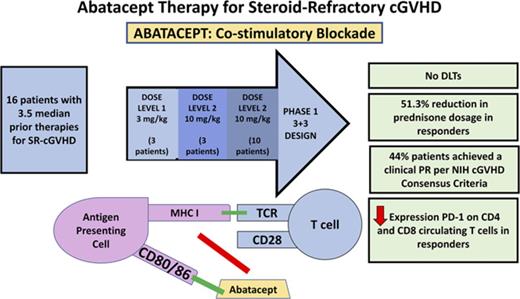
Key Points
Costimulatory blockade using abatacept represents a novel therapeutic approach for the treatment of cGVHD.
Abatacept resulted in a clinical response in 44% of patients with both decreased prednisone use and T-cell PD-1 expression in responders.
Abstract
Steroid-refractory chronic graft-versus-host disease (SR-cGVHD) remains a major cause of morbidity and mortality after allogeneic stem cell transplantation. Innovative immunotherapeutic strategies are urgently needed for the treatment of SR-cGVHD. We conducted a phase 1 clinical trial to evaluate the safety, efficacy, and immune effects of abatacept, a novel immunomodulatory drug that acts as an inhibitor of T-cell activation via costimulatory blockade, in the treatment of SR-cGVHD. The study followed a 3+3 design with 2 escalating abatacept doses: 3 mg/kg and 10 mg/kg, with an expansion cohort treated at 10 mg/kg. Abatacept was well-tolerated with no dose-limiting toxicities. Of the 16 evaluable patients, 44% achieved a clinical partial response per 2005 National Institutes of Health Consensus Criteria. Importantly, abatacept resulted in a 51.3% reduction in prednisone usage in clinical responders (mean baseline, 27 vs 14 mg; P = .01). Increased PD-1 expression on circulating CD4 (P = .009) and CD8 (P = .007) T cells was observed in clinical responders. In summary, abatacept was safe and led to a marked improvement in National Institutes of Health cGVHD scores and a significant reduction in prednisone use. In this cohort of heavily pretreated patients, the results suggest abatacept may be a promising therapeutic agent for SR-cGVHD, and a phase 2 trial has been initiated. This trial was registered at www.clinicaltrials.gov as #NCT01954979.
Introduction
Allogeneic stem cell transplantation (alloSCT) is a curative therapy for select patients with hematologic malignancies.1 Despite marked improvements in graft-versus-host disease (GVHD) prophylaxis,2 chronic GVHD (cGVHD) remains a major source of morbidity after alloSCT,3 with a cumulative incidence of nearly 50%.4
Corticosteroids remain the first-line therapy for cGVHD.5,6 However, only a subset of patients respond to steroids alone, and steroid-refractory (SR) cGVHD remains the major source of long-term complications in survivors of alloSCT. In addition, steroid-mediated complications including avascular necrosis, glaucoma, and hypertension contribute to major morbidity and impaired quality of life.7 Treatment options for SR-cGVHD remain limited, and response rates to therapy are low.8-12 Although the pathophysiology of cGVHD is complex, activated T cells driven by alloantigen stimulation play a critical role in mediating cGVHD.13,14 As such, inhibition of T-cell activation via blockade of costimulation has potential as a therapeutic target in SR-cGVHD.
Abatacept, a recombinant soluble fusion protein, is the first drug in a class of selective costimulation modulators. Immunomodulation with abatacept is used in the treatment of rheumatologic diseases including rheumatoid arthritis, which, similar to cGVHD, is driven in part by aberrant T-cell activation.15,16 Abatacept is biochemically composed of the extracellular domain of human cytotoxic T lymphocyte (T cell)-associated antigen 4 (CTLA-4) linked to the modified Fc (hinge, CH2, and CH3 domains) portion of human immunoglobulin G1 (IgG1) to prevent complement fixation/antibody-dependent cell-mediated cytotoxicity. The CTLA-4 moiety binds to costimulatory receptors, CD80 and CD86, on antigen-presenting cells with a higher affinity than does their native costimulatory ligand, CD28, thereby attenuating T-cell activation. By abrogating T-cell activation, abatacept has the potential to mitigate cGVHD. In murine models, administration of a CTLA-4 antibody prevented both acute GVHD (aGVHD) and cGVHD and reversed manifestations of cGVHD.17 In this study, we conducted a phase 1 clinical trial to assess the safety, efficacy, and immunologic effects of abatacept in the treatment of SR-cGVHD.
Methods
Study design
This phase 1 trial was designed to investigate the safety, efficacy, and immunologic effects of abatacept (Bristol-Myers Squibb, New York City) in the treatment of patients with SR-cGVHD. The protocol was reviewed and approved by the Dana-Farber Cancer Institute Institutional Review Board. Informed consent was obtained in accordance with the Helsinki Protocol. The study followed a 3+3 design with 2 escalating doses of abatacept to determine the maximum tolerated dose: 3 mg/kg and 10 mg/kg. Dose-limiting toxicities (DLTs) were defined as grade 3 or 4 toxicities judged to be probably or definitely related to abatacept. Infection was not considered a DLT, as infections are a reflection of the immunosuppression associated with cGVHD and its treatment; however, all infections were captured and reported. Infections are a leading cause of morbidity after alloSCT, and patients with cGVHD are especially at risk for disease caused by encapsulated microbes.18,19 The DLT observation period was from the first abatacept dose to 1 month after 6 abatacept infusions. Dose escalation to level 2 (10 mg/kg) was allowed if no DLTs occurred in the first 3 evaluable patients receiving dose level 1 (3 mg/kg) and if all 3 patients completed at least 8 weeks of treatment. Abatacept was administered for a total of 6 doses. Doses 1 to 3 were administered at 2-week intervals. One month after dose 3, abatacept was given at 4-week intervals for 3 doses (doses 4-6). One month after the sixth dose, participants experiencing clinical benefit (complete response or partial response [PR]; minor response not meeting National Institutes of Health (NIH) criteria for PR) with acceptable toxicity were permitted to continue receiving extended-duration abatacept treatment (10 mg/kg) at the discretion of the treating physician. Patients received monthly doses of abatacept for up to a total of 12 doses of extended-duration therapy. The primary objective of the trial was to determine the maximum tolerated dose of abatacept, as well as to assess abatacept’s toxicity profile. Secondary objectives of this trial were to investigate the efficacy of abatacept and patient prednisone usage and to elucidate the immunologic effects associated with the administration of abatacept in patients with SR-cGVHD. Clinical response to abatacept therapy was documented by 2005 NIH Consensus Criteria for cGVHD scoring for several organ sites20 and by changes in steroid dose usage in patients receiving the study drug, which were recorded at each treatment visit.
Patient eligibility
Patients with progressive SR-cGVHD who had persistent signs and symptoms despite their current immunosuppressive regimen (including systemic steroids) were recruited to participate in this study. Study patients were classified as having mild, moderate, or severe cGVHD (defined by the 2005 National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft versus Host Disease) requiring systemic treatment. SR-cGVHD was defined as persistent signs and symptoms of cGVHD despite the use of prednisone at least 0.5 mg/kg/day (or equivalent) for at least 4 weeks. Of note, while enrolled in the study, patients were allowed to remain on steroids and on the immune suppression they had been on before starting abatacept. The protocol excluded the addition or subtraction of immune suppression within 4 weeks of starting abatacept therapy, and steroid doses could not be modified within 2 weeks of starting therapy. Patients were excluded if they had ongoing or active infection, active cardiac disease, or active malignancy. Patients could not have received other experimental drugs or therapies within 28 days of starting treatment with abatacept, nor could they have received biologic antibody therapy for cGVHD with rituximab, alemtuzumab, or anti-thymocyte globulin within 3 months of starting treatment. Patients could not have had an ongoing prednisone requirement of more than 1 mg/kg/day (or equivalent), as ongoing use of greater than 1 mg/kg prednisone in patients with SR-cGVHD is associated with significant morbidity without clear benefit.
Clinical cGVHD assessment
Each patient’s cGVHD score was calculated according to the 2005 NIH scoring system at baseline (study entry) and, subsequently, at each treatment visit.20 Organ sites considered for scoring included skin, mouth, eyes, gastrointestinal tract, liver, lungs, joints and fascia, and the female genital tract. Each organ or site was scored according to a 4-point scale (0-3), with 0 representing no involvement and 3 reflecting severe impairment. Performance status was calculated on a 0 to 3 scale.
Correlative studies
Patient peripheral blood samples were collected at baseline, before each abatacept administration, and 1 month after 6 doses of the drug. The effect of abatacept on PD-1 expression on CD4 and CD8 T cells was assessed. The cells were incubated with anti-CD-4 (clone RPA-T4) pacific blue (PB), anti-CD8 (clone HIT8A) fluorescein isothiocyanate (FITC; BD Biosciences, Franklin Lakes, NJ), and anti-PD-1 (clone eBioJ105) R-phycoerythrin (PE) (eBioscience, San Diego, CA) monoclonal antibody. PE mouse IgG1k (clone G18-145) was used as the isotype control. Using flow cytometry, the T cells were selected by size, using the lymphocyte gate. Within this gate, the CD4/PD-1-positive and CD8/PD-1-positive cells were quantified and analyzed using Kaluza software (Beckman Coulter, Brea, CA). To determine the percentage of activated vs regulatory T cells, the cell preparations were incubated with FITC-conjugated anti-CD4 (clone RPA-T4), FITC anti-CD25 (clone M-A251), and PE-conjugated anti-CD69 (clone FN50; BD Biosciences). Next, cells were permeabilized and cultured with PE-conjugated antibody directed against FoxP3 (clone 150D/E4; eBioscience). PE rat IgG2a,k (clone R35-95) was used as the isotype control. Cells were subsequently analyzed by multichannel flow cytometry. Finally, to determine Th1/Th2 polarization, peripheral blood mononuclear cells were pulsed with GolgiStop (1 µg/mL; Pharmingen, San Diego, CA) and stimulated with phorbol myristate acetate/ionomycin for 4 to 6 hours at 37°C before analysis. Cells were next harvested and labeled with CD4-PB and CD8-FITC, as described earlier. Cells were then permeabilized by incubation in Cyto-fix/Cytoperm plus (Pharmingen) containing formaldehyde and saponin for 30 minutes at 4°C, washed twice in Perm/Wash solution (Pharmingen), and incubated with PE-conjugated interferon (IFN)-γ (clone B27; Invitrogen, Camarillo, CA) for 30 minutes. Alternatively, the cells were incubated with PE mouse anti-human IgG isotype control (clone G18-145). Cells were washed in 1× Perm/Wash solution before flow cytometric analysis. To determine whether abatacept affects B-cell activating factor (BAFF) levels, soluble BAFF in patient plasma samples was measured using a commercially available enzyme-linked immunosorbent assay and the manufacturer's recommended procedures (R&D Systems, Minneapolis, MN).
Statistical analysis
A paired Student t test was used for paired group comparison, and Student t test was used for unpaired comparison. All tests are 2-sided and P =.05 was considered significant. All statistical analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC).
Results
Baseline characteristics and cGVHD scoring
Seventeen subjects were enrolled between April 2014 and October 2015. Three patients were treated at a dose of 3 mg/kg without DLT. An initial 3 evaluable patients completed treatment on cohort 2 at a dose of 10 mg/kg without DLT. A fourth participant withdrew consent after 1 dose of treatment before the DLT assessment, and therefore was not evaluable. Ten additional patients were treated at a dose of 10 mg/kg. Baseline patient characteristics are summarized in Table 1. The median age of enrolled patients was 54 years (range, 24-72 years). Seven patients (44%) had received myeloablative conditioning, and 9 (56%) received nonmyeloablative conditioning transplant regimens. The majority of patients (n = 13, 81%) underwent a matched unrelated donor transplant, and 9 (56%) of 16 patients were transplanted for acute myeloid leukemia. This cohort was heavily pretreated for SR-cGVHD, with a median of 3.5 prior regimens (range, 1-8) before study enrollment (supplemental Table 1, available on the Blood Web site). Patients continued receiving the immune suppression they had been receiving before starting abatacept (supplemental Table 2).
Patient characteristics
| Patient . | Age, y . | Sex . | Disease . | Transplant . | Number of prior regimens for cGVHD . | Overall cGVHD severity . | Time from transplant to initiation of abatacept, y . | Time from GVHD onset to initiation of abatacept, y . | Dose level . | Doses received . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1* | 53 | F | MDS | NMA; MMUD | 6 | Severe | 4.01 | 3.94; P | 1 | 5 |
| 2 | 72 | M | AML | NMA; MUD | 3 | Moderate | 2.50 | 2.14; DN | 1 | 6 |
| 3* | 24 | F | ALL | MA; MUD | 8 | Severe | 3.19 | 2.68; DN | 1 | 18 |
| 4 | 53 | F | MM | NMA; MUD | 8 | Severe | 3.75 | 2.39; DN | 2 | 18 |
| 6 | 53 | F | AML | MA ; MUD | 5 | Moderate | 4.02 | 3.96; Q | 2 | 7 |
| 7 | 62 | M | AML | MA; MUD | 4 | Severe | 0.90 | 0.44; DN | 2 | 4 |
| 8* | 66 | F | AML | NMA; MUD | 6 | Moderate | 1.87 | 1.43; DN | 2 | 6 |
| 9* | 65 | M | CLL | NMA; MUD | 2 | Severe | 3.61 | 3.19; DN | 2 | 18 |
| 10* | 55 | F | AML | MA; MUD | 1 | Moderate | 0.92 | 0.16; DN | 2 | 11 |
| 11* | 61 | F | CML | NMA; MUD | 3 | Severe | 2.24 | 1.59; DN | 2 | 6 |
| 12* | 71 | M | NHL | NMA; MUD | 2 | Severe | 2.88 | 1.86; DN | 2 | 18 |
| 13 | 59 | M | CLL | NMA; MUD | 2 | Moderate | 4.17 | 3.48; DN | 2 | 6 |
| 14 | 61 | M | AML | MA; MRD | 3 | Severe | 2.41 | 1.50; DN | 2 | 18 |
| 15 | 39 | M | AML | MA; MUD | 5 | Severe | 4.20 | 1.28; DN | 2 | 6 |
| 16 | 31 | M | AML | MA; MUD | 7 | Severe | 9.30 | 9.77; P | 2 | 6 |
| 17 | 39 | F | AML | NMA; MRD | 3 | Moderate | 1.67 | 1.02; DN | 2 | 11 |
| Patient . | Age, y . | Sex . | Disease . | Transplant . | Number of prior regimens for cGVHD . | Overall cGVHD severity . | Time from transplant to initiation of abatacept, y . | Time from GVHD onset to initiation of abatacept, y . | Dose level . | Doses received . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1* | 53 | F | MDS | NMA; MMUD | 6 | Severe | 4.01 | 3.94; P | 1 | 5 |
| 2 | 72 | M | AML | NMA; MUD | 3 | Moderate | 2.50 | 2.14; DN | 1 | 6 |
| 3* | 24 | F | ALL | MA; MUD | 8 | Severe | 3.19 | 2.68; DN | 1 | 18 |
| 4 | 53 | F | MM | NMA; MUD | 8 | Severe | 3.75 | 2.39; DN | 2 | 18 |
| 6 | 53 | F | AML | MA ; MUD | 5 | Moderate | 4.02 | 3.96; Q | 2 | 7 |
| 7 | 62 | M | AML | MA; MUD | 4 | Severe | 0.90 | 0.44; DN | 2 | 4 |
| 8* | 66 | F | AML | NMA; MUD | 6 | Moderate | 1.87 | 1.43; DN | 2 | 6 |
| 9* | 65 | M | CLL | NMA; MUD | 2 | Severe | 3.61 | 3.19; DN | 2 | 18 |
| 10* | 55 | F | AML | MA; MUD | 1 | Moderate | 0.92 | 0.16; DN | 2 | 11 |
| 11* | 61 | F | CML | NMA; MUD | 3 | Severe | 2.24 | 1.59; DN | 2 | 6 |
| 12* | 71 | M | NHL | NMA; MUD | 2 | Severe | 2.88 | 1.86; DN | 2 | 18 |
| 13 | 59 | M | CLL | NMA; MUD | 2 | Moderate | 4.17 | 3.48; DN | 2 | 6 |
| 14 | 61 | M | AML | MA; MRD | 3 | Severe | 2.41 | 1.50; DN | 2 | 18 |
| 15 | 39 | M | AML | MA; MUD | 5 | Severe | 4.20 | 1.28; DN | 2 | 6 |
| 16 | 31 | M | AML | MA; MUD | 7 | Severe | 9.30 | 9.77; P | 2 | 6 |
| 17 | 39 | F | AML | NMA; MRD | 3 | Moderate | 1.67 | 1.02; DN | 2 | 11 |
AML, acute myeloid leukemia; CML, chronic myelogenous leukemia; DN, de novo chronic GVHD; F, female; M, male; MA, myeloablative; MDS indicates myelodysplastic syndrome; MMUD, mismatched unrelated donor; MRD, matched related donor; MUD, matched unrelated donor; NHL, non-Hodgkin lymphoma; NMA, nonmyeloablative; P, progressive from onset of acute GVHD; Q, quiescent from onset of acute GVHD.
Clinical responder.
Safety
An initial cohort of 3 patients was treated with 3 mg/kg abatacept per dose (dose level 1). No DLTs were seen in the first 3 evaluable patients, and all 3 patients completed at least 8 weeks of treatment. A second cohort of 3 patients was treated with 10 mg/kg (dose level 2). There were no DLTs at dose level 2. An additional 10 patients were treated at dose level 2 with no DLTs. Adverse events possibly related to abatacept therapy are summarized in Table 2. The most common serious adverse events were pulmonary infections (grade 4, n = 1; grade 3, n = 2), which occurred at both dose levels; these infections resolved. We did not observe reactivation of viral infections, including cytomegalovirus and Epstein-Barr virus. Abatacept-related adverse events included grade 2 gastritis (n = 1), grade 2 pain (n = 1), and grade 1 diarrhea (n = 2), fatigue (n = 2), rash (n = 1), and skin pain (n = 1).
Adverse events
| Adverse event . | Grade . | Number of adverse events . |
|---|---|---|
| Rash | 1 | 1 |
| Fatigue | 1 | 2 |
| Skin pain | 1 | 1 |
| Diarrhea | 1 | 2 |
| Gastritis | 2 | 1 |
| Pain | 2 | 1 |
| Pulmonary infection | 3 | 2 |
| Pulmonary infection | 4 | 1 |
| Adverse event . | Grade . | Number of adverse events . |
|---|---|---|
| Rash | 1 | 1 |
| Fatigue | 1 | 2 |
| Skin pain | 1 | 1 |
| Diarrhea | 1 | 2 |
| Gastritis | 2 | 1 |
| Pain | 2 | 1 |
| Pulmonary infection | 3 | 2 |
| Pulmonary infection | 4 | 1 |
Clinical response
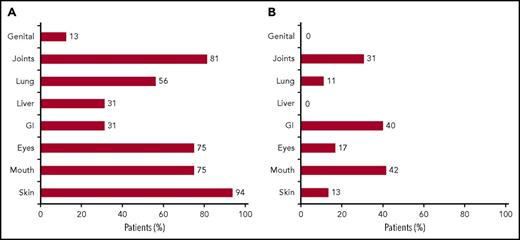
Of the 16 evaluable patients, 7 patients (44%) achieved a clinical PR 1 month after 6 doses of abatacept, as defined by improvement in 2 disease systems based on the 2005 NIH Consensus Criteria published by Filipovich et al.20 In total, 14 patients received the 6 planned abatacept doses, with a median number of 6.5 doses in this cohort. Site-specific scores and overall cGVHD scores in clinical responders at baseline and 1 month after the sixth dose of abatacept are summarized in Table 3. At baseline, the most common sites of involvement in the 16 evaluable patients, including responders and nonresponders, were skin (94%), joints (81%), mouth (75%), and eyes (75%; Figure 1A). The sites with greatest improvement among these 16 evaluable patients included mouth (42%) and gastrointestinal tract (40%), followed by joints, eyes, skin, and lung (Figure 1B). Thirteen of 15 patients with baseline skin cGVHD had deep sclerosis. Notably, patient 3 achieved a complete resolution of grade 2 pulmonary cGVHD. Before enrollment, patient 3 had normal PFTs until January 2014, at which time, the patient was diagnosed with grade 1 lung cGVHD with a forced expiratory volume in 1 second of 70% and mild symptoms (shortness of breath after climbing 1 flight of steps). Nine months later, at the time of enrollment to this study, the patient had progressive grade 2 lung cGVHD, with a baseline forced expiratory volume in 1 second of 61% and moderate symptoms (shortness of breath after walking on flat ground). One month after 6 abatacept infusions, forced expiratory volume in 1 second increased to 83% and the patient subjectively reported no lung symptoms consistent with a GVHD score of 0. Neither a bronchoscopy nor a computed tomography chest scan was performed at the time of these assessments during the enrolled study period. Importantly, abatacept resulted in decreased prednisone usage. In clinical responders, there was a 51.3% reduction in prednisone usage with a mean baseline dose of 27 vs 14 mg 1 month after the 6 abatacept infusions (P = .01; Figure 2).
Site-specific disease scores
| Patient . | GVHD grade . | Skin . | Mouth . | Eyes . | Gastrointestinal . | Liver . | Lung . | Joints . | Genital . | Overall . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1* | Baseline | 2 | 2† | 3† | 0 | 1 | 0 | 2† | 0 | Severe† |
| Final | 2 | 1† | 1† | 0 | 1 | 0 | 1† | 0 | Moderate† | |
| 2 | Baseline | 2 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | Moderate |
| Final | 2 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | Moderate | |
| 3* | Baseline | 3 | 0 | 0 | 1† | 0 | 2† | 2† | 0 | Severe† |
| Final | 3 | 0 | 0 | 0† | 0 | 0† | 1† | 0 | Severe† | |
| 4 | Baseline | 3 | 1 | 3 | 0 | 0 | 1 | 3 | 0 | Severe |
| Final | 3 | 1 | 2 | 1 | 0 | 1 | 3 | 0 | Severe | |
| 6 | Baseline | 2 | 2 | 1 | 1 | 0 | 1 | 2 | 0 | Moderate |
| Final | 2 | 1 | 0 | 1 | 0 | 1 | 3 | 0 | Severe | |
| 7 | Baseline | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Severe |
| Final | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Severe | |
| 8* | Baseline | 2 | 1† | 2 | 0 | 0 | 0 | 2† | 1 | Moderate |
| Final | 2 | 0† | 2 | 0 | 0 | 0 | 1† | 1 | Moderate | |
| 9* | Baseline | 3† | 0 | 1 | 0 | 0 | 1 | 2† | 0 | Severe |
| Final | 2† | 1 | 1 | 0 | 0 | 1 | 1† | 0 | Moderate | |
| 10* | Baseline | 2† | 2† | 0 | 1† | 0 | 0 | 0 | 0 | Moderate |
| Final | 0† | 1† | 0 | 0† | 0 | 0 | 0 | 0 | Mild | |
| 11* | Baseline | 3 | 2† | 3 | 1 | 1 | 0 | 3 | 0 | Severe |
| Final | 3 | 1† | 3 | 1 | 1 | 1 | 3 | 0 | Severe | |
| 12* | Baseline | 3 | 2† | 3† | 0 | 0 | 1 | 2 | 0 | Severe |
| Final | 3 | 1† | 2† | 0 | 0 | 1 | 2 | 0 | Severe | |
| 13 | Baseline | 0 | 1 | 1 | 2 | 0 | 1 | 0 | 0 | Moderate |
| Final | 0 | 1 | 2 | 1 | 0 | 1 | 0 | 0 | Moderate | |
| 14 | Baseline | 3 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | Severe |
| Final | 3 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | Severe | |
| 15 | Baseline | 3 | 0 | 0 | 0 | 1 | 3 | 3 | 0 | Severe |
| Final | 3 | 0 | 0 | 0 | 1 | 3 | 3 | 0 | Severe | |
| 16 | Baseline | 3 | 1 | 2 | 0 | 1 | 2 | 3 | 2 | Severe |
| Final | 3 | 1 | 2 | 0 | 1 | 2 | 3 | 0 | Severe | |
| 17 | Baseline | 2 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | Moderate |
| Final | 2 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | Moderate |
| Patient . | GVHD grade . | Skin . | Mouth . | Eyes . | Gastrointestinal . | Liver . | Lung . | Joints . | Genital . | Overall . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1* | Baseline | 2 | 2† | 3† | 0 | 1 | 0 | 2† | 0 | Severe† |
| Final | 2 | 1† | 1† | 0 | 1 | 0 | 1† | 0 | Moderate† | |
| 2 | Baseline | 2 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | Moderate |
| Final | 2 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | Moderate | |
| 3* | Baseline | 3 | 0 | 0 | 1† | 0 | 2† | 2† | 0 | Severe† |
| Final | 3 | 0 | 0 | 0† | 0 | 0† | 1† | 0 | Severe† | |
| 4 | Baseline | 3 | 1 | 3 | 0 | 0 | 1 | 3 | 0 | Severe |
| Final | 3 | 1 | 2 | 1 | 0 | 1 | 3 | 0 | Severe | |
| 6 | Baseline | 2 | 2 | 1 | 1 | 0 | 1 | 2 | 0 | Moderate |
| Final | 2 | 1 | 0 | 1 | 0 | 1 | 3 | 0 | Severe | |
| 7 | Baseline | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Severe |
| Final | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Severe | |
| 8* | Baseline | 2 | 1† | 2 | 0 | 0 | 0 | 2† | 1 | Moderate |
| Final | 2 | 0† | 2 | 0 | 0 | 0 | 1† | 1 | Moderate | |
| 9* | Baseline | 3† | 0 | 1 | 0 | 0 | 1 | 2† | 0 | Severe |
| Final | 2† | 1 | 1 | 0 | 0 | 1 | 1† | 0 | Moderate | |
| 10* | Baseline | 2† | 2† | 0 | 1† | 0 | 0 | 0 | 0 | Moderate |
| Final | 0† | 1† | 0 | 0† | 0 | 0 | 0 | 0 | Mild | |
| 11* | Baseline | 3 | 2† | 3 | 1 | 1 | 0 | 3 | 0 | Severe |
| Final | 3 | 1† | 3 | 1 | 1 | 1 | 3 | 0 | Severe | |
| 12* | Baseline | 3 | 2† | 3† | 0 | 0 | 1 | 2 | 0 | Severe |
| Final | 3 | 1† | 2† | 0 | 0 | 1 | 2 | 0 | Severe | |
| 13 | Baseline | 0 | 1 | 1 | 2 | 0 | 1 | 0 | 0 | Moderate |
| Final | 0 | 1 | 2 | 1 | 0 | 1 | 0 | 0 | Moderate | |
| 14 | Baseline | 3 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | Severe |
| Final | 3 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | Severe | |
| 15 | Baseline | 3 | 0 | 0 | 0 | 1 | 3 | 3 | 0 | Severe |
| Final | 3 | 0 | 0 | 0 | 1 | 3 | 3 | 0 | Severe | |
| 16 | Baseline | 3 | 1 | 2 | 0 | 1 | 2 | 3 | 2 | Severe |
| Final | 3 | 1 | 2 | 0 | 1 | 2 | 3 | 0 | Severe | |
| 17 | Baseline | 2 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | Moderate |
| Final | 2 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | Moderate |
Clinical responder.
Sites of response in clinical responders only.
Sites of GVHD involvement. (A) The bar graph displays the percentage of all evaluable patients with specified organ involvement at baseline: 94% of patients had skin cGVHD involvement at baseline. (B) The bar graph displays the percentage of all evaluable patients with site-specific clinical improvement after 6 abatacept infusion: 42% of patients experienced improvement in mouth cGVHD after exposure to abatacept.
Sites of GVHD involvement. (A) The bar graph displays the percentage of all evaluable patients with specified organ involvement at baseline: 94% of patients had skin cGVHD involvement at baseline. (B) The bar graph displays the percentage of all evaluable patients with site-specific clinical improvement after 6 abatacept infusion: 42% of patients experienced improvement in mouth cGVHD after exposure to abatacept.
Prednisone use in clinical responders. Abatacept resulted in a 51.3% reduction in prednisone usage in clinical responders, with a mean baseline dose of 27 mg compared with a mean dose of 14 mg, 1 month after the sixth dose of abatacept (P = .01).
Prednisone use in clinical responders. Abatacept resulted in a 51.3% reduction in prednisone usage in clinical responders, with a mean baseline dose of 27 mg compared with a mean dose of 14 mg, 1 month after the sixth dose of abatacept (P = .01).
Immunomodulatory effects
The effect of therapy on PD-1 expression on circulating CD4 and CD8 T cell populations was assessed. At 1 month, after 6 doses of abatacept, PD-1 expression on CD4 (P = .009) and CD8 (P = .007) T cells was significantly increased in clinical responders. At baseline, there was no difference in CD4 PD-1 expression (P = .34), nor in CD8 PD-1 expression (P = .27), in responders vs nonresponders (Figure 3). The effect of therapy on T-cell polarization with respect to secretion of IFN-γ and IL-10 was assessed. In both clinical responders and nonresponders, there was not a statistically significant change in Th1 (IFN-γ) or Th2 (IL-10) cytokine secretion after therapy (Figure 4). The effect of abatacept on CD25+FOXP3+ T regulatory cells was assessed and remained unchanged in both responders and nonresponders.
CD4 and CD8 PD-1 T-cell expression in responders (R) and nonresponders (NR). Peripheral blood samples were collected before each dose of abatacept and 1 month after the administration of 6 doses of abatacept. Peripheral blood mononuclear cells were stained for CD4 (A) or CD8 (B) and PD-1 cell surface expression. Results were analyzed using flow cytometry. At baseline, there was no difference in CD4 PD-1 expression in responders vs nonresponders (P = .34). One month after 6 doses of abatacept, a significant increase in CD4 PD-1 expression was observed in clinical responders (P = .009) (A), with a representative example shown (C). Similarly, there was no difference at baseline in CD8 PD-1 expression when comparing responders with nonresponders (P = .27). One month after 6 doses of abatacept, a significant increase in CD8 PD-1 expression was noted (P = .007) in clinical responders (B), with a representative example shown (C). A representative example of CD4 and CD8 PD-1 expression in a nonresponder is shown in (D).
CD4 and CD8 PD-1 T-cell expression in responders (R) and nonresponders (NR). Peripheral blood samples were collected before each dose of abatacept and 1 month after the administration of 6 doses of abatacept. Peripheral blood mononuclear cells were stained for CD4 (A) or CD8 (B) and PD-1 cell surface expression. Results were analyzed using flow cytometry. At baseline, there was no difference in CD4 PD-1 expression in responders vs nonresponders (P = .34). One month after 6 doses of abatacept, a significant increase in CD4 PD-1 expression was observed in clinical responders (P = .009) (A), with a representative example shown (C). Similarly, there was no difference at baseline in CD8 PD-1 expression when comparing responders with nonresponders (P = .27). One month after 6 doses of abatacept, a significant increase in CD8 PD-1 expression was noted (P = .007) in clinical responders (B), with a representative example shown (C). A representative example of CD4 and CD8 PD-1 expression in a nonresponder is shown in (D).
CD4 IFN-γ and IL-10 expression in responders (R) and nonresponders (NR). Peripheral blood samples were collected before each dose of abatacept and 1 month after the administration of 6 doses of abatacept. Peripheral blood mononuclear cells were isolated and CD4 T cells were assessed for intracellular expression of IFN-γ and IL-10 by flow cytometry. (A) Comparing responders with nonresponders, there was no difference in baseline CD4 IFN-γ expression (P = .33). One month after 6 abatacept doses, there was no significant change in CD4 IFN-γ expression in clinical responders (P = .09). (B) When comparing responders vs nonresponders, no difference in baseline CD4 Il-10 expression (P = .14) was observed. One month after 6 doses of abatacept, there was no significant difference in CD4 IL-10 expression (P = .08) in clinical responders.
CD4 IFN-γ and IL-10 expression in responders (R) and nonresponders (NR). Peripheral blood samples were collected before each dose of abatacept and 1 month after the administration of 6 doses of abatacept. Peripheral blood mononuclear cells were isolated and CD4 T cells were assessed for intracellular expression of IFN-γ and IL-10 by flow cytometry. (A) Comparing responders with nonresponders, there was no difference in baseline CD4 IFN-γ expression (P = .33). One month after 6 abatacept doses, there was no significant change in CD4 IFN-γ expression in clinical responders (P = .09). (B) When comparing responders vs nonresponders, no difference in baseline CD4 Il-10 expression (P = .14) was observed. One month after 6 doses of abatacept, there was no significant difference in CD4 IL-10 expression (P = .08) in clinical responders.
BAFF levels have been implicated in the pathogenesis of cGVHD, with higher levels correlating with adverse outcomes.21-23 As such, we analyzed the plasma of patients to assess the degree of BAFF expression at baseline and after exposure to abatacept. We did not observe a significant difference in BAFF levels among clinical responders compared with nonclinical responders. It is, however, important to note that steroid use decreases BAFF levels, potentially limiting the utility of BAFF levels as a biomarker of response in this trial.24
Discussion
cGVHD disease remains a major complication after alloSCT and is the major cause of late post-alloSCT treatment-related mortality and morbidity.3 Although several steroid-sparing agents have been studied in clinical trials, few have been shown to have clinical benefit, and outcomes for patients with SR-cGVHD remain poor.25 The syndrome resembles autoimmune diseases such as scleroderma, Sjögren syndrome, primary biliary cirrhosis, and bronchiolitis obliterans. The pathophysiology of cGVHD is complex: both T-cell and B-cell-mediated immunity play a role in the initiation and maintenance of cGVHD.26 Because T-cell activation, driven by alloantigen stimulation, is critical to the initiation and maintenance of cGVHD, blockade of costimulation has been evaluated as a means to prevent and to treat GVHD in preclinical models.27 In a murine model, blockade of costimulation with CTLA4Ig was shown to prevent both aGVHD and cGVHD, as well as reverse manifestations of cGVHD.17
The use of abatacept to reverse autoimmunity was first pioneered in rheumatoid arthritis in patients with inadequate response to disease-modifying antirheumatic drugs. In this context, abatacept has been safely used in patients for extended durations up to 8 years, with ongoing efficacy and improvement in quality-of-life metrics.28-30 Moreover, the use of abatacept in rheumatoid arthritis has been shown to result in decreased prednisone use.31 As such, immunomodulation via costimulatory blockade with abatacept represents a highly novel and promising approach for the treatment of cGVHD.
In a clinical trial, abatacept showed promise in preventing aGVHD,32 and a randomized phase 2 trial is underway (NCT01743131). Moreover, in hyperacute GVHD in the pediatric population, the combination of abatacept and a CD25 monoclonal antibody demonstrated efficacy.33 In a single case report, improvement in skin and gut aGVHD after orthotopic liver transplantation was observed in response to abatacept.34
In this study, we evaluated the safety, efficacy, and immune effects of abatacept in patients with SR-cGVHD. Abatacept was well-tolerated, with pulmonary infections being the only grade 3 or 4 possibly related adverse events observed in this phase 1 study. Infections are common in this patient population as a result of both immunosuppression related to cGVHD and the chronic immune suppression that is used for treatment. All pulmonary infections resolved, and patients were able to continue to receive therapy with abatacept without recurrence of pulmonary infections. Importantly, we did not observe viral reactivation, including cytomegalovirus and Epstein-Barr virus, in this cohort, nor did patients develop invasive fungal infections. Clinically, 44% patients (7 of the 16 evaluable patients) achieved a PR, as evidenced by improvement in at least 2 disease sites, per 2005 NIH Consensus Criteria.20 Moreover, a 51.3% reduction in prednisone usage was observed among clinical responders. The reduction in prednisone use is critical in protecting patients from the morbid adverse events incurred from prolonged steroid use. With the small sample size in this phase 1 trial, it is difficult to assess whether a particular organ is most susceptible to treatment with abatacept. Moreover, the lack of improvement in skin cGVHD may have been underestimated, given the fact that most patients with skin GVHD at baseline (13/15) had deep sclerosis, impairing response assessment. This will be studied further in a phase 2 trial. It is important to point out that given that the study was not randomized or blinded, there is a potential for investigator bias in reducing prednisone doses for individual patients. Investigators may have decreased prednisone doses merely because their patients were receiving a novel adjunctive immunosuppressive agent with abatacept; thus, the reduction in prednisone reported in this study may be overestimated. Nonetheless, the reduction in steroid requirement with ongoing improvement in cGVHD manifestations in a subgroup of patients is encouraging.
Understanding the mechanism by which therapies result in improvement of the manifestations of cGVHD and developing biomarkers that can predict for response to therapy is critically important. In exploratory studies, we assessed T cell polarization with respect to secretion of IFN-γ and IL-10. GVHD pathophysiology is characterized by the loss of regulatory T cells and increased pro-inflammatory-secreting T cells including Th1 (IFN-γ) and Th17 cells (IL-17A, IL-17F, IL-22, and IL-21).35-39 Although levels of Th1 and Th2 cytokines were measured, there was not a statistically significant change in clinical responders or in nonresponders, which may be a function of the small sample size for correlative studies in this phase 1 study. It is possible that the IL-10-secreting CD4 cells identified in the correlative studies represent FOXP3-negative, type 1 regulatory cells, as type 1 regulatory cells have the capability to produce high amounts of IL-10. Through the production of IL-10, type 1 regulatory cells suppress T-cell responses, and thereby contribute to peripheral T-cell tolerance.40
In addition, an increase in PD-1 expression on circulating CD4 and CD8 T cells was observed in clinical responders in contrast to nonresponders at 1 month, after 6 infusions of the drug. There has been recent interest in evaluating the role of PD-1/PD-L1 in contributing to the pathogenesis of GVHD.41-45 This pathway is instrumental in modulating peripheral tolerance and T cell exhaustion.46-49 In a murine model, stimulation of the PD-1 pathway contributed to the suppression of Th17/Th1-mediated cGVHD, thereby ameliorating cGVHD.42 Moreover, patients with cGVHD with severe disease demonstrate increased PD-L1 expression on donor T cells, and in a murine model, animals receiving T cells from PD-L1-negative donors experienced less severe manifestations of cGVHD.50 Interestingly, in patients with cGVHD treated with IL-2, clinical improvement of GVHD was associated with elevated levels of PD-1 on regulatory T cells.51 In this phase 1 study, increased PD-1 levels on T cells was noted in clinical responders to abatacept. These findings support the notion that the PD-1/PD-L1 pathway is critical in maintaining tolerance and halting cGVHD, and will be explored further in a phase 2 trial.
In summary, abatacept is well-tolerated in the treatment of SR-cGVHD with no DLTs in this phase 1 study. Abatacept resulted in the improvement in NIH cGVHD scores in 44% of patients who were heavily pretreated for SR-cGVHD, with a median of 3.5 prior regimens. Importantly, a significant decrease in prednisone usage was noted in clinical responders. An increase in PD-1 T cell expression was observed only in clinical responders. Based on this promising data, a phase 2 trial is underway.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This study was supported by research funding from Bristol-Myers Squibb (J.R.).
Authorship
Contribution: H.T.K., D.T., and J.R. conceived and designed the study; M.R.N., R.J.S., E.P.A., J.A., R.J., J.H.A., V.T.H., J.D.L., M.M., S.J., A.H., M.P.B., E.K.L., J.B., J.S., A.J., S.S., A.W., and L.C. acquired the data; M.R.N., R.J.S., H.T.K., D.S., S.L., D.A., and J.R. analyzed and interpreted the data; M.R.N., R.J.S., H.T.K., D.E.A., and J.R. wrote and revised the manuscript for important intellectual content; M.R.N., H.T.K., S.L., and J.R. performed statistical analysis; M.P.B., E.K.L., J.B., J.S., A.J., S.S., A.P., and R.K.L. provided administrative, technical, or material support; and R.J.S. and J.R. supervised the study.
Conflict-of-interest disclosure: J.R. has served on Advisory Summits for and has received research funding from Bristol-Myers Squibb. The remaining authors declare no competing financial interests.
Correspondence: Myrna R. Nahas, Beth Israel Deaconess Medical Center, Harvard Medical School, 330 Brookline Ave, KS 106-1, Boston, MA 02215; e-mail: mnahas1@bidmc.harvard.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal