Key Points
Pinometostat demonstrates first evidence of DOT1L target inhibition and clinical responses in a subset of MLL-r advanced leukemia patients.
The observed safety profile of pinometostat shows potential for exploration of combination therapies in leukemia.
Abstract
Pinometostat (EPZ-5676) is a first-in-class small-molecule inhibitor of the histone methyltransferase disrupter of telomeric silencing 1-like (DOT1L). In this phase 1 study, pinometostat was evaluated for safety and efficacy in adult patients with advanced acute leukemias, particularly those involving mixed lineage leukemia (MLL) gene rearrangements (MLL-r) resulting from 11q23 translocations. Fifty-one patients were enrolled into 6 dose-escalation cohorts (n = 26) and 2 expansion cohorts (n = 25) at pinometostat doses of 54 and 90 mg/m2 per day by continuous intravenous infusion in 28-day cycles. Because a maximum tolerated dose was not established in the dose-escalation phase, the expansion doses were selected based on safety and clinical response data combined with pharmacodynamic evidence of reduction in H3K79 methylation during dose escalation. Across all dose levels, plasma pinometostat concentrations increased in an approximately dose-proportional fashion, reaching an apparent steady-state by 4-8 hours after infusion, and rapidly decreased following treatment cessation. The most common adverse events, of any cause, were fatigue (39%), nausea (39%), constipation (35%), and febrile neutropenia (35%). Overall, 2 patients, both with t(11;19), experienced complete remission at 54 mg/m2 per day by continuous intravenous infusion, demonstrating proof of concept for delivering clinically meaningful responses through targeting DOT1L using the single agent pinometostat in MLL-r leukemia patients. Administration of pinometostat was generally safe, with the maximum tolerated dose not being reached, although efficacy as a single agent was modest. This study demonstrates the therapeutic potential for targeting DOT1L in MLL-r leukemia and lays the groundwork for future combination approaches in this patient population. This clinical trial is registered at www.clinicaltrials.gov as NCT01684150.
Introduction
Patients with acute myeloid leukemia (AML) or acute lymphoblastic leukemia (ALL) with translocations involving the mixed lineage leukemia (MLL) gene (also termed KMT2A) at chromosome locus 11q23 generally have a poor prognosis.1-4 MLL translocations disproportionately affect patients between the ages of 30 and 50 years, are more commonly seen in patients with therapy-related AML, and are difficult to treat, even with the use of allogeneic hematopoietic stem cell transplantation.5 The MLL locus normally encodes a histone 3 lysine 4 methyltransferase that is variably rearranged in leukemia to form different fusion proteins with conserved functions in leukemogenesis.6-8 Chimeric MLL proteins recruit the histone 3 lysine 79 (H3K79) methyltransferase disrupter of telomeric silencing 1-like (DOT1L) to aberrant target sites, promoting ectopic gene expression.9-12 Increased levels of H3K79 methylation and gene transcription are detected at loci associated with hematopoietic transformation, including HOXA9 and MEIS1.13-15 Using in vitro and in vivo genetic systems, DOT1L has been proposed to be a necessary catalyst for the development of leukemia in patients with MLL translocations.16-18
DOT1L is the only known H3K79 methyltransferase, making it an attractive therapeutic target for acute leukemia.19-23 Pinometostat is a potent and selective small-molecule DOT1L inhibitor with subnanomolar affinity for DOT1L and >37 000-fold selectivity against other histone methyltransferases.23-26 Pinometostat selectively inhibits intracellular H3K79 methylation in a concentration- and time-dependent manner. In cells harboring the MLL translocation, inhibition of H3K79 methylation results in concentration- and time-dependent inhibition of downstream gene expression and consequent cell killing, with half-maximal inhibitory concentration values for cell growth in the <1 μM range.24 In addition, pinometostat has activity against leukemia involving MLL rearrangements (MLL-r) in in vivo rodent xenograft studies.24,26 In in vitro and in vivo studies, prolonged exposure to pinometostat has been shown to be required to induce cell death in target cells and tumor regression, respectively. Because the sustained exposures required for antitumor activity with pinometostat could not be achieved using oral formulations, a prolonged continuous intravenous (CIV) infusion was chosen for initial clinical development.26 Herein are reported the first-in-human phase 1 study results for the safety, pharmacokinetics (PK), pharmacodynamics (PD), and efficacy of pinometostat in patients with relapsed/refractory hematologic malignancies.
Materials and methods
Study design
This was an open-label dose-escalation (DEsc) and dose-expansion multicenter study of pinometostat in the treatment of adult relapsed/refractory acute leukemias. The DEsc phase was initiated with successive single-patient treatment cohorts per institution (maximum of 2 patients), in which patients were administered pinometostat as a CIV infusion for 21 days of a 28-day cycle. The starting dose of 12 mg/m2 per day was doubled until a drug-related toxicity ≥ grade 2 occurred, after which DEsc proceeded using the common 3 + 3 design in dose increments of 25% to 50%, with consideration of the cumulative toxicity profile of the drug. Patient safety data from baseline through cycle 1 day 28 were reviewed by a Clinical Safety Review Committee for recommendations regarding DEsc in subsequent cohorts. A dose-limiting toxicity (DLT) was defined as a clinically significant grade ≥3 nonhematologic or grade >3 hematologic suspected adverse reaction or abnormal laboratory value assessed as unrelated to disease progression, intercurrent illness, or concomitant medications occurring within the first 28 days on study. The escalation-phase doses were 12, 24, 36, 54, and 80 mg/m2 per day. A review of safety data, PK, and H3K79 methylation data during DEsc led to the addition of a sixth escalation cohort treated at 90 mg/m2 per day. Because plasma pinometostat concentrations and H3K79 inhibition were found to decline rapidly between days 21 and 28 after discontinuation of the infusion, patient dosing was also amended to a 28-day CIV infusion. Based upon the safety, PK, and preliminary activity noted during DEsc, 2 expansion-phase cohorts were initiated to evaluate doses of 54 and 90 mg/m2 per day with 28-day CIV infusions in patients with MLL-r or MLL partial tandem duplication (MLL-PTD) leukemia.
The minimum targeted duration of treatment was 2 cycles. However, patients who experienced a clinical response could elect to continue treatment with investigator consent for additional cycles until disease progression, unacceptable toxicity, or achievement of a best response in the opinion of the investigator.
Patient eligibility
Patients in the escalation and expansion phases of the study were required to be ≥18 years of age with a minimal life expectancy ≥3 months, have an Eastern Cooperative Oncology Group performance status of 0 to 2, and have white blood cell counts (with or without hydroxyurea) of <30 000/μL at the start of the study. Key inclusion criteria included a history of relapsed/refractory disease that had failed all known effective therapies and was considered ineligible for treatment by allogeneic stem cell transplantation. Key exclusion criteria included uncontrolled intercurrent illness, recent active heart or cerebrovascular disease, and bleeding diathesis.
Patients in the DEsc phase had histologically confirmed AML, ALL, mixed lineage leukemia (MLL), chronic myelomonocytic leukemia (CMML), myeloproliferative neoplasm, or high-risk myelodysplastic syndrome. Expansion-phase participants were restricted to patients with 11q23 MLL-r or MLL-PTD aberrations and relapsed/refractory AML, ALL, or acute MLL. MLL-r were identified by karyotyping, fluorescence in situ hybridization (FISH), next-generation sequencing (NGS), or polymerase chain reaction (PCR). In addition, expansion-phase patients were required to have biopsy or bone marrow aspirate blast counts >10% or biopsy-documented leukemia cutis or myeloid sarcoma. Patients enrolled in both phases must have attempted prespecified disease-appropriate chemotherapy regimens prior to enrollment unless they were >60 years of age or significant comorbid medical conditions were present. Institutional Review Board approval was obtained from each participating center. All participants provided written informed consent.
Study assessments
Patient safety was monitored by Clinical Safety Review Committee evaluations of adverse events (AEs), serious AEs (SAEs), 12-lead electrocardiograms, vital signs, physical examinations, and a review of laboratory findings for biochemistry, hematology (including bone marrow assessment), and urinalysis. Clinical activity, PK, and/or PD were assessed by clinical examination, bone marrow aspiration, and blood and/or biopsy samples collected predose and at specified postdose time points. Disease assessments, based on disease-specific response criteria, were initially completed at screening and at the end of every other treatment cycle, beginning with cycle 2. Following a protocol amendment, efficacy assessments were subsequently changed to the first day of each treatment cycle, beginning with cycle 3. Plasma and urine pinometostat concentrations were determined with validated methods performed by BASi (West Lafayette, IN), and all PK analyses were conducted by ProPharma Services (Superior, CO). In addition to cellularity and blast percentage, blood samples were assessed for cytogenetic changes, genetic alterations, target gene expression, or dimethylated H3K79 (H3K79me2) levels. Methods for NGS, real-time quantitative reverse transcription PCR, and chromatin immunoprecipitation sequencing (ChIP-seq) are detailed in supplemental Materials, available on the Blood Web site.
Investigators used the 2003 International Working Group standardized response criteria for AML, 2002 National Comprehensive Cancer Network response guidelines for ALL, or additional disease-appropriate standardized criteria to assess patient responses. The incidence and duration of disease responses were determined.
Statistical methods
The primary objective of the study was to identify the maximum tolerated dose (MTD) of, and to determine the safety and tolerability profile for, pinometostat as a 21- or 28-day CIV infusion in acute hematologic malignancies with 11q23 rearrangements involving the MLL gene. The secondary objectives were to evaluate the PK and PD response profile (H3K79me2 inhibition) of pinometostat and identify early evidence of treatment efficacy in the acute leukemia study population. All AEs were coded using the Medical Dictionary for Regulatory Activities version 15.0. Severity was assessed by the investigator using the National Cancer Institute’s Common Terminology Criteria for Adverse Events, version 4.03. Treatment-related AEs were defined as AEs classified by the investigator as definitely, probably, or possibly related to the study drug. Patients with a missing best response were treated as nonresponders and, therefore, were included in the denominator for objective response rate. Objective response rates in patients were calculated using exact 80% confidence intervals. Statistical analyses for PK and PD data, unless otherwise noted, are reported as the mean, SD, or SEM.
Results
Baseline characteristics
A total of 51 adult patients with relapsed/refractory acute leukemias, including 26 patients in the DEsc cohorts and 25 patients in the MLL-r/MLL-PTD restricted/expansion phase, was enrolled and treated between September 2012 and October 2015 in this phase 1 study conducted at 6 study centers in the United States and 1 in The Netherlands. Patient demographics and disease characteristics are detailed in Table 1. The median age of the study population was 50.0 years, with more male patients (n = 29) than female patients (n = 22). A total of 43 of 51 patients (84%) had a primary diagnosis of AML, 6 patients (12%) had ALL, and 1 patient (2%) each had a primary diagnosis of CMML or acute MLL. Approximately 75% of patients received ≥3 systemic treatments for their disease prior to enrollment. Locally determined enrollment enabling cytogenetic rearrangements were captured, and MLL fusion partners were identified centrally using NGS methods (Invivoscribe, San Diego, CA; Supplemental Table 1). Of the 25 patients with locally determined MLL-r using FISH/cytogenetics, 24 were confirmed centrally via NGS. Notably, NGS, in addition to confirming MLL-r status, enabled full identification of the specific fusion partners in all t(11:19) patients (eg, ELL or ENL/MLLT1) and the presence of additional somatic mutations. In total, 42 of 51 study participants had confirmed MLL-r, MLL-PTD, or both alterations using a combination of these methodologies. All 51 patients have discontinued drug treatment (Table 2). The most common primary reasons for treatment discontinuation were disease progression (20 patients), lack of efficacy (11 patients), and AEs (10 patients), as discussed in greater detail in the Safety section.
Patient characteristics
| Demographic/characteristic . | Total patients (N = 51) . |
|---|---|
| Sex, n (%) | |
| Female | 22 (43) |
| Male | 29 (57) |
| Age, median (range), y | 50.0 (19-81) |
| Disease at study entry, n (%) | |
| AML (n = 43) | |
| MLL-r | 33 (65) |
| MLL-PTD | 2 (4) |
| MLL-r and MLL-PTD | 2 (4) |
| MLL-wt | 6 (12) |
| ALL (n = 6) | |
| MLL-r | 4 (8) |
| MLL-wt | 2 (4) |
| MLL (n = 1) | |
| MLL-PTD | 1 (2) |
| CMML (n = 1) | |
| MLL-wt | 1 (2) |
| Prior systemic therapy regimens, n (%) | |
| 1 | 2 (4) |
| 2 | 11 (22) |
| ≥3 | 38 (75) |
| Prior allogeneic hematopoietic cell transplants, n (%) | |
| 1 | 19 (37) |
| 2 | 1 (2) |
| Demographic/characteristic . | Total patients (N = 51) . |
|---|---|
| Sex, n (%) | |
| Female | 22 (43) |
| Male | 29 (57) |
| Age, median (range), y | 50.0 (19-81) |
| Disease at study entry, n (%) | |
| AML (n = 43) | |
| MLL-r | 33 (65) |
| MLL-PTD | 2 (4) |
| MLL-r and MLL-PTD | 2 (4) |
| MLL-wt | 6 (12) |
| ALL (n = 6) | |
| MLL-r | 4 (8) |
| MLL-wt | 2 (4) |
| MLL (n = 1) | |
| MLL-PTD | 1 (2) |
| CMML (n = 1) | |
| MLL-wt | 1 (2) |
| Prior systemic therapy regimens, n (%) | |
| 1 | 2 (4) |
| 2 | 11 (22) |
| ≥3 | 38 (75) |
| Prior allogeneic hematopoietic cell transplants, n (%) | |
| 1 | 19 (37) |
| 2 | 1 (2) |
MLL-r and MLL-PTD were identified locally through standard karyotyping or 11q23 FISH (MLL-r) or NGS- or PCR-based methods (MLL-PTD). MLL-wt = no MLL rearrangement detected.
Patient disposition
| Patient disposition (N = 51) . | n (%) . |
|---|---|
| Received study drug | 51 (100) |
| Completed study* | 3 (6) |
| Discontinued study | 48 (94) |
| Reason for treatment discontinuation | |
| Disease progression | 20 (39) |
| Lack of efficacy | 11 (21) |
| AE | 10 (20) |
| Death† | 4 (8) |
| Withdrawal by patient | 4 (8) |
| Required for alternative therapy | 1 (2) |
| Other | 1 (2) |
| Patient disposition (N = 51) . | n (%) . |
|---|---|
| Received study drug | 51 (100) |
| Completed study* | 3 (6) |
| Discontinued study | 48 (94) |
| Reason for treatment discontinuation | |
| Disease progression | 20 (39) |
| Lack of efficacy | 11 (21) |
| AE | 10 (20) |
| Death† | 4 (8) |
| Withdrawal by patient | 4 (8) |
| Required for alternative therapy | 1 (2) |
| Other | 1 (2) |
Patients who attended a final follow-up visit 30 d after last study drug dose met the definition for completing study.
Classified as unlikely or definitely not related to study drug by the investigator.
PK analysis
Pinometostat concentration increased rapidly within the first 4 hours after CIV infusion and reached an apparent steady-state level by 8 hours. In patients treated with 21-day CIV infusions, the decline in plasma pinometostat concentrations was biphasic, with a short α-elimination phase (T1/2 ∼ 0.8-2.0 hours), followed by a prolonged β-phase (T1/2 of 102-298 hours), potentially relating to slow drug release from tissues (Figure 1A). Mean plasma pinometostat concentrations declined to low levels between days 21 and 28 with the 21-day infusion, but they remained elevated throughout these time points during the 28-day infusion. Plasma pinometostat concentrations were variable during the infusion period (Figure 1A-B). An increase in the infusion rate did not result in greater mean plasma pinometostat concentrations at any time point relative to the concentrations at lower doses at each dose level. However, an analysis of plasma pinometostat concentrations from all 51 patients following 21- or 28-day CIV infusion showed that the mean steady-state concentration (Css) of pinometostat increased with dose and that the magnitude of the increase was generally proportional to dose (Figure 1C). At doses from 24 to 90 mg/m2 per day, a 3.75-fold increase in dose resulted in an approximately fourfold increase in mean Css. Furthermore, the greatest median pinometostat value for maximum plasma concentration (Cmax) was observed in the 90 mg/m2 per day 28-day CIV-dose cohort.
Summary of plasma pinometostat PK. (A) Mean (SD) plasma pinometostat concentration–time profile after administration of 24, 36, or 54 mg/m2 per day with 21-day CIV infusion. Pinometostat concentrations in plasma rose quickly, reaching an apparent steady-state within 4-8 hours postinfusion, followed by a biphasic decline. A pinometostat concentration–time profile for the 80 mg/m2 per day group (n = 3) is not displayed, because only a median profile was available. (B) Mean (SD) plasma pinometostat concentration–time profiles after administration of 54 or 90 mg/m2 per day with 28-day CIV infusion. (C) Summary of PK data for each treatment cohort.
Summary of plasma pinometostat PK. (A) Mean (SD) plasma pinometostat concentration–time profile after administration of 24, 36, or 54 mg/m2 per day with 21-day CIV infusion. Pinometostat concentrations in plasma rose quickly, reaching an apparent steady-state within 4-8 hours postinfusion, followed by a biphasic decline. A pinometostat concentration–time profile for the 80 mg/m2 per day group (n = 3) is not displayed, because only a median profile was available. (B) Mean (SD) plasma pinometostat concentration–time profiles after administration of 54 or 90 mg/m2 per day with 28-day CIV infusion. (C) Summary of PK data for each treatment cohort.
PD analysis
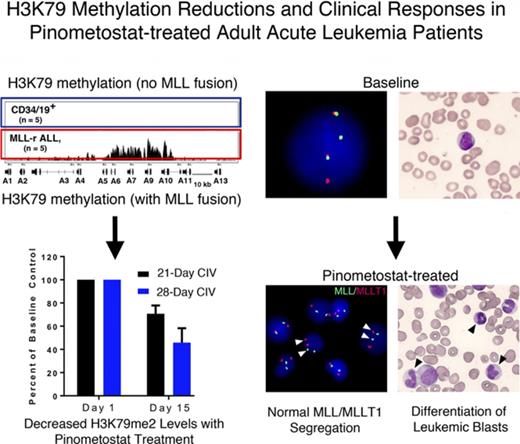
H3K79me2 ChIP-seq performed on leukemic blasts collected pre- and postdose demonstrated reductions in H3K79me2 levels globally and locally at specific MLL-r target genes13 in most patients analyzed (supplemental Figure 1A). H3K79me2 levels at the loci for HOXA9 and MEIS1 were reduced in most patients, although decreases did not necessarily correlate with clinical outcome. Although these results provided evidence of DOT1L inhibition in a relevant tissue compartment (leukemic blasts), the degree of H3K79me2 reduction was variable, even between patients treated with the same doses of pinometostat. HOXA9 has been intensively investigated with regard to its role in promoting leukemogenesis, and continued overexpression during disease treatment has been associated with poor clinical outcome.11,15,27-30 Analysis of MLL-r target genes by quantitative reverse-transcription PCR confirmed that HOXA9 expression was also decreased in treated patients, although the level of inhibition was similarly variable (supplemental Figure 1B).
PK–PD relationship
In preclinical studies, pinometostat inhibited DOT1L enzymatic activity, as well as in vitro and in vivo global H3K79 methylation, and reduced the proliferation of MLL-r leukemia cell lines.24 Global H3K79me2 levels in peripheral blood mononuclear cells (PBMCs) were reduced following pinometostat exposure and ranged from 7% to 88% (Figure 2A). These results suggested that varying degrees of DOT1L inhibition were achieved in most patients, although the level of suppression was only weakly correlated with the drug plasma concentration.
Sustained global H3K79me2 inhibition is dependent on uninterrupted DOT1L inhibition. (A) Global H3K79me2 inhibition in bulk PBMCs representing patients from all dosing cohorts vs the mean Css. Percent inhibition was calculated by taking the cycle 1 time point with maximum H3K79me2 inhibition relative to a pretreatment baseline sample. Decreases in H3K79me2 levels were exhibited by all patients who could be reliably measured, but the magnitude of inhibition was only weakly proportional to Css. (B) Inhibition of H3K79me2 levels in bulk PBMCs collected from patients at days 15 and 28 relative to preinfusion baseline controls. Inhibition levels from patients treated continuously for 21 days, followed by a 7-day-off period, were compared with those from patients treated for the full 28-day treatment cycle. After initial decreases with both regimens, global H3K79me2 levels recovered toward baseline in patients receiving the 7-day break in treatment compared with those patients treated continuously for 28 days (P = .005, Mann-Whitney 2-tailed Student t test). Consistent with rapid drug clearance, continuous treatment with pinometostat was needed to sustain H3K79me2 inhibition. Data are mean ± SEM.
Sustained global H3K79me2 inhibition is dependent on uninterrupted DOT1L inhibition. (A) Global H3K79me2 inhibition in bulk PBMCs representing patients from all dosing cohorts vs the mean Css. Percent inhibition was calculated by taking the cycle 1 time point with maximum H3K79me2 inhibition relative to a pretreatment baseline sample. Decreases in H3K79me2 levels were exhibited by all patients who could be reliably measured, but the magnitude of inhibition was only weakly proportional to Css. (B) Inhibition of H3K79me2 levels in bulk PBMCs collected from patients at days 15 and 28 relative to preinfusion baseline controls. Inhibition levels from patients treated continuously for 21 days, followed by a 7-day-off period, were compared with those from patients treated for the full 28-day treatment cycle. After initial decreases with both regimens, global H3K79me2 levels recovered toward baseline in patients receiving the 7-day break in treatment compared with those patients treated continuously for 28 days (P = .005, Mann-Whitney 2-tailed Student t test). Consistent with rapid drug clearance, continuous treatment with pinometostat was needed to sustain H3K79me2 inhibition. Data are mean ± SEM.
During the course of the study, we compared the kinetics of global H3K79me2 inhibition over the 28-day treatment cycle in PBMCs collected from patients administered pinometostat using the 21- or the 28-day CIV regimen (Figure 2B). With the 21-day CIV schedule, H3K79me2 levels decreased up to day 15, but they regressed to baseline at day 28 following the 7-day interval between treatments, in parallel with the decrease in plasma pinometostat during this period (Figure 1A). H3K79me2 levels were also decreased at day 15 in patients dosed with the continuous 28-day CIV cycle, but they remained suppressed throughout the duration of treatment. This finding of sustained inhibition, in combination with PK and clinical data, supported a modification in the dosing schedule to a continuous 28-day CIV infusion for the remainder of the study. This was implemented with the 90 mg/m2 per day DEsc cohort.
Safety
DEsc phase.
The mean duration of exposure to pinometostat in this study was 5.1 weeks, with a range of 0.4 to 27 weeks. The only DLT to occur during the DEsc phase of the study was a grade 4 cardiac failure event, classified as possibly related by the investigator, which occurred after 13 days of treatment. Infusions of pinometostat were discontinued in this patient, and the event resolved 6 days later. Because the DLT occurred in 1 of 6 patients in the 90 mg/m2 per day 28-day CIV infusion cohort, the threshold for MTD determination was not established. Pinometostat doses of 54 mg/m2 per day inhibited H3K79me2 levels and achieved a Css that was within the lower range of the effective drug concentration predicted from rat xenograft models.24 Because of the combined clinical observation of a DLT and the correlative PD and PK data showing maximal exposure and methylation inhibition at 90 mg/m2 per day, the DEsc phase was suspended, and the expansion-phase dose was declared to be 90 mg/m2 per day. Furthermore, the preliminary observation of clinical benefit in some patients at lower drug thresholds during the DEsc phase, combined with the PD and PK data, warranted the inclusion of a second expansion cohort treated at 54 mg/m2 per day using the 28-day CIV schedule.
Dose expansion and safety population.
All patients in the study experienced ≥1 treatment-emergent AE (TEAE), with the majority being ≤ grade 2 (Table 3). The most commonly identified events within the patient population, regardless of attribution to treatment, were fatigue (39%), nausea (39%), constipation (35%), and febrile neutropenia (35%). Definitive differences in the number of AEs between doses could not be established from the small cohort sizes, particularly during DEsc (supplemental Table 2). There were apparent increases in the percentage of patients who experienced hypocalcemia, respiratory failure, and peripheral edema when dosed at 90 mg/m2 per day relative to 54 mg/m2 per day by 28-day CIV infusion, although the study was not designed to show statistical significance between dose levels, and these AEs were not considered treatment related by the investigators. Overall, the spectrum of TEAEs was generally typical for the patient population under investigation.
Treatment-emergent AEs regardless of attribution (N = 51)
| Adverse Event . | All grades* >15%, n (%) . | Grades ≥3, n (%) . |
|---|---|---|
| Patients reporting ≥1 AE | 51 (100) | 46 (90) |
| Fatigue | 20 (39) | 4 (8) |
| Nausea | 20 (39) | 0 |
| Constipation | 18 (35) | 0 |
| Febrile neutropenia | 18 (35) | 17 (33) |
| Hypokalemia | 16 (31) | 4 (8) |
| Hypocalcaemia | 15 (29) | 7 (14) |
| Edema peripheral | 13 (25) | 0 |
| Vomiting | 13 (25) | 0 |
| Hypomagnesaemia | 12 (24) | 0 |
| Diarrhea | 11 (22) | 0 |
| Leukocytosis | 11 (22) | 10 (20) |
| Cough | 11 (22) | 0 |
| Dyspnea | 11 (22) | 2 (4) |
| Pyrexia | 10 (20) | 1 (2) |
| Abdominal pain | 10 (20) | 2 (4) |
| Anemia | 10 (20) | 8 (16) |
| Mucosal inflammation | 9 (18) | 2 (4) |
| Pneumonia | 9 (18) | 7 (14) |
| Adverse Event . | All grades* >15%, n (%) . | Grades ≥3, n (%) . |
|---|---|---|
| Patients reporting ≥1 AE | 51 (100) | 46 (90) |
| Fatigue | 20 (39) | 4 (8) |
| Nausea | 20 (39) | 0 |
| Constipation | 18 (35) | 0 |
| Febrile neutropenia | 18 (35) | 17 (33) |
| Hypokalemia | 16 (31) | 4 (8) |
| Hypocalcaemia | 15 (29) | 7 (14) |
| Edema peripheral | 13 (25) | 0 |
| Vomiting | 13 (25) | 0 |
| Hypomagnesaemia | 12 (24) | 0 |
| Diarrhea | 11 (22) | 0 |
| Leukocytosis | 11 (22) | 10 (20) |
| Cough | 11 (22) | 0 |
| Dyspnea | 11 (22) | 2 (4) |
| Pyrexia | 10 (20) | 1 (2) |
| Abdominal pain | 10 (20) | 2 (4) |
| Anemia | 10 (20) | 8 (16) |
| Mucosal inflammation | 9 (18) | 2 (4) |
| Pneumonia | 9 (18) | 7 (14) |
Graded according to National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03.
A total of 10 patients discontinued treatment primarily due to TEAEs. These included intracranial hemorrhage and infections (2 patients each), acute renal failure, increased blast count, and enterovesical fistula (1 patient each), which were not attributed to the study drug. Two patients discontinued treatment due to congestive cardiac failure, which was considered treatment related by the investigator, and 1 patient discontinued due to contemporaneous respiratory failure (not related) and cardiac failure (possibly related). SAEs were reported in 35 patients (69%), the most common being febrile neutropenia (13 patients, 25%), respiratory failure (6 patients, 12%), and pneumonia (5 patients, 10%). There were 22 deaths on study, 12 of which were attributed, by the investigator, to fatal SAEs, 9 to progressive disease, and 1 (in a patient with pneumonia, neutropenic fever, and chronic renal failure) occurring in the setting of progressive ALL. No fatal SAE was considered treatment related, with the exception of the death of a 73-year-old patient in the 90 mg/m2 per day 28-day CIV infusion DEsc cohort who had an extensive cardiac medical history. This patient experienced cardiorespiratory arrest 4 days after pinometostat discontinuation.
Overall, 22 patients experienced treatment-related TEAEs, including 9 patients with events classified as grade 3 or greater (Table 4). These treatment-related TEAEs were more commonly hematologic in nature (4 patients), such as leukocytosis, white blood cell increase and anemia, or cardiac (4 patients), including 1 patient with congestive heart failure, 1 patient with cardiac failure, 1 patient with prolonged electrocardiogram QT interval, and 1 patient with congestive cardiac failure, decreased ejection fraction, and cardiopulmonary arrest.
Drug-related AEs grade ≥3
| AE . | Dose (mg/m2 per day) . | N (%) . |
|---|---|---|
| Patients with ≥1 drug-related TEAE ≥ grade 3 | All | 9 (18) |
| Leukocytosis | 36, 80, 90 | 3 (6) |
| Cardiac failure congestive | 90 | 2 (4) |
| Cardiac failure | 90 | 1 (2) |
| Ejection fraction decreased* | 90 | 1 (2) |
| Cardiorespiratory arrest* | 90 | 1 (2) |
| Liver function test abnormal† | 54 | 1 (2) |
| White blood cell count increased | 54 | 1 (2) |
| Anemia | 54 | 1 (2) |
| Electrocardiogram QT prolonged | 54 | 1 (2) |
| Hypophosphatemia | 90 | 1 (2) |
| AE . | Dose (mg/m2 per day) . | N (%) . |
|---|---|---|
| Patients with ≥1 drug-related TEAE ≥ grade 3 | All | 9 (18) |
| Leukocytosis | 36, 80, 90 | 3 (6) |
| Cardiac failure congestive | 90 | 2 (4) |
| Cardiac failure | 90 | 1 (2) |
| Ejection fraction decreased* | 90 | 1 (2) |
| Cardiorespiratory arrest* | 90 | 1 (2) |
| Liver function test abnormal† | 54 | 1 (2) |
| White blood cell count increased | 54 | 1 (2) |
| Anemia | 54 | 1 (2) |
| Electrocardiogram QT prolonged | 54 | 1 (2) |
| Hypophosphatemia | 90 | 1 (2) |
AEs co-occurred in patient with congestive cardiac failure.
Laboratory values included concurrent grade 3 alanine aminotransferase and grade 4 aspartate transaminase and bilirubin increases.
Efficacy
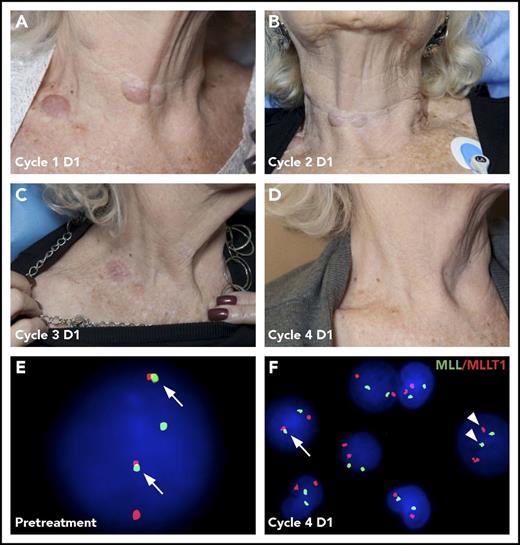
The clinical activity observed across all doses is shown in Table 5. Two patients achieved a complete remission (CR). The first patient was diagnosed with t(11:19) CMML and given 1 cycle of 5-azacitidine (75 mg/m2 for 7 days). Because of toxicity and a rapid increase in the number and size of leukemic skin lesions, she opted to discontinue 5-azacitidine. She then enrolled in the 54 mg/m2 per day DEsc cohort of pinometostat by 21-day CIV infusion. She achieved a best response of CR at 113 days. The duration of response was 85 days and was ongoing at cycle 7 day 29, when she withdrew from participation in the study. In addition to clearance of leukemic blasts in the marrow, the patient had resolution of leukemia cutis (Figure 3A-D; supplemental Figure 2) and a cytogenetic remission, as assessed by FISH (Figure 3E-F). Within 3 weeks of discontinuation of pinometostat, the patient developed AML.
Clinical activity by dose
| Dose (mg/m2 per day) . | Number of patients (N = 51) . | Leukemia cutis resolved (n = 3) . | Leukocytosis/differentiation (n = 9) . | Overall response (n = 2) . |
|---|---|---|---|---|
| 12 | 1 | 0 | 0 | |
| 24 | 5 | 0 | 1 | |
| 36 | 4 | 1 | 2 | |
| 54 | 6 | 1 | 1 | 1 CR |
| 54 (28-d CIV) | 8 | 1 | 1 | 1 CR |
| 80 | 3 | 0 | 2 | |
| 90 (28-d CIV) | 24 | 0 | 2 |
| Dose (mg/m2 per day) . | Number of patients (N = 51) . | Leukemia cutis resolved (n = 3) . | Leukocytosis/differentiation (n = 9) . | Overall response (n = 2) . |
|---|---|---|---|---|
| 12 | 1 | 0 | 0 | |
| 24 | 5 | 0 | 1 | |
| 36 | 4 | 1 | 2 | |
| 54 | 6 | 1 | 1 | 1 CR |
| 54 (28-d CIV) | 8 | 1 | 1 | 1 CR |
| 80 | 3 | 0 | 2 | |
| 90 (28-d CIV) | 24 | 0 | 2 |
Resolution of leukemia cutis and cytogenetic changes following pinometostat treatment. Cutaneous leukemia cutis in an 81-year-old patient presenting with MLL-r CMML that was treated with 54 mg/m2 per day of pinometostat by 21-day CIV infusion. Leukemia cutis neck lesions that were apparent on day 1 (D1) at the start of treatment (A) progressively resolved over the course of subsequent treatment cycles (B-D). (E) Translocation-positive cell in a peripheral blood sample detected by FISH from the same patient showing the t(11:19) MLL-r product colocalizing with the MLLT1 fusion partner (arrows). (F) The number of translocation-positive cells (arrow) decreased from 90% pretreatment to 0.2% at the start of the fourth pinometostat treatment cycle, with most cells demonstrating normal segregation of MLL and MLLT1 signals (arrowheads).
Resolution of leukemia cutis and cytogenetic changes following pinometostat treatment. Cutaneous leukemia cutis in an 81-year-old patient presenting with MLL-r CMML that was treated with 54 mg/m2 per day of pinometostat by 21-day CIV infusion. Leukemia cutis neck lesions that were apparent on day 1 (D1) at the start of treatment (A) progressively resolved over the course of subsequent treatment cycles (B-D). (E) Translocation-positive cell in a peripheral blood sample detected by FISH from the same patient showing the t(11:19) MLL-r product colocalizing with the MLLT1 fusion partner (arrows). (F) The number of translocation-positive cells (arrow) decreased from 90% pretreatment to 0.2% at the start of the fourth pinometostat treatment cycle, with most cells demonstrating normal segregation of MLL and MLLT1 signals (arrowheads).
A second patient with relapsed t(11:19) MLL-r AML that was refractory to reinduction with mitoxantrone, etoposide, and cytarabine achieved a CR at day 49, with a duration of response of 92 days, while receiving 54 mg/m2 per day pinometostat by 28-day CIV infusion. The patient underwent treatment through cycle 4 day 30 before discontinuation from the study in anticipation of an allogeneic bone marrow transplant. The patient subsequently died 29 days after the end of treatment from complications related to his allogeneic transplant. Two additional patients had clearance of leukemia cutis without a bone marrow response. Seven nonresponding patients had morphologic changes in the bone marrow consistent with myeloid differentiation (appearance of mature forms; supplemental Figure 3).
Discussion
This first-in-human DEsc and expansion cohort study investigated the safety and tolerability of pinometostat, a small-molecule inhibitor of the DOT1L histone methyltransferase, for the treatment of adult acute leukemias. Ectopic DOT1L activation is a consequence of MLL-r that arises in 5% to 10% of patients with acute leukemias of lymphoid, myeloid, or mixed/indeterminate lineage.31,32 The current absence of effective treatment options for patients with MLL-r–based leukemias highlights the need for novel therapeutic approaches and makes DOT1L an attractive target for new drug development.20,33-35 In preclinical testing, pinometostat inhibited the proliferation of leukemia cell lines harboring MLL-r and induced sustained regressions in MLL-r rat xenograft models.24 The 37 000-fold selectivity of pinometostat for DOT1L over other histone methyltransferases suggests a high degree of specificity for MLL-r fusion protein targets.24
DOT1L is the only H3K79 methyltransferase identified to date.21 As such, any H3K79me2 reductions achieved by patients are a sensitive indicator for DOT1L inhibition. PBMCs isolated from measurable samples collectively showed consistent inhibition of H3K79 methylation in all subjects, despite the fact that the levels of suppression achieved varied significantly and did not correlate with plasma pinometostat Css. In preclinical xenograft models, pinometostat induced tumor stasis or regressions at plasma Css > 600 ng/mL.24 The mean Css of patients dosed at 36 mg/m2 per day in this study was 714 ng/mL, indicating that patients treated with drug concentrations at or above this level largely reached the target Css for preclinical in vivo efficacy. This finding is further supported by the decreased H3K79me2 levels detected in individual patients at the MLL-r fusion protein target loci, HOXA9 and MEIS1, by ChIP-seq analysis and by inhibition of HOXA9 transcription in PBMCs of some patients, including 1 participant who achieved a CR in this study. An additional patient with a PD response had progressive disease, however, underscoring that achieving reductions in HOXA9 mRNA expression does not necessarily correlate with clinical outcomes.
Pinometostat was well tolerated, in general, by the patient population at the therapeutic doses required for DOT1L inhibition. The majority of AEs that occurred were grade 1 or 2, although there were 9 patients with drug-related grade 3 or higher events reported. Several of these patients experienced hematologic TEAEs, such as leukocytosis and anemia, which may also reflect the underlying disease. Less apparent is the relationship between the cardiac failure and congestive heart failure TEAEs that occurred in 3 patients. Potential connections between DOT1L inhibition and heart disease have not been identified previously. Of note, the presence of preexisting cardiac conditions and the advanced age of 2 of these patients may also have been contributing factors.
Despite achieving pinometostat exposure levels ≥ 36 mg/m2 per day, which were sufficient to induce antitumor activity in preclinical models24 and inhibit DOT1L activity in patient leukemic blasts, formal clinical responses were limited to 2 of 51 patients treated. Clearly, these data indicate that the levels of DOT1L inhibition attained by pinometostat as a stand-alone therapy are not sufficient to achieve clinical benefit in most adult patients with relapsed/refractory MLL-r leukemia. One patient who attained a CR had rapid progression of disease after stopping pinometostat. This may suggest that maintaining remission requires continued inhibition of DOT1L, although this would need validation in future studies.
There are several potential reasons for the absence of greater activity among patients in this study. First, the MTD was not reached, so higher doses may have achieved greater suppression of H3K79me2 in leukemia patients leading, in turn, to more patients with clinical responses. Nevertheless, significant reductions in H3K79me2 were attained at the doses explored in this study, and the 2 CRs achieved occurred at doses ∼50% less than the highest dose infused. Additionally, higher doses may be less well tolerated. Secondly, it may be that different MLL-r fusion proteins lead to differential sensitivity to DOT1L inhibition. Indeed, it is noteworthy that both patients who achieved CRs had t(11;19), although the resulting fusion proteins differed (1 DOT1L-ELL and 1 DOT1L-ENL). However, not all patients with evidence of such fusions received detectable clinical benefit from pinometostat treatment.
The evidence of clinical activity, such as leukocytosis, differentiation, and resolution of leukemia cutis, in patients who did not meet formal International Working Group criteria for a response, indicates that the clinical impact of inhibition of DOT1L on leukemia progression was not limited to the 2 patients with observed CRs. Additionally, the general tolerability profile of pinometostat suggests that further clinical investigations, potentially in combination with other antileukemia agents, are warranted. Pinometostat, in combination with standard-of-care agents, such as Ara-C, or hypomethylating agents, such as azacytidine, demonstrated improved in vitro cell killing and tolerability in in vivo animal models.25 In addition, other small molecule inhibitors that target the MLL fusion complex, including menin inhibitors and syk inhibitors, are in clinical development, and the combination of these agents with DOT1L inhibitors may be reasonable to pursue if supported by robust preclinical models.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients and families who participated in this study. Additionally, they acknowledge Alice McDonald, Carly Campbell, Natalie Warholic, Richard Koche, and Katie Yang Li for generation and analysis of the PD end points. Tim Henion and Virginia Kelly of Acumen Medical Communications, LLC provided medical writing and editorial assistance. We thank Robert Copeland and Peter Ho for manuscript review and comments.
This work was supported by Epizyme, Inc.
Authorship
Contribution: E.M.S., E.H., R.P., B.L., S.A.A., M.S.T., and J.D. participated in trial design, protocol writing, and data analysis; E.M.S., G.G.-M., D.A.R., R.T., J.G.B., M.R.S., M.J.-L., and J.K.A. enrolled patients in the trial, reviewed data, and identified toxicities; E.M.S., M.S.T., J.D., S.J.B., and A.C. wrote the manuscript; B.T., E.H., A.C., and E.M.S. performed clinical data analysis; N.J.W. and A.B.S. performed PK data generation and analysis; S.R.D., S.J.B., and A.K. performed PD data generation and analysis; S.R.D. and S.J.B. analyzed NGS of tumor tissue; and all authors participated in manuscript review and editing.
Conflict-of-interest disclosure: E.M.S. received research funding and personal fees from Celgene Corporation and Agios Pharmaceuticals, Inc. R.T. received institutional research funding for trial participation. J.G.B. has received funding support from AbbVie, Amgen, Bluebird, Bristol-Myers Squibb, Celgene, Constellation, Curis, Janssen, Novartis, Takeda, Teva, and Vivolux. M.R.S. owns stock in Karyopharm Therapeutics; has a consulting/advisory role at Amgen, Astex, Celgene, Gilead, Incyte, Karyopharm Therapeutics, and TG Therapeutics; and receives research funding from Astex, Incyte, Sunesis, Takeda, and TG Therapeutics. J.K.A. has an advisory role at Immune Pharmaceuticals; received personal fees from and has an advisory role at Syros, Janssen Pharmaceuticals, Novartis, Bristol-Myers Squibb, Celgene, and Astellas; received institutional research funding for trial participation from Celgene, Astellas, Fujifilm, Genentech, ARIAD, Bayer, Celator, Cyclacel, Epizyme, Incyte, GlaxoSmithKline, Pfizer, Agios, and Boehringer Ingelheim; and received nonfinancial support from MC2. B.T., S.J.B., S.R.D., N.J.W., A.B.S., A.C., R.P., and E.H. are current or former employees of Epizyme and/or stockholders. S.A.A. is a consultant for Epizyme. J.D. is an employee and shareholder of Celgene. The remaining authors declare no competing financial interests.
The current affiliation for R.T. is Clinical Leukemia Program, NYU School of Medicine & Perlmutter Cancer Center NYU Langone Health, New York, NY.
Correspondence: Eytan M. Stein, Leukemia Service, Department of Medicine, Memorial Sloan Kettering Cancer Center, 1275 York Ave, New York, NY 10065; e-mail: steine@mskcc.org.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal