Key Points
Hematopoietic stem/progenitor mutation burden is not increased in SCN.
Clonal hematopoiesis due to mutations of TP53 is present in the majority of patients with SDS.
Abstract
Severe congenital neutropenia (SCN) and Shwachman-Diamond syndrome (SDS) are congenital neutropenia syndromes with a high rate of leukemic transformation. Hematopoietic stressors may contribute to leukemic transformation by increasing the mutation rate in hematopoietic stem/progenitor cells (HSPCs) and/or by promoting clonal hematopoiesis. We sequenced the exome of individual hematopoietic colonies derived from 13 patients with congenital neutropenia to measure total mutation burden and performed error-corrected sequencing on a panel of 46 genes on 80 patients with congenital neutropenia to assess for clonal hematopoiesis. An average of 3.6 ± 1.2 somatic mutations per exome was identified in HSPCs from patients with SCN compared with 3.9 ± 0.4 for healthy controls (P = NS). Clonal hematopoiesis due to mutations in TP53 was present in 48% (13/27) of patients with SDS but was not seen in healthy controls (0/17, P < .001) or patients with SCN (0/40, P < .001). Our SDS cohort was young (median age 6.3 years), and many of the patients had multiple TP53 mutations. Conversely, clonal hematopoiesis due to mutations of CSF3R was present in patients with SCN but was not detected in healthy controls or patients with SDS. These data show that hematopoietic stress, including granulocyte colony-stimulating factor, do not increase the mutation burden in HSPCs in congenital neutropenia. Rather, distinct hematopoietic stressors result in the selective expansion of HSPCs carrying specific gene mutations. In particular, in SDS there is enormous selective pressure to expand TP53-mutated HSPCs, suggesting that acquisition of TP53 mutations is an early, likely initiating event, in the transformation to myelodysplastic syndrome/acute myeloid leukemia in patients with SDS.
Introduction
Severe congenital neutropenia (SCN) is a rare syndrome characterized by chronic neutropenia present from birth and by recurring bacterial infections. Mutations of ELANE are the most common cause of SCN, accounting for ∼50% of cases, with mutations of HAX1 and G6PC3 accounting for an additional 10% to 20% of cases.1-4 Treatment with granulocyte colony-stimulating factor (G-CSF) is the standard of care for SCN, because it increases the level of circulating neutrophils and reduces infection-related mortality.5 Shwachman-Diamond syndrome (SDS) is a recessive disorder characterized by exocrine pancreatic insufficiency, bone marrow dysfunction, and skeletal abnormalities. SDS is caused in most cases by biallelic mutations of SBDS.6 Current studies support a model of disease pathogenesis in which SBDS mutations lead to impaired ribosome assembly.7-9
A shared feature of SCN, SDS, and several other bone marrow failure syndromes that feature neutropenia is a marked propensity to develop a myelodysplastic syndrome (MDS) or acute myeloid leukemia (AML). The cumulative incidence of MDS/AML in patients with SCN treated with G-CSF is 22%.10 Of note, MDS/AML has been reported in SCN secondary to ELANE, HAX1, G6PC3, or WAS mutations.11 The cumulative incidence of MDS/AML in SDS is ∼20%.12 Acquired gain-of-function mutations of CSF3R, encoding the G-CSF receptor, are present in ∼40% of cases of SCN and are associated with the development of MDS/AML.13,14 Mutations of RUNX1 are present in ∼65% of patients with SCN who develop AML/MDS.15 In SDS, a recent study showed that mutations of TP53 are common in MDS that develops in patients with SDS.16 Whether the TP53 mutations represent an early, leukemia initiating event, or a late progression event (as has been shown for some types of secondary AML),17 is unknown. Despite these observations, the molecular mechanisms contributing to transformation to MDS/AML in congenital neutropenia syndromes are poorly understood, limiting the development of new therapies or strategies for risk stratification or early detection.
The accumulation of mutations in hematopoietic stem cells (HSCs) with age results in the production of a genetically heterogeneous cell population, with each HSC possessing its own unique set of private mutations.18 HSCs that acquire somatic mutations that confer a competitive fitness advantage relative to their normal counterparts may clonally expand. Indeed, several groups have documented the presence of clonal hematopoiesis in healthy individuals.19-21 Factors that increase the rate at which mutations accumulate in HSCs may increase the frequency of clonal hematopoiesis and ultimately MDS/AML. The common mutations causing SCN are not known to be directly involved in DNA repair, suggesting the possibility that noncell autonomous mechanisms may contribute to the high rate of leukemic transformation. For example, G-CSF expression is induced by neutropenia and may increase the rate at which HSCs accumulate mutations by inducing their replication.22 Of note, prior studies have demonstrated that the G-CSF receptor (CSF3R) is expressed on HSCs.23 Factors that select for HSCs carrying deleterious mutations also may increase the risk of MDS/AML. For example, we previously showed that HSCs carrying mutations in TP53 are selected by exposure to chemotherapy.24 Thus, it is possible that HSC-cell autonomous and/or noncell autonomous alterations in congenital neutropenia may confer a competitive fitness advantage to HSCs that carry leukemia-associated mutations. To test these possibilities, we measured the mutation burden in individual hematopoietic stem/progenitor cells (HSPCs) and characterized clonal hematopoiesis in patients with congenital neutropenia.
Methods
Human subjects
A total of 101 human blood or bone marrow samples were obtained for our study. These samples were divided into 5 cohorts: patients with SCN (40), patients with SDS (28), patients with cyclic neutropenia (13), healthy volunteers (17), and cord blood (3). Coded blood or bone marrow samples from patients with congenital neutropenia were obtained from the Severe Chronic Neutropenia International Registry (https://depts.washington.edu/registry/), the SDS Registry (http://sdsregistry.org), or from various other academic institutions. Anonymized cord blood samples were obtained from the Saint Louis Cord Blood Bank (http://www.slcbb.org). Coded blood or bone marrow samples were obtained from healthy volunteers, with the following exclusion criteria: a personal history of cancer, the use of cytotoxic drugs for nonmalignant disease, a history of radiation therapy, or known infections with hepatitis B or C, human T-cell leukemia virus, or HIV. In each case, the banking and distribution of these samples were approved by institutional review boards at the involved institution; written informed consent was obtained from all participants.
Hematopoietic progenitor cell expansion
The low-density mononuclear cell fraction was isolated from peripheral blood or bone marrow by centrifugation at 400g for 30 minutes over a Histopaque 1077 gradient (Sigma); in some cases, red blood cells were lysed by incubating in Tris-buffered ammonium chloride (pH 7.4). An aliquot (1 × 106) of unselected mononuclear cells was removed, and genomic DNA was prepared using the QIAmp DNA Mini kit (Qiagen), per the manufacturer’s instructions. The remainder of the cells was stained with a panel of fluorescein-conjugated lineage markers (CD3, CD19, CD14, and CD16), phycoerythrin-conjugated CD34, and allophycocyanin-conjugated CD38. CD34+ CD38− lineage− cells were sorted at 1 cell per well into a 96-well plate using a Cytomation MoFlow or Sony Synergy cell sorter. Prior to sorting, the 96-well plate was seeded with irradiated (2000 cGy) AFT024 stromal cells (ATCC: SCRC-1007) at a density of 2.5 × 104 cell per well. CD34+ CD38− lineage− cells were cultured for 2 to 3 weeks in Iscove’s Modified Dulbecco’s medium supplemented with 10% fetal calf serum, 1 mM l-glutamine, and the following human recombinant cytokines: stem cell factor (10 ng/mL), FLT3 ligand (25 ng/mL), thrombopoietin (20 ng/mL), interleukin-3 (10 ng/mL), and G-CSF (10 ng/mL). The cultures were maintained at 37°C with 5% CO2 and ambient oxygen and supplemented with fresh media every 5 days. After 2 to 3 weeks of culture, wells with visible hematopoietic cell growth were expanded into a 24-well plate without AFT024 feeder cells for another week. Hematopoietic cells from the colonies were then harvested and counted and genomic DNA was prepared using the Qiagen QIAmp DNA Micro Kit (Qiagen).
HSPC clone whole exome sequencing and variant calling
Genomic DNA was fragmented, and exome capture was performed using a customized version of the Agilent SureSelect Human All Exon v2 kit, which targets 99.01% of CCDS exons, 93.29% of RefSeq genes, and 90% of known microRNA genes (MiRbase 14). Median sequence coverage was 104X (range 46.2-232X). Sequence was aligned to reference sequence build GRCh37-lite-build37, as previously described.25 Putative somatic mutations were identified via direct pairwise comparisons of each HSPC clone to the unfractionated total peripheral blood/bone marrow cells from which it was derived. Single nucleotide variants (SNVs) and insertions/deletions (Indels) were detected as previously described.25 SNVs and Indels that exceeded 0.1% frequency in the 1000 genomes or National Heart, Lung, and Blood Institute exome sequencing projects were removed. To remove nonclonal events, reference and variant read counts were compared with a binomial distribution of the same number of reads, assuming a variant allele frequency of 50%. Sites that significantly differed (Fisher’s exact test, P > .05) from the expected binomial distribution were removed. Additional filters required the number of reference reads in the control sample to be >30, the number of variant reads in the HSPC clone sample to be >5, and the variant allele frequency in the control sample to be <3%. Finally, sites were manually reviewed to remove other classes of alignment artifacts.
Error-corrected (Haloplex) targeted sequencing and variant detection
Error-corrected sequencing was performed using the Agilent Haloplex HS Target Enrichment System, as previously described.26 A customized HaloPlex HS Target Enrichment assay targeting 46 genes mutated in clonal hematopoiesis and/or MDS/AML (supplemental Table 1, available on the Blood Web site) was designed using the Agilent SureDesign platform. The probes had dual indices: a unique molecular barcode to allow for error-corrected sequencing and a sample index to allow for sample multiplexing. Genomic DNA (500 ng) was hybridized to the custom probes, ligated, captured with streptavidin, and polymerase chain reaction amplified (×24 cycles) to create read families each with its own molecular bar code. Median sequence coverage was 17 824X (range 9491-34 895). Variants were required to be supported by 3 read families. Filters were applied to remove artifacts appearing at homopolymer runs of length >4 and alignment artifacts appearing in >5% of a panel of normal samples. Next, background noise calculation was performed on a position-by-position basis for each identified variant as follows: For each variant, read counts were gathered from all other samples, excluding those sites with variant allele frequency >25%, which were assumed to be germ line SNPs. In a single case (SCN51), we retained a truncation CSF3R mutation with a variant allele frequency of 39%. A P value was obtained via Fisher's exact test, comparing the reference and variant reads at a site to the number of reference and variant reads at that site in all other samples. Multiple testing correction was applied with the P value adjust function (default parameters). Those variants with an adjusted P value of <.1 were retained. The same process is repeated with subsequent background calculations excluding all variants retained in previous rounds until no new variants were identified. Finally, we interrogated the Exome Aggregation Consortium database (http://exac.broadinstitute.org) to remove variants with an adjusted allele frequency <0.0005. Following manual review, we reported somatic variants that generated missense, nonsense, or splice site mutations.
Statistical analysis
Continuous and count variables are described with means and standard deviations when distributions appear symmetrical and by medians when the distributions appear skewed. Categorical variables are described by percentages. Pearson correlations are used when the distributions of variables appear symmetrical and Spearman correlations when they appear asymmetrical or there are outlying values that would inflate a Pearson correlation. Independence between dichotomous variables in 2-way tables is evaluated with Fisher’s exact tests, whereas exact χ2 tests are used in 2-way tables with small sample sizes where one or both variables are not dichotomous. Independent-sample Student t tests are used to compare 2 groups with respect to symmetrically distributed continuous variables. Analysis of covariance is used as an alternative to analysis of variance in order to adjust for confounding variables such as age. The Kruskal-Wallis nonparametric alternative to analysis of variance is used when there are multiple groups and the dependent variable is asymmetric. The analysis is followed by Bonferroni-adjusted pairwise Wilcoxon tests. Logistic regression is used when the dependent variable is dichotomous with results reported as odds ratios with 95% confidence intervals. For all tests, a P value <.05 was used to indicate statistical significance. Statistical analyses were done with SAS Version 9.4 for Windows and the R statistical package.
Results
Hematopoietic progenitor mutation burden
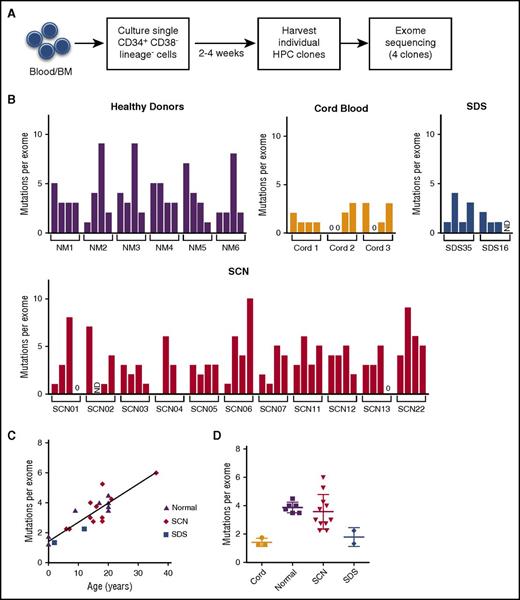
To determine whether chronic neutropenia and/or long-term treatment with G-CSF increased mutation burden in hematopoietic progenitors, we sequenced the exomes of individual, ex vivo expanded HSPCs from patients with congenital neutropenia (Figure 1A). These sequences were compared with the exome sequence of total leukocytes from the same patient to identify clonal somatic mutations (see “Methods”). Whereas we were successful in generating hematopoietic colonies of sufficient size for exome sequencing from the majority of SCN, healthy donors, and cord blood samples, only 2 of 15 (13.3%) SDS samples generated such colonies. This is consistent with a study showing that HSPCs from patients with SDS have impaired growth.27 In total, we sequenced hematopoietic colonies from 11 patients with SCN, 2 with SDS, 6 healthy donors, and 3 umbilical cord blood samples; patients with evidence of MDS or AML at the time of tissue collection were excluded (Table 1; supplemental Tables 2 and 3). All of the patients with SCN for whom information was available carried heterozygous germ line mutations of ELANE and were being treated chronically with G-CSF. Of note, the 2 patients with SDS were younger than those in the SCN cohort and had not received prior G-CSF treatment.
Hematopoietic progenitor mutation burden. (A) Experimental schema. CD34+ CD38− lineage− cells from blood (Nm1, Nm4, and cord blood) or bone marrow were sorted 1 cell per well and expanded on stromal support for 2 to 4 weeks. Exome sequencing was performed on 4 hematopoietic progenitor clones isolated from 6 healthy donors, 3 cord blood, 11 SCN, or 2 SDS patients. Somatic mutations were identified by comparison with exome sequence data from matched unfractionated blood or bone marrow leukocytes. (B) The number of somatic SNVs per exome for each clone. (C) The average number of somatic SNVs per hematopoietic progenitor exome vs age at sample collection. (D) The average number of somatic SNVs per exome. The mean ± standard error of the mean is shown. BM, bone marrow; HPC, hematopoietic progenitor cell; ND, not determined; NM, healthy volunteers.
Hematopoietic progenitor mutation burden. (A) Experimental schema. CD34+ CD38− lineage− cells from blood (Nm1, Nm4, and cord blood) or bone marrow were sorted 1 cell per well and expanded on stromal support for 2 to 4 weeks. Exome sequencing was performed on 4 hematopoietic progenitor clones isolated from 6 healthy donors, 3 cord blood, 11 SCN, or 2 SDS patients. Somatic mutations were identified by comparison with exome sequence data from matched unfractionated blood or bone marrow leukocytes. (B) The number of somatic SNVs per exome for each clone. (C) The average number of somatic SNVs per hematopoietic progenitor exome vs age at sample collection. (D) The average number of somatic SNVs per exome. The mean ± standard error of the mean is shown. BM, bone marrow; HPC, hematopoietic progenitor cell; ND, not determined; NM, healthy volunteers.
Demographic and disease characteristics HPC mutation burden cohort
| Characteristic . | Normal (N = 6) . | SCN (N = 11) . | SDS (N = 2) . |
|---|---|---|---|
| Age, y | |||
| Mean ± SD | 17.7 ± 4.4 | 16.6 ± 7.9 | 7.0 ± 7.1 |
| Median (range) | 20 (9-20) | 15.5 (6-36) | 7.0 (2-12) |
| Female sex, n (%) | 2 (33.3) | 5 (45.4) | 1 (50) |
| G-CSF treatment, n (%)* | 0 (0) | 10 (100) | 0 (0) |
| ANC per mm3† | |||
| Mean ± SD | na | 62 ± 99 | 1170 ± 1198 |
| Median (range) | na | 0 (0-300) | 1170 (330-2020) |
| ELANE mutation, n (%)‡ | na | 10 (100) | na |
| SBDS mutation, n (%) | na | na | 2 (100) |
| Characteristic . | Normal (N = 6) . | SCN (N = 11) . | SDS (N = 2) . |
|---|---|---|---|
| Age, y | |||
| Mean ± SD | 17.7 ± 4.4 | 16.6 ± 7.9 | 7.0 ± 7.1 |
| Median (range) | 20 (9-20) | 15.5 (6-36) | 7.0 (2-12) |
| Female sex, n (%) | 2 (33.3) | 5 (45.4) | 1 (50) |
| G-CSF treatment, n (%)* | 0 (0) | 10 (100) | 0 (0) |
| ANC per mm3† | |||
| Mean ± SD | na | 62 ± 99 | 1170 ± 1198 |
| Median (range) | na | 0 (0-300) | 1170 (330-2020) |
| ELANE mutation, n (%)‡ | na | 10 (100) | na |
| SBDS mutation, n (%) | na | na | 2 (100) |
ANC obtained prior to G-CSF therapy is shown. Percentage of patients with germ line heterozygous ELANE or biallelic SBDS mutations is shown.
na, not available; SD, standard deviation.
Data not available for 1 patient with SCN.
Data not available for 2 patients with SCN.
Data not available for 1 patient with SCN.
Across all samples, the number of genic somatic mutations detected in the progeny of each HSPC ranged from 0 to 10 (Figure 1B; supplemental Table 4). As reported previously, a strong correlation between HSPC mutation burden and the age of the patient was observed (Pearson r = 0.83, P < .001) (Figure 1C).18 The lowest number of mutations was present in the cord blood samples, with only 1.4 ± 0.29 mutations per HSPC exome (Figure 1D). The number of mutations detected in the exomes of HSPCs from healthy donors (3.9 ± 0.38) is similar to that observed from patients with SCN (3.6 ± 1.2) or SDS (1.8 ± 0.65). After adjusting for age, there was no difference in HSPC mutation burden in the different cohorts (P = .34 by analysis of covariance). Somatic copy number alterations were not identified in any of the hematopoietic colonies (data not shown). These data suggest that the rate at which mutations accumulate in HSPCs in patients with congenital neutropenia is not increased compared with that of healthy individuals.
Clonal hematopoiesis
We used a sensitive error-corrected sequencing approach to look for clonal hematopoiesis in the blood or bone marrow of patients with congenital neutropenia. Using this sequencing technique, we were able to reliably detect mutations with a variant allele frequency of at least 0.1%, corresponding to 1 cell in 500 carrying a mutation. We interrogated 46 genes that have been reported to be mutated in individuals with clonal hematopoiesis or MDS/AML (supplemental Table 1). We analyzed 17 healthy individuals, 40 with SCN, and 27 with SDS (Table 2; supplemental Tables 2 and 3). We also analyzed 13 patients with cyclic neutropenia. Cyclic neutropenia is characterized by intermittent neutropenia and is caused, in most cases, by mutations of ELANE. However, in contrast to SCN, it rarely transforms to MDS/AML.28 All of the patients with cyclic neutropenia or SCN (for whom information was available) carried heterozygous germ line mutations of ELANE, and all of the patients with SDS carried biallelic germ line mutations of SBDS. As expected, the baseline (pre-G-CSF treatment) absolute neutrophil count (ANC) was significantly lower in patients with SCN compared with patients with SDS or cyclic neutropenia (P < .001 and P < .01, respectively, using Bonferroni-corrected Wilcoxon tests). All of the patients with cyclic neutropenia or SCN (for whom information was available) were treated chronically with G-CSF, compared with 10 of 25 (40%) cases of SDS (for which information was available). Ten of 40 patients (25%) of patients with SCN underwent allogenic stem cell transplantation, compared with a single patient with cyclic neutropenia, and 1 patient with SDS. Only 2 patients with SCN in our cohort are known to have developed AML or MDS.
Demographic and disease characteristics clonal hematopoiesis cohort
| Characteristic . | Normal (N = 17) . | Cyclic (N = 13) . | SCN (N = 40) . | SDS (N = 27) . |
|---|---|---|---|---|
| Age, y | ||||
| Mean ± SD | 17.2 ± 10.1 | 24.5 ± 14.1 | 11.6 ± 10.3 | 7.9 ± 5.0 |
| Median (range) | 17 (4-34) | 26 (3-47) | 10 (0.25-45) | 6.3 (2-19) |
| Female sex, n (%) | 8 (47.0) | 4 (30.7) | 23 (57.5) | 11 (37.9) |
| G-CSF treatment, n (%)* | 0 (0) | 10 (100) | 38 (100) | 10 (40) |
| ANC per mm3† | ||||
| Mean ± SD | na | 613 ± 554 | 140 ± 150 | 1080 ± 1070 |
| Median (range) | na | 400 | 90 (0-650) | 770 (0-4490) |
| ELANE mutation, n (%)‡ | na | 9 (100) | 39 (100) | na |
| SBDS mutation, n (%) | na | na | na | 27 (100) |
| Allogenic stem cell transplant, n (%) | na | 1 (7.6%) | 10 (25%) | 1 (3.7) |
| AML or MDS, n (%)§ | na | 0 (0) | 2 (5.0%) | 0 (0) |
| Characteristic . | Normal (N = 17) . | Cyclic (N = 13) . | SCN (N = 40) . | SDS (N = 27) . |
|---|---|---|---|---|
| Age, y | ||||
| Mean ± SD | 17.2 ± 10.1 | 24.5 ± 14.1 | 11.6 ± 10.3 | 7.9 ± 5.0 |
| Median (range) | 17 (4-34) | 26 (3-47) | 10 (0.25-45) | 6.3 (2-19) |
| Female sex, n (%) | 8 (47.0) | 4 (30.7) | 23 (57.5) | 11 (37.9) |
| G-CSF treatment, n (%)* | 0 (0) | 10 (100) | 38 (100) | 10 (40) |
| ANC per mm3† | ||||
| Mean ± SD | na | 613 ± 554 | 140 ± 150 | 1080 ± 1070 |
| Median (range) | na | 400 | 90 (0-650) | 770 (0-4490) |
| ELANE mutation, n (%)‡ | na | 9 (100) | 39 (100) | na |
| SBDS mutation, n (%) | na | na | na | 27 (100) |
| Allogenic stem cell transplant, n (%) | na | 1 (7.6%) | 10 (25%) | 1 (3.7) |
| AML or MDS, n (%)§ | na | 0 (0) | 2 (5.0%) | 0 (0) |
Data not available for 3 patients with cyclic neutropenia, 2 patients with SCN, and 2 patients with SDS.
ANC obtained prior to G-CSF therapy is shown. Data not available for 8 patients with cyclic neutropenia and 3 patients with SCN.
Percentage of patients with germ line heterozygous ELANE or biallelic SBDS mutations is shown. Data not available for 4 patients with cyclic neutropenia and 1 patient with SCN.
Subsequent development of AML or MDS. No patient had MDS or AML at the time of sample analysis.
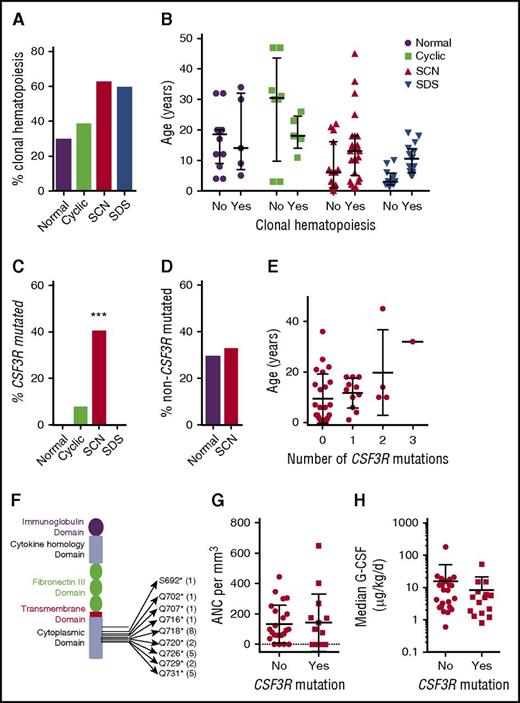
Clonal hematopoiesis due to any mutation was identified in 5 of 17 (29%) healthy individuals, 5 of 13 (38%) patients with cyclic neutropenia, 25 of 40 (62%) SCN cases, and 16 of 27 (59%) of SDS cases (P = .08 by Pearson χ2 of independence) (Figure 2A; supplemental Table 5). No consistent effect of tissue origin (ie, blood vs bone marrow) was observed on the incidence of clonal hematopoiesis (supplemental Figure 1A). Although clonal hematopoiesis was not detected in the 3 cord blood samples tested, we observed no difference in the median age of individuals with or without clonal hematopoiesis, which may reflect the rather narrow age range of cases analyzed in this study (Figure 2B).
Clonal hematopoiesis with CSF3R mutations. (A) Percentage of cases with clonal hematopoiesis due to any gene mutation. (B) Age of individuals with or without clonal hematopoiesis due to any gene mutation. (C) Percentage of cases with clonal hematopoiesis due to CSF3R mutations. ***P = .003 compared with healthy donors. (D) Percentage of cases with clonal hematopoiesis due to mutations in genes besides CSF3R. (E) Age of patients with SCN based on the number of CSF3R mutations. (F) The CSF3R mutations, with the number of times the mutation was seen in parentheses. (G) ANC prior to G-CSF treatment. (H) The median G-CSF dose. The mean ± standard deviation is shown.
Clonal hematopoiesis with CSF3R mutations. (A) Percentage of cases with clonal hematopoiesis due to any gene mutation. (B) Age of individuals with or without clonal hematopoiesis due to any gene mutation. (C) Percentage of cases with clonal hematopoiesis due to CSF3R mutations. ***P = .003 compared with healthy donors. (D) Percentage of cases with clonal hematopoiesis due to mutations in genes besides CSF3R. (E) Age of patients with SCN based on the number of CSF3R mutations. (F) The CSF3R mutations, with the number of times the mutation was seen in parentheses. (G) ANC prior to G-CSF treatment. (H) The median G-CSF dose. The mean ± standard deviation is shown.
Clonal hematopoiesis with CSF3R mutations
Consistent with prior reports,14,29 clonal hematopoiesis due to mutations of CSF3R was detected in 40% (16 of 40) of patients with SCN, compared with 0 of 17 of healthy controls (Fisher’s exact test, P = .003; Figure 2C). CSF3R mutations were detected in a single patient with cyclic neutropenia (1/17, 7.7%, P = .04) and in no patients with SDS (0/27; P < .001). Of note, after removing CSF3R mutations, the percentage of cases with clonal hematopoiesis was similar between healthy controls and patients with SCN (Figure 2D). The size of the hematopoietic clone carrying a CSF3R mutation ranged from 0.26% to 78% of cells in the blood samples (median 0.70%). The number of CSF3R mutations per patient with SCN ranged from 0 to 3 (Figure 2E). Consistent with prior reports, all of the CSF3R variants are nonsense mutations that truncate the cytoplasmic domain of the G-CSF receptor (Figure 2F). Logistic regression, both adjusted for age and unadjusted, showed that the presence of CSF3R mutations was not related to standard measures of disease severity in SCN, including baseline ANC or median dose of G-CSF (Figure 2G-H).
Clonal hematopoiesis with TP53 mutations
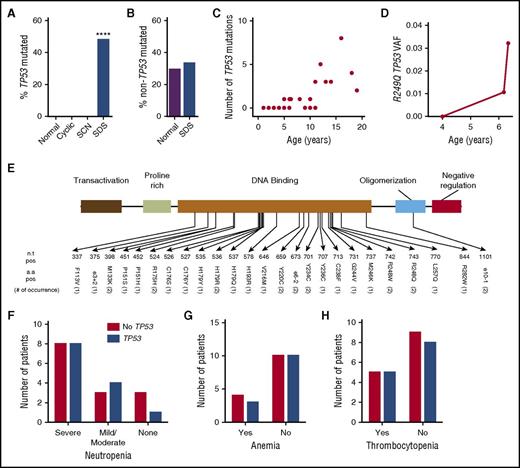
Clonal hematopoiesis due to mutations of TP53 was observed in 48% (13/27) of patients with SDS, but was not detected in 17 healthy donors (P < .001 by Fisher’s exact test; Figure 3A). No mutations of TP53 were detected in any of the patients with SCN (0/40, P < .001) or cyclic neutropenia (0/13, P = .003). TP53 mutations were detected at similar frequency in blood and bone marrow samples from patients with SDS (supplemental Figure 1B). After removing TP53 mutations, the percentage of cases with clonal hematopoiesis was similar between healthy controls and patients with SDS (Figure 3B). A significant relationship between the presence of TP53 mutations and age was observed with an odds ratio of 1.53 (95% confidence interval: 1.12-2.09, P < .01) (Figure 3C). Remarkably, 80% (8/10) of patients ≥10 years of age had at least 1 TP53 mutation, including 1 patient with 8 different TP53 mutations. The TP53 mutations were present in a low percentage of bone marrow cells (median 0.76%, range 0.1% to 7.7%). For 1 case (SDS34), multiple bone marrow samples were analyzed and showed that an R249Q TP53 mutation not detected at age 4 was present at a variant allele fraction of 1.1% at age 6.2 years, with an increase to 3.2% 1 month later (Figure 3D). The TP53 mutations clustered in the DNA binding domain of the gene, and all were present in the International Agency of Research on Cancer TP53 Database (Figure 3E). Logistic regression after adjusting for age showed that the presence of TP53 mutations was not related to the degree of neutropenia, G-CSF treatment, or presence of anemia or thrombocytopenia (Figure 3F-H and data not shown).
Clonal hematopoiesis with TP53 mutations. (A) Percentage of cases with clonal hematopoiesis due to TP53 mutations. (B) Percentage of cases with clonal hematopoiesis due to mutations in genes besides TP53. ****P < .001 compared with healthy donors. (C) Number of TP53 mutations per patient with SDS vs age. (D) Variant allele frequency (VAF) for R249Q TP53 in serial bone marrow samples obtained for patient SDS34. (E) The TP53 mutations, with the number of times the mutation was seen in parentheses. (F) Number of patients with severe neutropenia (ANC < 500 per mm3), mild/moderate neutropenia (ANC 500-1500 per mm3), or no neutropenia. (G) Number of patients with anemia. (H) Number of patients with thrombocytopenia. a.a., amino acid; n.t., nucleotide; pos, position.
Clonal hematopoiesis with TP53 mutations. (A) Percentage of cases with clonal hematopoiesis due to TP53 mutations. (B) Percentage of cases with clonal hematopoiesis due to mutations in genes besides TP53. ****P < .001 compared with healthy donors. (C) Number of TP53 mutations per patient with SDS vs age. (D) Variant allele frequency (VAF) for R249Q TP53 in serial bone marrow samples obtained for patient SDS34. (E) The TP53 mutations, with the number of times the mutation was seen in parentheses. (F) Number of patients with severe neutropenia (ANC < 500 per mm3), mild/moderate neutropenia (ANC 500-1500 per mm3), or no neutropenia. (G) Number of patients with anemia. (H) Number of patients with thrombocytopenia. a.a., amino acid; n.t., nucleotide; pos, position.
Discussion
In this report, we show that the mutation burden in HSPCs from patients with SCN is comparable to that of age-matched healthy individuals. Because of the limited number of SDS samples analyzed, firm conclusions about mutation burden in SDS HSPCs are not possible. Nonetheless, these data suggest that an elevated mutation rate in HSPCs is not solely responsible for the marked increased risk of MDS/AML in congenital neutropenia. Although the published data are limited, this conclusion is supported by reports showing that the overall mutation burden in MDS or AML arising in the setting of SCN or SDS is comparable to that of de novo MDS/AML.16,30
Here, we provide evidence for the selective expansion of HSPCs carrying specific gene mutations in congenital neutropenia. In SDS, nearly 50% of patients have clonal hematopoiesis because of mutations of TP53. Multiple TP53 mutations per patient were often detected. Of note, the variant allele frequencies of the TP53 mutations in a given patient often varied considerably. For example, in patient SDS31, in whom 8 different TP53 mutations were identified, the variant allele frequency ranged from 0.12% to 3.0%. Although single-cell genotyping is needed to confirm, this observation suggests that in patients with multiple TP53 mutations, the mutations likely arose in distinct HSPCs. The frequency of TP53 mutations increases with age in patients with SDS and was not seen in any patients with SCN or cyclic neutropenia, or healthy controls. These observations show that specific stressors are present in SDS that strongly and specifically select for HSPCs carrying TP53 mutations.
Mutations of SBDS that are present in the great majority of cases of SDS result in impaired ribosome biogenesis.8,9,31 There is evidence that ribosome biogenesis stress induces p53 expression, which in turn results in growth arrest. For example, mutations in genes encoding for ribosomal proteins RPS19 in Diamond-Blackfan syndrome or RPS14 in 5q− syndrome result in impaired ribosome biogenesis and induction of TP53 expression in erythroid progenitors.32,33 Importantly, genetic or pharmacologic inhibition of p53 rescues the defect in erythropoiesis in RPS19-deficient cells, establishing the importance of increased TP53 expression in these disorders.34-36 Increased p53 expression also has been identified in hematopoietic cells from patients with SDS or in Sbds-deficient murine hematopoietic cells.31,37 Moreover, in mice with targeted disruption of Sbds in pancreatic cells, genetic ablation of Trp53 rescues the severe atrophy in pancreatic acinar cells.38 Together, these observations suggest a model in which elevated p53 expression due to ribosome biogenesis stress in SDS HSPCs results in impaired HSPC growth and/or survival. Mutations of TP53 in HSPCs are predicted to attenuate this growth arrest, resulting in their selective expansion in patients with SDS. Our data suggest that the acquisition of TP53 mutations is an early, initiating event, for the transformation to MDS/AML in SDS patients. Consistent with this conclusion, a recent study showed that 7 of 7 (100%) cases of MDS arising in the setting of SDS carried TP53 mutations.16 However, because nearly all of the older patients with SDS had at least 1 TP53 mutation, other mutations, including mutations of the residual TP53 allele, are likely to be required for leukemic transformation. The predictive value of clonal hematopoiesis due to TP53 mutations for the development of MDS/AML in patients with SDS is currently unclear. However, 8 of 10 (80%) of our SDS patients >10 years had at least 1 TP53 mutation. Thus, it is unlikely that the simple presence of TP53 mutations in blood/bone marrow will be a useful biomarker for the development of MDS/AML in these patients. Whether the number of TP53 mutations, maximum variant allele fraction, or increase in TP53 allele burden over time is predictive will require prospective longitudinal studies.
We confirm prior studies showing a high incidence of CSF3R truncation mutations in patients with SCN.13,14,29,39 The truncated G-CSF receptor, although remaining dependent on G-CSF, transmits a sustained, increased signal in response to G-CSF.40-42 Expression of the truncated G-CSF receptor confers a competitive advantage to HSCs in mice that is dependent on chronic G-CSF treatment.43 Together, these observations suggest that the very high level of G-CSF present in patients (through either endogenous production or pharmacologic administration) is driving the expansion of HSPCs carrying CSF3R mutations. Of note, no increase in clonal hematopoiesis due to other gene mutations was observed, demonstrating the highly selective nature of CSF3R-dependent clonal expansion in SCN. There is evidence that CSF3R mutations contribute to the development of MDS/AML. A study showed that 13 of 18 (72%) patients with SCN who developed MDS/AML carried CSF3R mutations, compared with 43 of 125 (34%) without MDS/AML.14 Moreover, truncation mutations of Csf3r cooperate with the PML-RAR oncogene to induce AML in mice.44 On the other hand, there are reports of MDS/AML arising in patients with SCN prior to the availability of G-CSF.45-47 The predictive value of CSF3R mutations in patients with SCN for MDS/AML is uncertain. Our study does not resolve this issue, because only 2 patients in our SCN cohort are known to have developed MDS/AML. Of note, prior studies show that CSF3R mutations can persist for many years (and occasionally disappear) without developing MDS/AML in some patients with SCN.14,30 A recent report showed that mutations of RUNX1 were present in the majority (64.5%) of patients with SCN who developed MDS or AML.15 Interestingly, we identified no RUNX1 mutations in our SCN cohort, which includes 2 patients who later developed AML. This is consistent with the conclusion by Skokowa et al that RUNX1 mutations are a late step in leukemic transformation in patients with SCN.15
Our study has several limitations. Most importantly, the number of samples in which HSPC mutation burden was determined, especially for SDS, was small. Only a minority of SDS samples generated hematopoietic colonies of sufficient size for analysis, and it is not clear whether these cases are representative of SDS in general. Our studies of clonal hematopoiesis were limited to a panel of 46 genes; it is possible that mutations in other genes contribute to clonal hematopoiesis in congenital neutropenia.
In summary, our data suggest that both HSPC–cell-intrinsic and non–cell-intrinsic changes may determine the competitive fitness of individual HSPCs. In the case of SCN, the persistently high levels of G-CSF drive the expansion of HSPCs carrying mutations of CSF3R. In SDS, impaired ribosome biogenesis induces p53-mediated growth inhibition and drives expansion of HSPCs carrying TP53 mutations. It is likely that additional stressors may influence the development of clonal hematopoiesis. For example, a recent study reported that exposure to chemotherapy results in a higher incidence of clonal hematopoiesis carrying TP53 or PPM1D mutations.48 Identifying cell-intrinsic and non–cell-intrinsic stressors that shape the expansion of HSPCs may provide novel insights into the pathogenesis of AML or MDS.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was funded by Department of Defense grant BM130173 (D.C.L.) and the National Cancer Institute, National Institutes of Health grants PO1 CA101937 (D.C.L.) and P50 CA171963 (D.C.L.). Technical support was provided by McDonnell Genome Institute and High-Speed Cell Sorting Core at Washington University School of Medicine, which are supported by National Cancer Institute grant P30 CA91842.
Authorship
Contribution: J.X. and D.C.L. wrote the paper, designed and performed the research, and analyzed and interpreted the data; J.X., A.R., and M.R.M.J. processed samples; C.A.M. and R.S.F. provided bioinformatics support for the sequence data analysis; J.B. provided statistical support for data analyses; and T.P.V., M.A.C., K.J.W., L.A.B., V.M., A. A. Bolyard, D.C.D., M.C.D., D.B.W., A.V., K.C.M., R.J.R., A. A. Bertuch, and A.S. provided samples for this study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Daniel C. Link, Division of Oncology, Department of Internal Medicine, Washington University School of Medicine, 660 S Euclid Ave, Campus Box 8007, St. Louis, MO 63110; e-mail: danielclink@wustl.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal