Abstract
Current approaches to prevent and treat graft-versus-host disease (GVHD) after stem cell transplantation rely principally on pharmacological immune suppression. Such approaches are limited by drug toxicity, nonspecific immune suppression, and a requirement for long-term therapy. Our increased understanding of the regulatory cells and molecular pathways involved in limiting pathogenic immune responses opens the opportunity for the use of these cell subsets to prevent and/or GVHD. The theoretical advantages of this approach is permanency of effect, potential for facilitating tissue repair, and induction of tolerance that obviates a need for ongoing drug therapy. To date, a number of potential cell subsets have been identified, including FoxP3+ regulatory T (Treg) and FoxP3negIL-10+ (FoxP3-negative) regulatory T (Tr1), natural killer (NK) and natural killer T (NKT) cells, innate lymphoid cells, and various myeloid suppressor populations of hematopoietic (eg, myeloid derived suppressor cells) and stromal origin (eg, mesenchymal stem cells). Despite initial technical challenges relating to large-scale selection and expansion, these regulatory lineages are now undergoing early phase clinical testing. To date, Treg therapies have shown promising results in preventing clinical GVHD when infused early after transplant. Results from ongoing studies over the next 5 years will delineate the most appropriate cell lineage, source (donor, host, third party), timing, and potential exogenous cytokine support needed to achieve the goal of clinical transplant tolerance.
Introduction
Despite improved understanding of graft-versus-host disease (GVHD) mechanisms and new prophylaxis approaches, GVHD continues as a significant source of morbidity and mortality following allogeneic hematopoietic stem cell transplantation (allo-HSCT). Steroids remain the major first-line treatment of inflammatory acute GVHD (aGVHD) and acellular, tissue fibrosis associated with pathogenic antibody production and chronic GVHD (cGVHD). With the exception of Imbruvica (ibrutinib) for adult cGVHD patients failing prior therapies, there is no US Food and Drug Administration–approved or gold-standard second-line GVHD therapy. New treatments are needed to improve outcomes and reduce steroid complications.
Regulatory cell populations can control immune homeostasis, reduce detrimental T-cell responses to foreign antigens, and facilitate tissue repair. Preclinical studies have demonstrated the efficacy of regulatory cells to reduce GVHD. Some have been tested as GVHD prophylaxis or therapy in patients. We will summarize available preclinical and clinical cell infusional data and discuss potential clinical applications and challenges for cell therapies not yet in the clinic.
Nonlymphoid cells
Mesenchymal stem cells (MSCs) have been studied worldwide for treatment of steroid-refractory aGVHD. Both MSCs and multipotent adult progenitor cells (MAPCs), distinct from MSCs, share the dual mechanisms of immune modulatory and tissue reparative properties. Myeloid-derived suppressor cells (MDSCs) that can be granulocytic, monocytic, or early-stage hematopoietic cells have all been infused in preclinical models, although human MDSC trials have not begun.
MSCs
MSCs are MAPCs that can be rapidly expanded in vitro and, under appropriate conditions, differentiate into mesenchymal lineages, including chondrocytes, osteoblasts, and adipocytes.1,2 MSCs reside in most tissues as rare populations,3 although the bone marrow (BM) is the most commonly used source. MSC functional are largely nonimmunogenic and immunosuppressive and can promote tissue repair and hematopoiesis. Absent MHC class II and costimulatory molecules, and low MHC class I expression, predict MSCs are more immunoprivileged and support use across MHC barriers. Recent preclinical studies have suggested that human MSCs home to the splenic marginal zones in vivo and regulate T-cell function therein via prostaglandin E2 (PGE2).4
MSCs use multiple mechanisms to constrain both innate and adaptive immune responses that promote GVHD. MSC-derived interleukin-6 (IL-6) can inhibit dendritic cell (DC) maturation, and transforming growth factor (TGF-β), indoleamine 2,3-dioxygenase (IDO), nitric oxide (NO), and PGE2 released from MSCs attenuate T-cell proliferation.5,6 IDO induces T effector cell apoptosis and regulatory T (Treg) cell differentiation and, through cell contact and PGE2 and IL-10-mediated mechanisms, inhibit helper T (Th)17 differentiation.7-9 MSCs express the checkpoint inhibitor programmed cell death ligand-1 (PD-L1), upregulated by interferon-γ (IFN-γ) produced during GVHD, which enhances their suppressive capacity; MSCs produce soluble PD-L1 and PD-L2.10,11 MSCs limit T effector migration into target tissues by downregulating chemokines (CCL1, CCL3, CCL8, CCL17, and CCL22) and chemokine receptor expression on CD4 T cells (CCR4 and CCR8) and monocytes/macrophages (CCR1).12 Cultured MSC exosomes have been shown to exert immunosuppressive effects on NK, B, and T cells through their CD73, PD-L1, galectin-1, and IL-10 messenger RNA content.13-16
MSCs can contribute to tissue repair through their promotion of angiogenesis, regeneration, and remodeling, through their production of soluble mediators, including connective tissue growth factor, vascular endothelial growth factor-α, keratinocyte growth factor, angiopoietin-1, and stromal derived factor-1.17-19
The clinical safety and feasibility of MSC GVHD therapy were established by Le Blanc et al, who administered haploidentical MSCs to a pediatric patient with severe steroid-refractory GVHD.20 The response was dramatic with a rapid and significant decrease in GVHD symptoms. This study prompted a plethora of case reports and clinical trials using autologous, haploidentical, or third-party HLA mismatched MSCs for steroid-refractory aGVHD.21-28 Results have been mixed with some reporting efficacy, including in a large collaborative European phase 2 study,23 whereas a phase 3, randomized, double-blind study (#NCT00366145), evaluating the commercial MSC product Prochymal, showed no significant difference in clinical outcomes between the placebo control and allogeneic MSC groups. MSC studies to treat established aGVHD in preclinical models also have mixed results (reviewed in Baron and Storb29 ). However, Prochymal was approved for GVHD therapy in pediatric patients30 in the United States, Canada, and New Zealand. The heterogeneous clinical products studied, knowledge gap in biodistribution, persistence, and mechanism(s) of action in suppressing established GVHD in patients and mixed results have meant the use of MSC for the treatment of GVHD has generally not found widespread acceptance in the United States to date.
In cGVHD, 3 single-arm studies demonstrated safety and some benefit, although small patient numbers (n = 4-19) limit interpretation.31-33 MSCs also attenuated murine sclerodermatous cGVHD.12 MSCs appear a safe treatment option of steroid-refractory aGVHD and cGVHD, and although promising, well-designed prospective randomized trials are required to establish efficacy. Although most studies of MSCs have been for the treatment of GVHD, there have also been a number of interventions in the setting of GVHD prophylaxis (ie, prevention). A recent meta-analysis of these trials has been published and could not ascertain clear effects of MSCs infused within 24 hours of transplantation on subsequent engraftment or GVHD.34
MAPCs
Similar to MSCs, MAPCs are nonimmunogenic expanded BM-derived adult stem cells with immunomodulatory, immunosuppressive, and tissue regenerative capacity being explored in GVHD. MAPC exhibit a broader differentiation capacity than MSCs, including mesenchymal, endothelial, and endodermal lineages.35,36 MAPCs have a greater expansion potential, permitting large-scale off-the-shelf products from a single donor, reducing product variability.37,38 MAPCs are able to suppress allogeneic T-cell responses through PGE2 and IDO-mediated suppression of proliferation and Th1, Th22, and Th17 differentiation,39-41 independent of cell contact, Treg, IL-10, or TGF-β.39,40 MAPCs as GVHD prophylaxis significantly decreased murine GVHD,40,41 if the MAPCs were delivered intrasplenically prior to T cells infusion, highlighting the need to ensure MAPCs home to sites of allopriming.40 In a phase 1 dose escalation study using MultiStem, a commercial MAPC product (Athersys, Inc), feasibility and safety were established, and encouraging GVHD outcomes were reported.42
MDSCs
MDSCs are a heterogeneous population of immunosuppressive myeloid cells that undergo systemic expansion during inflammation and cancer. MDSCs were first described in cancer patients, shown to infiltrate tumors and dampen antitumor T-cell responses, identifying their potential for GVHD therapy. MDSC can be fractionated into 3 subsets based on their phenotype: in humans, granulocytic polymorphonuclear leukocyte (PMN)-MDSCs are defined by CD11b+CD14−CD15+, myeloid (M)-MDSC by CD11b+CD14+HLA-DR−/loCD15−, and immature/early MDSC by CD33+ in the absence of lymphoid lineage and HLA-DR antigens.43 Large numbers of functionally suppressive MDSCs are predominantly generated from BM (mouse) or peripheral blood mononuclear cells (human) as a starting source.44,45 Depending on the seeding population, duration of culture, and cytokines used, which are generally those associated with the tumor microenvironment (eg, granulocyte colony-stimulating factor, granulocyte-macrophage colony-stimulating factor, IL-13, IL1β, IL-6), the phenotype and immune proteome of the resultant MDSC are variable.46,47
MDSCs suppress T cells via multiple pathways, including arginase-1, NO, reactive oxygen species (ROS), heme oxygenase-1, TFG-β, and IL-10. MDSCs suppress NK cytotoxicity and B-cell proliferation and promote Tregs. Subsets use distinct, but overlapping, immunosuppressive mechanisms. For example, in mice, PMN-MDSC containing large ROS amounts suppress antigen-specific T cells in a contact-dependent manner, whereas M-MDSC produced NO and arginase-1 and suppress nonspecifically.48 Whether 1 subset holds more potential is unclear; however, on a per-cell basis, M-MDSCs are more potent than PMN-MDSCs, the latter numerically more common in tumors,49 suggesting that M-MDSC may limit GVHD risk while protecting graft-versus-leukemia (GVL) responses.
There are no reports of MDSC infusions in GVHD patients; however, preclinical studies using murine MDSC derived from either embryonic stem cells or adult BM, demonstrated that when coinfused with the stem cell graft, MDSC potently inhibited GVHD without aborting GVL responses.46,47 Embryonic stem cell–derived MDSCs resembled an immature MDSC and expressed inducible nitric oxide synthetase and TGF-β, in contrast to those derived from the BM, which exhibited an M-MDSC phenotype and suppressed GVHD via arginase-1. MDSCs expanded in vivo by granulocyte-macrophage colony-stimulating factor administration alleviated GVHD while maintaining GVL.50,51
Innate immune system cells
Lymphoid members of the innate immune system include natural killer (NK) cells, innate lymphoid cells (ILCs),52 and NKT53 cells that bridge the innate and adaptive immune systems. Such cells are poised for rapid responses to foreign antigens and injured cells.54 Donor NK (reviewed in Simonetta et al55 ), ILC type 2 (ILC2),56 and NKT57 cells each have been infused in preclinical models and shown to reduce GVHD. Donor NK cells also have been tested in the clinic with unclear conclusions as to GVHD effects.55
NK cells
NK cells respond to viral infections, tumors, and alloantigens.58 Donor NK cells are exquisitely sensitive to and fail to mature during GVHD59,60 because of IL-15 consumption by conventional T (Tcon) cells.60 This results in defects in donor NK-dependent pathogen and leukemia-specific immunity. Adoptive NK cell transfer will be most effective in the absence of GVHD and/or with IL-15 administration. Donor-activated murine NK cell transfer reduced aGVHD while preserving GVL.61-63 Donor T cells and to a lesser extent Tregs acquire susceptibility to NK killing in GVHD mice.62 Murine NK cells can eliminate host antigen-presenting cells (APCs), limiting donor T-cell expansion.61 Donor NK cell numerical quantification as well as alloreactivity was associated with reduced GVHD in allo-HSCT patients.55 In some clinical studies, NK cells have been linked to causing aGVHD, although most donor NK cell depletion studies do not support a cause-and-effect relationship with GVHD.55
A comprehensive review summarized outcomes following allogeneic NK cell infusion predominantly used to reduce leukemia burden.55 In 5 studies performed in non-HSCT recipients, none of 132 patients receiving NK cell infusion and lymphodepletion without (n = 119) or with (n = 13) IL-2 developed aGVHD. Seven studies included allo-HSCT patients (n = 116). Fourteen of 86 (16%) recipients of haploidentical or HLA-mismatched NK cells given from day +3 to day +50 posttransplant without exogenous IL-2 developed aGVHD, 8 (9%) of which were severe. No studies have been reported giving donor NK cell infusion post–allo-HSCT with exogenous IL-2. In a pilot study of patients given IL-15/4-1BB ligand-activated NK cells from HLA-matched sibling or HLA-matched unrelated donors from day +7 to day +35, 5 of 9 (55%) experienced aGVHD, 3 (33%) being severe.64 Donor NK cells were found in GVHD biopsies, although T cells may have contributed to GVHD.
Infusion timing may be critical for outcome. When given early post–bone marrow transplantation (BMT) with exogenous IL-2, activated donor NK cells suppressed GVHD, subverted by anti–TGF-β monoclonal antibody, whereas a 3-day delay worsened lethality.63 Conversely, activated NK cells can produce proinflammatory cytokines that can contribute to murine and human GVHD. However, day −8 pre-HSCT haploidentical NK cell infusion with exogenous IL-2 support did not preclude subsequent aGVHD65 ; aGVHD was diagnosed in 7 of 21 (33%), 2 (10%) of which were severe, albeit with an uncertain cause-and-effect relationship.
We conclude that haploidentical NK cells infused into non–allo-HSCT patients did not appear to generate aGVHD, pre–allo-HSCT donor NK cell infusions with IL-2 supplementation do not appear to obviate aGVHD risk, and post–allo-HSCT NK infusions without IL-2 supplementation have a relatively low aGVHD risk, although not directly compared with comparable patients not receiving donor NK cells.
In NK cells, a small heterogeneous population can express CD4 and/or CD8 or neither along with NK-specific molecules (reviewed in Bendelac et al and Godfrey et al53,66 ). NKT cells respond to lipid antigens presented by an MHC-like structure, CD1d with 5 semi-invariant NKT (iNKT) cell and noninvariant NKT cells that have greater T-cell receptor (TCR) diversity. Th1 and Th2 cytokines can be produced in copious amounts, and iNKT cells rapidly respond to limit proinflammatory (eg, IFN-γ; tumor necrosis factor-α) and danger signals by releasing anti-inflammatory Th2 cytokines, suppressing aGVHD in an IL-4–dependent manner.57
In rodents, donor or host CD4+ iNKT cell infusion increased Treg expansion, ameliorating aGVHD without compromising GVL at ratios of 1:20 iNKT/T cell, in contrast to required freshly isolated Tregs/T-cell ratios of 1:1.57 Although third-party iNKT cells were rejected early posttransplant, aGVHD lethality was diminished. In patient donor grafts, higher CD4+ iNKT cell numbers correlated with reduced aGVHD.67 Total lymphoid irradiation conditioning is linked to a low aGVHD incidence, associated with increased host NKT cells and iNKT/T cell ratios,68,69 pointing to higher iNKT cell frequencies as diminishing aGVHD risk. In a cGVHD multiorgan system model with bronchiolitis obliterans, iNKT cells were deficient.70 Purified iNKT cell infusion on days 1 and 14 prevented cGVHD, whereas transfer on days 28 and 42 reversed cGVHD pulmonary and immunological manifestations that were dependent upon donor Tregs but not IL-4.70
Together, these data point to a translational pathway for testing of iNKT cell infusions in patients to prevent or treat aGVHD or cGVHD.
ILCs
ILCs that lack T- and B-cell receptors are poised at mucosal surfaces as rapid pathogen responders.52,54 Among these are group 1 (ILC1, IFN-γ producing; includes NK cells), group 2 (ILC2 IL-4, -5, -13 producing), and group 3 (including lymphoid tissue-inducer and mucosal IL-17 and/or IL-22 producing ILC3s). IL-22 is a pleotropic cytokine with both anti- and proinflammatory properties. It is clear that the endogenous secretion of IL-22 by recipient ILC3 or exogenous IL-22 administration early after BMT can attenuate murine GVHD in the gastrointestinal (GI) tract, an effect postulated to be due to the protection and/or stimulation of intestinal stem cells.71,72 Conversely, IL-22 production from conventional (Th22) cells promotes cutaneous GVHD.73 Much of the focus in allo-HSCT has resided on ensuring high ILC2s, and ILC3s (or their secreted products) are present in the small intestine and colon lamina propria post–allo-HSCT, given their known roles in immune regulation and intestinal injury repair and key role of the gut in aGVHD.74
In patients analyzed 12 weeks after allo-HSCT, circulating ILC2s were significantly decreased.75 Conversely, patients with high levels of activated (CD69+) ILCs pretransplant had reduced aGVHD and mucositis. Other studies revealed decreased ILCs in older individuals and those receiving cord blood transplants or nonmyeloablative conditioning.76 In allo-HSCT mice, lower GI tract ILCs but not lung ILC2s were depleted by chemo- or radiotherapy conditioning regimens and slow to repopulate.56 Impaired donor-derived gut ILC2 was observed.56 Ex vivo expanded donor murine ILC2 infusion ameliorated GVHD when given on day 0 or day 7 as therapy, diminishing gut injury, reducing donor Th1 and Th17 cells, increasing ILC2-derived IL-13-induced MDSCs, and providing ILC2-derived amphiregulin for tissue repair.56 Multiple donor ILC2 infusions were superior in reducing aGVHD lethality, while retaining GVL.
Lamina propria CCR6+NKp46-IL7Rα+ ILC3 (lymphoid tissue-inducer–like) cells secrete IL-22, support intestinal stem cell epithelial regeneration,72 and remain predominantly host derived up to 3 months post–allo-HSCT.71 Because GVHD depletes murine gut ILC3s,71 infusion of either donor ILC3s that may confer tissue regenerative, antimicrobial and DC tolerogenic properties, or common ILC precursors77,78 that can differentiate into ILC1s, ILC2s, and ILC3s deserve preclinical and future clinical testing.
Adaptive lymphoid cells
T cells with regulatory capability are a diverse group of cells that for the purposes of this review bear αβ TCRs and suppress Tcon responses. The best studied are CD4 T cells expressing high levels of CD25 (high-affinity IL-2 receptor) and transcription factor FoxP3 (in response to TGF-β and TCR). FoxP3 can be stably expressed at an early stage of thymic development (so-called natural or thymic Treg referred to hereafter as nTreg), or induced after activation in vitro or in the periphery (referred to as induced Tregs, iTregs). FoxP3 is stably expressed in a demethylated state in nTreg but characteristically methylated and unstable in iTreg. By nature of their TCR expression, nTreg and iTreg recognize and respond to specific antigens within MHC class II. In allogeneic BMT and GVHD, a failure of Treg development occurs due to impaired thymopoiesis and propensity to death in the periphery,82 which promotes aGVHD and particularly cGVHD, the latter a result of an acquired impairment in antigen presentation in the periphery.83 Transfer of nTreg can prevent aGVHD84,85 and reverse cGVHD in preclinical systems.83,86 In contrast, iTreg can be phenotypically unstable when infused after transplant and loose FoxP3 expression,87,88 making them unreliable when used prophylactically to prevent GVHD, although this may be partially overcome by the coadministration of IL-2 and rapamycin.88
Translation to the clinic is difficult; Tregs represent only 2% to 10% of CD4 T cells in peripheral blood, and the transfer of meaningful numbers likely requires in vitro expansion protocols that are expensive and technically challenging. Nevertheless, good manufacturing process grade, large-scale nTreg expansion has been demonstrated,89 and studies infusing third-party, umbilical cord blood–derived nTreg as a component of GVHD prophylaxis demonstrate promising results, with very low rates of GVHD.90,91 The infusion of nTregs (with or without IL-2 administration), that are selected directly from donors, is being trialed at several sites for the treatment of cGVHD. These expansion protocols primarily use polyclonal anti-CD3/28 antibody-based expansion protocols, and the feasibility and activity of using recipient APC to antigen-specific products remain unknown. Insertion of chimeric-antigen receptors specific for alloantigen (eg, recipient HLA-A2) represents an interesting and testable new approach to achieve this, especially in solid organ transplant recipients.92
At this time, nTreg infusions represent the most promising cellular therapy for preventing and treating GVHD. The effect of nTreg on GVL has varied in preclinical systems from complete neutrality (ie, no impairment) to clear impairment, depending on the model and malignancy used (eg, cell line or primary leukemia, requirement for CD4 help, MHC expression, etc).84,88,93,94 The mechanisms by which Treg may attenuate GVHD include release of regenerative cytokines (eg, amphiregulin),95 APC function inhibition (eg, via CTLA4), and the inhibition of Tcon by the release of inhibitory molecules (eg, adenosine, TGF-β, IL-35, and IL-10)96 and/or IL-2 consumption.97
A second Treg type is iTregs, a subset of which is CD8+ and HLA class I restricted.88,98 CD8 iTregs do not express FoxP3 at steady state but can do so after intense stimulation in vivo,99,100 or in vitro in the presence of IL-2 and TGF-β.88,98 Although these CD8 iTregs can suppress Tcon responses in vitro, they are inherently unstable after BMT and can revert to conventional Th1 cells to exacerbate GVHD in murine systems.88 Some investigators have used humanized GVHD systems to suggest these cells may be used as an approach to modulate GVHD in the clinic,98 but the limitations of the preclinical model systems (reviewed in Markey et al101 ) make the ability to translate these findings less clear. Other CD8 Tregs have been described that are FoxP3 negative and specific for HLA-E (Qa-1 in mice),102 but have yet to be tested in GVHD.
A Treg subset producing high levels of IL-10 but FoxP3 negative is known as type-1 regulatory T (Tr1) cells.103 These cells characteristically secrete IFN-γ. The characterization of these cells has been difficult, but recent studies in mice and humans demonstrate that these cells are generated in response to potent alloantigen stimulation by recipient DC in the context of IL-27, secreted predominantly from donor monocytes-macrophages.104 Tbet and blimp-1 transcription factors are required in the initiation phase of these cells, but the stable persistence of Tr1 is dependent on Eomes.104 In aGVHD, where donor nTregs are profoundly deficient, Tr1 becomes the dominant Treg population and Tr1 deficiency exacerbates GVHD.104 Whether Tr1 can be effectively used in transfer studies has begun testing105 and will likely expand as new information allows the production of pure and stable populations.
As the cytokines controlling both Treg and Tr1 are increasingly understood, attempts are being made to modulate this environment to promote Treg expansion in vivo, with or without concurrent Treg transfer. This includes providing exogenous growth factor support in the form of IL-2,106,107 and new approaches to better target IL-2 to Treg are likely to begin clinical testing shortly. A second approach, involving tumor necrosis factor receptor-2 agonists, has been shown to be useful in expanding recipient rather than donor Treg to attenuate aGVHD.93 Alternatively, inhibition of cytokines that are known to subvert Treg differentiation108 is in clinical trials with IL-6 inhibition being foremost.109 IL-6 inhibition appears to enhance both Treg and Tr1 expansion, the latter by increasing sensitivity to IL-27, whose receptor shares gp130 subunits with that for IL-6.104 Unfortunately, IL-27 also has proinflammatory properties after BMT, making it unsuitable as treatment.110
Challenges and future directions
Table 1 summarizes and Figure 1 illustrates the range of cell infusions assessed for GVHD prevention or therapy in mice and humans. Excluding NK cells given to treat residual tumor burden, only MSCs and thymus-derived CD4 Tregs have been reported in multiple clinical trials that have assessed safety and GVHD, although in a phase 1 trial, MAPCs had a favorable safety profile, and at the highest dose level tested, a low incidence of aGVHD (1/9, 11%), limited to grade 2. Therefore, we will briefly review challenges for MSC and Treg cell therapies for ameliorating GVHD.
Selected preclinical and clinical use of cellular therapies in GVHD
| Cell . | Species mouse (m)/human (h) . | Origin recipient (r)/donor (d) . | GVHD . | GVL . | Reference (examples) . |
|---|---|---|---|---|---|
| nTreg | m h | d; r; 3rd party | ↓↓↓ | 83,84,-86,88 | |
| ↓↓/ongoing | → | 84,85 | |||
| ↓ | 88 | ||||
| Ongoing | 90 | ||||
| iTreg (CD4) | m h | d | ↑ | 88 | |
| ↓ | ↓ | 88,94 | |||
| → | Ongoing | 87 | |||
| Ongoing | 88,94 | ||||
| iTreg (CD8) | m | r | ↑ | 88 | |
| ↓ | ↑ | 94,98 | |||
| → | 94 | ||||
| 98 | |||||
| Tr1 | m h | d | ↓ | 104 | |
| Ongoing | ND | 105 | |||
| Ongoing | |||||
| ILC2 | m | d | ↓↓ | 56 | |
| → | 56 | ||||
| NK | m h | D | ↓ | 61,-63 | |
| → | ↑ | 61,63 | |||
| ↑ | → | 62 | |||
| ↑ | 119,120 | ||||
| 64,121 | |||||
| 120,121 | |||||
| iNKT | m | d, 3rd party | ↓↓ | 57,70,122 | |
| → | 57,122 | ||||
| MDSC | m | d | ↓↓ | 46,47,50,51 | |
| → | 47,51 | ||||
| MSC | m h | d d, 3rd party | ↓↓ | 12,29 | |
| → | ND | 20,,,,,,,-28,31,-33 | |||
| ND | |||||
| MAPC | m h | d 3rd party | ↓↓ | 40,41 | |
| ↓↓ | ND | 42 | |||
| ND |
| Cell . | Species mouse (m)/human (h) . | Origin recipient (r)/donor (d) . | GVHD . | GVL . | Reference (examples) . |
|---|---|---|---|---|---|
| nTreg | m h | d; r; 3rd party | ↓↓↓ | 83,84,-86,88 | |
| ↓↓/ongoing | → | 84,85 | |||
| ↓ | 88 | ||||
| Ongoing | 90 | ||||
| iTreg (CD4) | m h | d | ↑ | 88 | |
| ↓ | ↓ | 88,94 | |||
| → | Ongoing | 87 | |||
| Ongoing | 88,94 | ||||
| iTreg (CD8) | m | r | ↑ | 88 | |
| ↓ | ↑ | 94,98 | |||
| → | 94 | ||||
| 98 | |||||
| Tr1 | m h | d | ↓ | 104 | |
| Ongoing | ND | 105 | |||
| Ongoing | |||||
| ILC2 | m | d | ↓↓ | 56 | |
| → | 56 | ||||
| NK | m h | D | ↓ | 61,-63 | |
| → | ↑ | 61,63 | |||
| ↑ | → | 62 | |||
| ↑ | 119,120 | ||||
| 64,121 | |||||
| 120,121 | |||||
| iNKT | m | d, 3rd party | ↓↓ | 57,70,122 | |
| → | 57,122 | ||||
| MDSC | m | d | ↓↓ | 46,47,50,51 | |
| → | 47,51 | ||||
| MSC | m h | d d, 3rd party | ↓↓ | 12,29 | |
| → | ND | 20,,,,,,,-28,31,-33 | |||
| ND | |||||
| MAPC | m h | d 3rd party | ↓↓ | 40,41 | |
| ↓↓ | ND | 42 | |||
| ND |
ND, not determined.
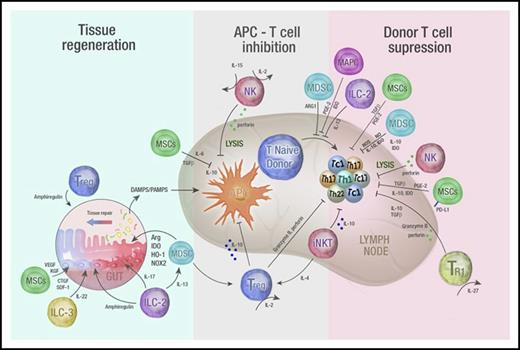
Immune regulatory cell therapy to prevent and treat GVHD. A schematic overview of mechanisms by which infused regulatory populations promote tissue regeneration and inhibit T-cell priming and differentiation to attenuate GVHD. Infused regulatory cells may prevent and/or treat GVHD by acting in 3 distinct phases. First, they may promote tissue regeneration following damage by conditioning (when used prophylactically) or from GVHD (when used as treatment). Intermediate secreted molecules from both adaptive and innate cells are highlighted and include amphiregulin, cytokines important in GI tract homeostasis such as IL-22 and IL-17, angiogenic factors such as connective tissue growth factor (CTGF), vascular endothelial growth factor (VEGF), and keratinocyte growth factor (KGF) as well as regulators of inflammatory responses such as arginase, IDO, heme oxygenase-1 (HO-1), and the reduced NAD phosphate oxygenase isoform (NOX2). Second, regulatory cells may inhibit the APC–T-cell interaction via the release of immunosuppressive cytokines (such as IL-10, TGF-β) or by directly lysing APCs themselves (eg, NK cells). Finally, regulatory populations may suppress donor T-cell differentiation and expansion via a number of secreted immunosuppressive molecules (eg, IL-10, TGF-β, IDO, NO, and ROS) or by directly lysing T cells (NK cells). DAMPS, damage-associated molecular patterns; PAMPS, pathogen-associated molecular patterns; SDF, stromal derived factor-1.
Immune regulatory cell therapy to prevent and treat GVHD. A schematic overview of mechanisms by which infused regulatory populations promote tissue regeneration and inhibit T-cell priming and differentiation to attenuate GVHD. Infused regulatory cells may prevent and/or treat GVHD by acting in 3 distinct phases. First, they may promote tissue regeneration following damage by conditioning (when used prophylactically) or from GVHD (when used as treatment). Intermediate secreted molecules from both adaptive and innate cells are highlighted and include amphiregulin, cytokines important in GI tract homeostasis such as IL-22 and IL-17, angiogenic factors such as connective tissue growth factor (CTGF), vascular endothelial growth factor (VEGF), and keratinocyte growth factor (KGF) as well as regulators of inflammatory responses such as arginase, IDO, heme oxygenase-1 (HO-1), and the reduced NAD phosphate oxygenase isoform (NOX2). Second, regulatory cells may inhibit the APC–T-cell interaction via the release of immunosuppressive cytokines (such as IL-10, TGF-β) or by directly lysing APCs themselves (eg, NK cells). Finally, regulatory populations may suppress donor T-cell differentiation and expansion via a number of secreted immunosuppressive molecules (eg, IL-10, TGF-β, IDO, NO, and ROS) or by directly lysing T cells (NK cells). DAMPS, damage-associated molecular patterns; PAMPS, pathogen-associated molecular patterns; SDF, stromal derived factor-1.
MSCs most often are generated from donor BM, separated by density gradient centrifugation, cultured with fetal bovine serum, and plated in flasks at a prespecified density. Once a minimum confluence level is reached, cells are harvested with trypsin/EDTA, replated, reharvested often after 2 to 3 passages, and frozen. With multiple manipulations, decision-making process during culture, and prescreened serum, it would not be surprising that MSC batches may have distinct characteristics. Of particular importance are cell density, confluence, sera batches, and replating techniques. Minimal criteria for defining include plastic adherence, CD105, CD73, and CD90 expression and lack of hematopoietic markers, together with osteoblast, adipocyte, and chondroblast differentiation capacity in vitro.111 Thus, it is essential that potential product variability be taken into account when evaluating outcomes. Although there is an extremely low risk of malignant transformation, assays should be employed to determine that the final product does not have premalignant or malignant features.112 Because MSCs typically are undetectable in vivo, mechanistic investigations at the tissue level are difficult to obtain, and such data would be highly useful to best understand the context and conditions in which MSCs are likely to be most successful.
A major Treg challenge is obtaining high-level purification from an infrequent starting population in peripheral blood or cord blood using good manufacturing process–compatible approaches. Although magnetic bead sorting for CD4+25hi cells or cell-sorter enrichment may incorporate CD127lo/neg, if purity is inadequate, CD25+ or CD25− T effectors can cause Tregs to lose suppression because residual Tcon cells have a substantial proliferative advantage over Tregs during ex vivo expansion. Although freshly sorted Tregs have been infused into allo-HSCT patients,113 when larger numbers are required, Tregs are ex vivo expanded using anti-CD3/28 antibody-coated beads or anti-CD3 antibody-loaded cell-based artificial APCs and high IL-2 concentration. Restimulation markedly increases yield within 12 to 18 days.89 Although surface phenotyping quantifies final purity, functional suppression is key for quality control, although such assays have an inherent variability, and results of a proliferation assay are often available only after Treg infusion. Furthermore, Tregs temporarily lose suppressor function upon freeze/thaw that can be restored after overnight IL-2 exposure, complicating the utilization of frozen Tregs that could be stored in a readily available cell bank.
Under inflammatory conditions in rodents, infused Tregs may convert into T effectors,114 although plasticity has not been observed to date in human trials. Treg tissue localization may prove critical to achieving high-level GVHD prevention or therapy to ensure Treg cell contact-dependent suppression or secretion of soluble factors juxtaposed to T effectors. In allo-HSCT patients, Tregs may rapidly disappear from peripheral blood, often ascribed to lack of IL-2 released by T effectors that have been suppressed or use of drugs (eg, cyclosporine) that inhibit IL-2 production.90 Steroids or broadly reactive T-cell–depleting antibodies used for GVHD prevention, or therapy may deplete infused Tregs. Immune suppressive populations may result in increased viral or fungal infection or higher relapse rates as a result of suppressed in vivo T effector function expansion and activation. However, neither relapse rates nor viral or fungal infections have been observed to be increased in the small studies reported to date compared with historical controls,90,91 with the exception of 1 study that revealed an increase in the density of opportunistic infection in the first 1 month with a trend toward decreased opportunistic infection from 1 to 6 months after allo-HSCT.115 Notably, the density of opportunistic infections in a subsequent study infusing a higher number of Tregs was found not to be significantly different compared with contemporary non-Treg patient controls.91 For acute myeloid leukemia and MDS patients receiving haploidentical HSCT with supplemental freshly isolated donor Tregs, relapse rates were significantly lower than historical controls, likely due to very low aGVHD coupled with the ability to provide high T effector numbers at allo-HSCT.116 Future prospective randomized studies will be needed to accurately assess the effect of Treg infusion on relapse rates and opportunistic infections.
Promising cell therapies for future development can be based upon preclinical (MDSCs, NKT cells, ILC2s, CD8 Tregs) or early clinical results (MAPCs, Tr1). Of these, only a phase 1 GVHD prevention study of allogeneic IL-10 anergized T cells enriched for Tr1 cells is currently registered on clinicaltrials.gov for testing. Challenges to overcome include loss of MDSC function upon inflammasome activation, low NKT cell frequency, and the need to optimize ILC2 and CD8+ Treg manufacturing before clinical applications.
Over the past ∼10 years, murine and human NK cells with memory cell characteristics such as antigen-specific function, recall responses, and long-term persistence have been identified after hapten or virus exposure, especially cytomegalovirus.58,117 In mice and humans, prior cytomegalovirus exposure results in clonal expansion leading to increased frequencies of NK memory cells. Inflammatory cytokines such as IL-12 and IL-18 can imbue NK cells with longevity and continued effector function. Altering the metabolic profile of conventional NK cells can drive NK memory cell conversion.118 Clinical studies of NK memory cell infusion to treat relapse in non–allo-HSCT patients have begun. Whether NK memory cells will prove to have distinct functions that might positively or negatively influence GVHD remains unknown.
Combining or sequentially delivering products will likely increase efficacy via any nonredundant mechanisms, differential homing or persistence, and functional retention. The latter may be accomplished by identifying conditioning and GVHD prevention or therapy regimens most compatible with a product or genetically modifying products to optimize their efficacy. Combinatorial approaches with proteins, antibodies, small molecules, or drugs that stimulate tissue repair may increase the therapeutic efficacy of cell therapies. Master cell bank generation for a variety of cell products, as has been done with MSCs, should reduce costs. Last, incorporating laboratory analyses such as biomarkers or transcriptomics may provide insights into which cell products at which time periods are optimal for infusion.
Acknowledgments
The authors thank members of their laboratories, their collaborators, and the scientific community for providing the foundation for this review. They also thank Madeleine Flynn for her excellent graphical support. The authors apologize to those investigators whose work they were unable to cite here.
This work was supported by the Australian National Health and Medical Research Council grant APP1031728 (K.P.A.M.), the National Institutes of Health (NIH) National Institute of Allergy and Infectious Diseases grant R37 AI34495, National Heart, Lung, NIH Blood Institute grants R01 HL56067 and HL11879, and NIH National Cancer Institute grants P01 CA142106 and P01 CA065493. G.R.H. is an NH&MRC Senior Principal Research Fellow.
Authorship
Contribution: B.R.B., K.P.A.M., and G.R.H. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bruce R. Blazar, MMC 109, University of Minnesota, 420 Delaware St SE, Minneapolis, MN 55455; e-mail: blaza001@umn.edu; and Geoffrey R. Hill, QIMR Berghofer Medical Research Institute, 300 Herston Rd, Herston, Queensland 4006, Australia; e-mail: geoff.hill@qimrberghofer.edu.au.
REFERENCES
Author notes
B.R.B. and G.R.H. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal