolerable, even in older patients, with minimal off-target toxicity. Although thrombocytopenia was a frequent finding in this study, serious bleeding was rare. Hepatic events were uncommon, including laboratory abnormalities of any grade; elevations of bilirubin, ALT, and AST each occurred in ≤5% of patients. SOS/VOD has not been observed in vadastuximab talirine trials outside of alloHSCT. Fourteen patients from this study received subsequent alloHSCT. One patient discontinued study treatment due to nonresponse, received subsequent off-protocol salvage therapy, and then underwent alloHSCT, developing SOS/VOD >100 days from the last dose of vadastuximab talirine. Continued monitoring of hepatobiliary serious AEs occurred across vadastuximab talirine protocols, including those that have enrolled a greater proportion of transplant-eligible patients.
Vadastuximab talirine was designed with a highly potent synthetic PBD dimer payload to overcome drug resistance. This is in contrast to GO’s cytotoxic payload, calicheamycin, a substrate for drug transporters (eg, P-glycoprotein and MRP1), which can limit intracellular accumulation.4 In preclinical testing, vadastuximab talirine was demonstrated to be more potent than GO across a panel of AML cell lines, including primary AML cells in vitro and in models with the multidrug-resistant phenotype.7 Encouragingly, this appears to have translated into single-agent clinical activity among studied patients; responses in this phase 1 study were achieved across the spectrum of AML patients, including those with traditionally poor disease risk factors, such as underlying myelodysplasia and adverse-risk cytogenetics.
In the dose-escalation experience, responses were observed across all dose levels. The likelihood of blast clearance (best response of at least mLFS, with or without PB count recovery) appeared to have a relationship with expression of CD33; this effect was driven by the high mLFS rate in the NPM1-mutated cohort. The apparent relationship with CD33 expression did not correlate with CRc or MRD-negative remission. Although formal MTD was not exceeded at the doses tested, an apparent dose-dependent relationship was observed in terms of blast clearance and myelosuppression. The 40 µg/kg-dose level was determined to provide an acceptable balance of safety and antileukemic activity. This dose was further tested in subsequent expansion cohorts, including a cohort of treatment-naive patients, where a CRc rate of 56% was observed. Deep remissions were achieved, as evidenced by a 40% MRD clearance rate among responders in the treatment-naive group. This included 44% of CRi patients achieving MRD negativity, suggesting that a subset of patients with incomplete platelet recovery may have attained deep remissions. The thrombocytopenia observed in these MRD-negative CRi patients may have been due to on-target myelosuppression rather than residual leukemia.
This phase 1 trial demonstrates single-agent biologic activity and a safety profile consistent with minimal nonhematologic toxicity. In an expansion arm of this study, vadastuximab talirine was subsequently combined with HMAs in older adults with previously untreated AML, resulting in encouraging response rates.12 Although most grade 3 or higher AEs were related to myelosuppression, early 60-day mortality was 8% in the phase 1 HMA combination cohort, similar to published studies using HMAs alone.13,14 However, a global, double-blinded, placebo-controlled phase 3 successor trial (CASCADE) comparing the combination of vadastuximab talirine and HMAs with HMAs alone was closed early due to increased infectious deaths in the experimental arm. Data from the CASCADE trial are currently being analyzed. The optimal approach to incorporating vadastuximab talirine into a therapeutic strategy for patients with AML has not yet been determined.
Presented in part at the 56th and 57th annual meetings of the American Society of Hematology, San Francisco, CA, 6-9 December 2014 and Orlando, FL, 5-8 December 2015.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Jenna Voellinger for her pharmacokinetic contribution, Niya Gu for statistical guidance, and Candace Larson for assistance in manuscript preparation as employees of Seattle Genetics, Inc. The authors would also like to thank Brent Wood and his laboratory for the work on this trial.
This work was supported by Seattle Genetics, Inc. R.W. is a Leukemia & Lymphoma Society Scholar in Clinical Research.
Authorship
Contribution: E.M.S. performed the research and wrote the paper; R.B.W., H.P.E., A.T.F. A.S.A., J.E.L., F.R., T.K., D.J.D., D.B., S.F., A.P.J., and A.S.S. were investigators and performed the research; and P.A.H., M.M.O., B.Z., and C.B.-S. designed the trial and analyzed the data.
Conflict-of-interest disclosure: E.M.S., R.B.W., H.P.E., A.T.F., A.S.A., J.E.L., F.R., T.K., D.J.D., D.B., S.F., A.P.J., and A.S.S. were investigators funded by Seattle Genetics, Inc. to do this study. A.T.F., A.S.A., F.R., H.P.E., and J.E.L. served as consultants to Seattle Genetics, Inc. A.T.F. was a member of a steering committee for Seattle Genetics, Inc. F.R. received an honoraria from Seattle Genetics, Inc. P.A.H., M.M.O., B.Z., and C.B.-S. are Seattle Genetics, Inc. employees.
Correspondence: Eytan M. Stein, Memorial Sloan-Kettering Cancer Center, 1275 York Ave, New York, NY 10065; e-mail: steine@mskcc.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal