In this issue of Blood, Jethwa et al identify a novel mechanism that facilitates the accumulation of mutant p53 (mutp53) as well as wild-type p53 (wtp53) that is induced by DNA damage in tumor cells. They demonstrate that transformation/transcription domain-associated protein (TRRAP), a constituent of multiple histone acetyltransferase (HAT) complexes, acetylates and stabilizes both mutant as well as DNA damage–induced wtp53 by preventing its nuclear export and subsequent degradation by MDM2, a negative regulator of p53.1
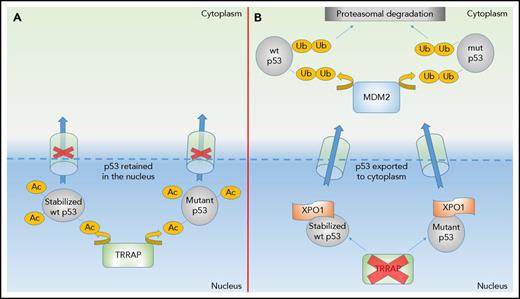
Mechanism of TRRAP action. (A) TRRAP acetylation (Ac) of either mutp53 or DNA damage–stabilized wtp53 prevents nuclear export and facilitates accumulation within the nucleus. (B) Loss of TRRAP leads to XPO1-directed nuclear export and subsequent binding to MDM2, targeting either mutant or DNA damage–stabilized p53 for destruction via the ubiquitin (Ub)-proteasome pathway.
Mechanism of TRRAP action. (A) TRRAP acetylation (Ac) of either mutp53 or DNA damage–stabilized wtp53 prevents nuclear export and facilitates accumulation within the nucleus. (B) Loss of TRRAP leads to XPO1-directed nuclear export and subsequent binding to MDM2, targeting either mutant or DNA damage–stabilized p53 for destruction via the ubiquitin (Ub)-proteasome pathway.
MDM2 is an E3 ubiquitin ligase that binds both mutp53 and wtp53 to trigger their degradation via the ubiquitin-dependent proteasome pathway.2-4 Under physiological conditions, the constitutive action of MDM2 keeps the levels of wtp53 low. DNA damage leads to the transient stabilization of p53, which transcriptionally induces many p53-responsive targets including MDM2. MDM2 in turn degrades p53 and restores it to basal levels.2,3 In contrast, missense mutations in p53 usually result in the stabilization and accumulation of mutp53 across tumor types. Mutations in p53 occur largely within the DNA-binding domain of which 6 “hotspot” mutations (R175H, R179H, G245, G248Q/W, R249, R282H) occur recurrently in cancer and exert either a dominant-negative effect or a gain of function that facilitates tumor progression.5 In B-cell lymphomas, p53 mutations occur in 30% to 40% of Burkitt lymphoma and in 40% of chronic lymphocytic leukemia that undergo Richter transformation into an aggressive lymphoma.6 Because MDM2 is overexpressed in lymphomas,7 there is great interest in understanding the mechanisms by which either mutp53 or wtp53 that is induced in response to DNA damage becomes stabilized.
In this issue, Jethwa et al demonstrated that TRRAP was well expressed in B cells and many lymphomas (see figure). Silencing of TRRAP expression using RNA interference strategies resulted in a decrease in the levels of mutp53 across lymphoma cell lines whereas overexpression of TRRAP led to an accumulation of mutp53. They identified that the HEAT repeat domain of TRRAP was crucial for stabilizing mutp53. Knockdown of TRRAP resulted in the binding of mutp53 to exportin-1 (XPO1), and its export from the nucleus to the cytoplasm where it bound to and was degraded by MDM2 via the ubiquitin-proteasome pathway. Pharmacological inhibition of XPO1 resulted in the nuclear retention of p53. Similarly, TRRAP knockdown-mediated loss of p53 was prevented by using proteasome inhibitors, confirming the role of both XPO1 and the ubiquitin-proteasome pathway in degrading mutp53. TRRAP silencing also prevented the stabilization of wtp53 that accumulated after DNA damage by the mechanism described above, suggesting that it could shield both mutant and DNA damage–activated wtp53 from XPO1-mediated nuclear transport and subsequent degradation by MDM2. These findings are intriguing because they uncover a previously unknown mechanism that regulates the stability of p53.
However, a number of unresolved questions remain. For instance, TRRAP is well expressed in normal B lymphocytes. So why does wtp53 under basal conditions not accumulate in normal cells? It is possible that TRRAP does not trigger the initial stabilization of p53 but rather TRRAP contributes to p53 stabilization by shielding it from MDM2-mediated degradation. This is significant because the mechanisms that stabilize wtp53 are well known whereas those that cause mutp53 to initially accumulate in tumor cells are less understood. The second unresolved issue comes from the knowledge that TRRAP is a component of multiple HAT complexes. Silencing of TRRAP led to a substantial decrease in the acetylation of mutp53. However, contrary to expectation, they also show that blocking the activity of histone deacetylases (HDACs) with HDAC inhibitors (which presumably favors HAT activity and increases the level of acetylation on histone and nonhistone proteins) surprisingly phenocopied the effect of TRRAP silencing and reduced acetylation of mutp53. Additional mechanistic work will be required to understand the complex mechanisms by which TRRAP regulates the stability of mutant as well as DNA damage–activated wtp53.
In summary, this study identifies TRRAP as a novel regulator of p53 stability that may represent a new therapeutic strategy to eliminate mutp53 in lymphomas and other cancers.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal