Key Points
TP53 expression (>50% positive cells) has shorter TTF and poor OS independent of both MIPI score and Ki67 index.
Abstract
Currently, prediction of time to treatment failure (TTF) and overall survival (OS) in mantle cell lymphoma (MCL) is based on the clinical factors included in the Mantle Cell Lymphoma International Prognostic Index (MIPI), and proliferation is assessed by Ki67. However, TP53 and SOX11 immunohistochemistry might improve risk stratification. We performed SOX11 and TP53 immunohistochemistry on the so far largest published cohort of lymphoma specimens (n = 365). All patients were treated in prospective trials of the European MCL Network. In multivariate analyses, including MIPI and Ki67, SOX11 expression was not associated with TTF, but patients with low SOX11 expression had shorter OS. On the contrary, high TP53 expression was a strong predictor of TTF and inferior OS compared with low TP53 expression in univariate and multivariate analyses adjusting for MIPI score and Ki-67 index (hazard ratio [HR], 2.0; P = .0054 for TTF, and HR, 2.1; P = .068 for OS). In particular, patients with high TP53 expression (>50% positive lymphoma cells) had a shorter TTF and poor OS independent of both MIPI score and Ki-67 index. Thus, TP53 immunohistochemistry is a suitable test for routine diagnostic practice to assess MCL prognosis.
Introduction
Mantle cell lymphoma (MCL) is an aggressive form of non-Hodgkin lymphoma characterized by the translocation t(11;14)/CyclinD1-immunoglobulin heavy chain, leading to overexpression of cyclin D1. Clinical outcomes are heterogeneous, and risk stratification is currently based on the clinical parameters composing the Mantle Cell Lymphoma Prognostic Index (MIPI) and a combined biological MIPI, which additionally includes Ki67.1,2 However, risk stratification might be further improved by incorporating biological markers, including SOX11 and TP53.3 Recently, a mainly nonnodal subset of MCL with good clinical outcome has been described that frequently lacks expression of the protein SOX11.4 The prognostic role of SOX11 expression in nodal MCL is controversial.5-9 TP53 expression might serve as a surrogate marker for TP53 mutations3,10 and has been shown to be of prognostic value independent of the biological MIPI score in only 1 previous report.8 We analyzed the prognostic impact of these 2 biomarkers in patients treated in prospective trials of the European MCL Network.
Study design
Patients and tissue specimen
Analysis of SOX11 and TP53
SOX11 immunohistochemistry was scored as negative (0% positive lymphoma cells), low (1%-10% positive lymphoma cells), or positive (>10% positive lymphoma cells). TP53 was scored as negative (0% positive lymphoma cells), low (1%-10% positive lymphoma cells), intermediate (10%-50% positive lymphoma cells) or high (>50% positive lymphoma cells). For further details, see supplemental Methods.
Statistical methods
Outcome variables were overall survival (OS) from trial registration to death from any cause and time to treatment failure (TTF) from treatment start to stable disease, progression, or death from any cause. Statistical methods are outlined in the supplemental Methods.
Results and discussion
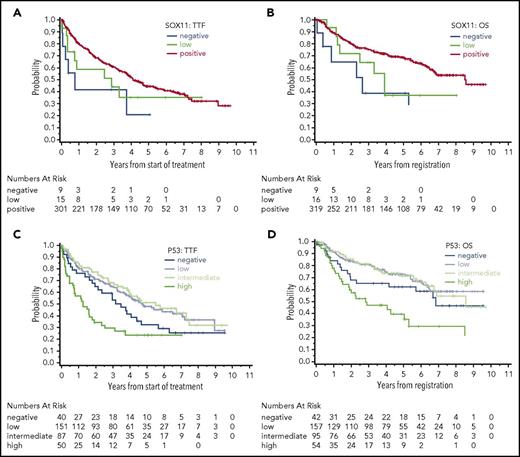
In total, 344 and 348 of 365 cases with available material were evaluable for SOX11 and TP53, respectively (supplemental Figure 1). To the best of our knowledge, this is the largest cohort so far tested for these 2 biomarkers. Nine cases (3%) were evaluated as SOX11 negative, 16 cases (5%) were evaluated as SOX11 low, and 319 cases (93%) were evaluated as SOX11 positive. Only 1 patient among those with SOX11 data had nonnodal MCL; this patient was SOX11 positive. For TP53, 42 cases (12%) scored negative, 157 cases (45%) scored low, 95 cases (27%) scored intermediate, and 54 cases (16%) scored high (supplemental Figure 3).
The group of patients that were SOX11 negative did not differ from SOX11-positive patients, whereas the group of patients that scored SOX11 low was slightly associated with features of unfavorable outcome, such as higher MIPI score, which probably reflects random variation (supplemental Table 2). SOX11 status did not show a strong association with TTF or OS (Figure 1A-B, supplemental Table 3). In contrast with the expectation, SOX11 negativity and low SOX11 positivity were associated with shorter OS compared with SOX11 positivity in univariate analyses (hazard ratio [HR], 3.5; P = .0032 and HR, 1.9; P = .073, respectively). However, this association was retained in a multivariate analysis that included the MIPI score and Ki-67 index only for low SOX11 positivity (HR, 2.3; P = .038, supplemental Table 4). Published data regarding the prognostic role of SOX11 expression in MCL are controversial. Retrospective case cohorts demonstrate an association of SOX11 negativity with molecular features, such as low genomic complexity and a mutated immunoglobulin heavy chain region, but also with clinical features like favorable prognosis and nonnodal or chronic lymphocytic leukemia–like presentation.7,9 However, a systematic retrospective analysis of large patient cohorts that have been previously published5,8 as well as our study fail to demonstrate an association of SOX11 negativity with patient outcome. Screening of MCL patients for SOX11 expression in our study and published studies was done by immunohistochemistry using tissue specimens. Although immunohistochemistry has proven to be a reliable surrogate assay for SOX11 expression status,13 nonnodal MCL might be underrepresented in our analyses as well as other published analyses. The reason for the assumed underrepresentation is that SOX11-negative MCL often presents clinically as a nonnodal variant. In these cases, the diagnosis might be established by combining flow cytometry and genetic analysis of peripheral blood only, and tissue biopsies might be less frequently obtained and thus lacking for analysis by immunohistochemistry. Moreover, because a tissue biopsy was requested for registration in the trials analyzed in the current study, nonnodal MCL might even be more underrepresented, with <1% in our analysis, potentially masking a prognostic effect of SOX11. Although SOX11 does not seem to be a suitable biomarker of outcome in nodal MCL, it is certainly a valuable marker to identify a biologically and clinically relevant group of MCL when combined with other features, such as clinical presentation as nonnodal MCL.
SOX11 and TP53 expression. Shown are the associations of SOX11 with (A) TTF and (B) OS and of TP53 expression with (C) TTF and (D) OS.
SOX11 and TP53 expression. Shown are the associations of SOX11 with (A) TTF and (B) OS and of TP53 expression with (C) TTF and (D) OS.
TP53 protein was correlated with features associated with adverse outcome. Patients in the TP53-high group had a higher median Ki67 index (28%; range, 6%-86%) compared with those in the TP53-negative (median, 19%), -low (median, 18%), and -intermediate groups (median, 20%, P = .012; supplemental Table 2). Furthermore, MIPI scores differed between TP53 expression groups, with the TP53-low group showing the lowest median MIPI score (median MIPI score, 5.92 for the TP53-high group, 5.85 for the TP53-intermediate group, 5.68 for the TP53-low group, and 5.82 for the TP53-negative group; P = .0096, supplemental Table 2). In contrast to SOX11, expression of TP53 was strongly associated with patient outcome. High TP53 expression was a strong predictor of inferior TTF (Figure 1C) and OS (Figure 1D) compared with low TP53 expression in univariate (TTF HR, 2.5; P < .0001; OS HR, 3.0; P < .0001; Table 1, supplemental Table 5) and multivariate analyses adjusting for MIPI score and Ki-67 index (TTF HR, 2.0; P = .0054; OS HR 2.1; P = .0068; Table 1, supplemental Table 5). Interestingly, a negative prognostic effect was not detectable in the subgroup of patients treated with intensive chemotherapy (R-CHOP [cyclophosphamide, hydroxydaunorubicin, vincristine, prednisone] / R-DHAP [rituximab, dexamethasone, cytarabine, cisplatin]) with autologous stem cell transplantation (data not shown). However, this finding needs further confirmation given that mutational analysis reported recently revealed potentially contradictory results.14
Multivariate analysis for OS using the parameters TP53 expression, Ki67 index, and MIPI score
| Variable and comparison . | P . | HR . | 95% confidence interval . | Events overall . |
|---|---|---|---|---|
| TP53 | ||||
| Negative vs low | .21 | 1.43 | 0.82-2.51 | 118/348 |
| Intermediate vs low | .88 | 1.04 | 0.64-1.67 | |
| High vs low | <.0001 | 3.01 | 1.87-4.83 | |
| TP53 | ||||
| Negative vs low | .19 | 1.52 | 0.81-2.87 | 96/276 |
| Intermediate vs low | .58 | 0.86 | 0.50-1.47 | |
| High vs low | .0043 | 2.20 | 1.28-3.78 | |
| Ki-67index | ||||
| +10% | .0002 | 1.20 | 1.09-1.32 | |
| TP53 | ||||
| Negative vs low | .17 | 1.56 | 0.82-2.94 | 96/276 |
| Intermediate vs low | .28 | 0.74 | 0.43-1.27 | |
| High vs low | .0068 | 2.09 | 1.23-3.55 | |
| Ki-67 index | ||||
| +10% | .012 | 1.13 | 1.03-1.25 | |
| MIPI score | ||||
| 1 | <.0001 | 2.00 | 1.46-2.74 |
| Variable and comparison . | P . | HR . | 95% confidence interval . | Events overall . |
|---|---|---|---|---|
| TP53 | ||||
| Negative vs low | .21 | 1.43 | 0.82-2.51 | 118/348 |
| Intermediate vs low | .88 | 1.04 | 0.64-1.67 | |
| High vs low | <.0001 | 3.01 | 1.87-4.83 | |
| TP53 | ||||
| Negative vs low | .19 | 1.52 | 0.81-2.87 | 96/276 |
| Intermediate vs low | .58 | 0.86 | 0.50-1.47 | |
| High vs low | .0043 | 2.20 | 1.28-3.78 | |
| Ki-67index | ||||
| +10% | .0002 | 1.20 | 1.09-1.32 | |
| TP53 | ||||
| Negative vs low | .17 | 1.56 | 0.82-2.94 | 96/276 |
| Intermediate vs low | .28 | 0.74 | 0.43-1.27 | |
| High vs low | .0068 | 2.09 | 1.23-3.55 | |
| Ki-67 index | ||||
| +10% | .012 | 1.13 | 1.03-1.25 | |
| MIPI score | ||||
| 1 | <.0001 | 2.00 | 1.46-2.74 |
Of note, TP53-negative patients showed a tendency toward inferior outcome, too (adjusted TTF HR, 1.4; P = .21; adjusted OS HR, 1.6; P = .17; Figure 1C-D). The fact that both extremes of TP53 expression (high and negative) present with high MIPI scores and an association with inferior outcome might be related to the fact that they both potentially reflect genomic TP53 alterations. As shown previously, high TP53 expression is a biomarker for TP53 gene mutation and/or heterozygous loss in MCL.10,15,16 Homozygous loss of the gene in MCL might be reflected by the complete absence of TP53 expression. However, among 10 patients with negative TP53 expression and data on copy number from a previous study of the same cohort,17 no patient showed a TP53 deletion. Certainly, MCL completely lacking TP53 expression needs to be studied in more detail in future analyses to understand whether absence of the protein is in fact related to (epi)genetic alterations.
Interestingly, among the SOX11-negative patients, 4 of 8 (50%) were in the TP53-high group (75% were in the intermediate group or the high group). In contrast, 0 of 16 (0%) patients with low SOX11 expression and only 15% of 310 SOX11-positive patients were in the TP53-high group. These results qualitatively confirm the results published by Nygren et al that SOX11-negative cases frequently show TP53 expression.5
This analysis extends our knowledge on the use of TP53 in MCL. We demonstrate a prognostic role of TP53 expression in MCL in patients treated within prospective randomized trials using the most recent therapeutic protocols and a large number of patients, further establishing TP53 expression as a prognostic biomarker independent of Ki67 index and MIPI score. The pathology panel of the European MCL Network completed a round-robin test for Sox11 and TP53 staining (Snjezana Dotlic, Elias Campo, S.H., G.R., Johan H. van Krieken, E.H., and W.K., manuscript in preparation). However, further standardization of the procedure of scoring will be necessary before TP53 assessment by immunohistochemistry will become part of a routine diagnostic test. Whether the analysis of a recently published gene expression–based proliferation signature18 will be superior to the immunohistochemical analysis of Ki67 and TP53 needs to be evaluated in future studies, ideally using samples from prospective trials. Independent of the result of such future studies, analysis of TP53 expression is, in contrast to gene expression profiling, a widely established assay in pathology laboratories. It thus seems feasible to introduce the assessment into the diagnostic workup for future risk-adapted treatment strategies of MCL.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
The authors thank Charlotte Botz von Drathen for excellent technical assistance.
Authorship
Contribution: S.M.A. and W.K. generated the data; E.H. performed the statistical analysis; A.R., D.C., G.R., C.T., and S.H. collected material; H.K.-N., O.H., and M.D. provided clinical data; M.-H.D.-L. performed the genetic analysis; S.M.A., E.H., M.D., and W.K. designed the research and wrote the manuscript; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Wolfram Klapper, Department of Pathology, University of Kiel, 24105 Kiel, Germany; e-mail: wklapper@path.uni-kiel.de.
References
Author notes
S.M.A., E.H., M.D., and W.K. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal