Abstract
Immune-based therapy has emerged as a paradigm shift in cancer therapy with dramatic responses observed in previously incurable disease. Cancer vaccines are being developed to disrupt tumor-associated tolerance and activate and selectively expand tumor-specific lymphocytes within the native effector cell repertoire while maintaining immune-regulatory protection against autoimmunity. Although individual antigen approaches result in immune response with a suggestion of clinical effect in some settings, broader efficacy may be dependent on presentation of multiple antigens that capture clonal diversity presented in the context of functionally potent antigen-presenting cells. The use of whole cell–based strategies such as dendritic cell/tumor fusions have yielded provocative results in single-arm studies and are currently being explored in multicenter randomized trials. The posttransplant setting is a potentially promising platform for vaccination due to cytoreduction and relative depletion of inhibitory accessory cells fostering greater immune responsiveness. Integration of these efforts with other immunotherapeutic strategies and agents that target the tumor microenvironment is being studied in an effort to generate durable immunologic responses with clinically meaningful impact on disease.
Introduction
The unique potency of immune effector cells to target and eliminate hematologic cancers is supported by the observation that allogeneic transplantation is curative for a subset of patients due to the graft-versus-disease effect mediated by alloreactive lymphocytes and natural killer (NK) cells.1 The critical role of cell-mediated immunity has been highlighted by the association of relapse with T-cell depletion of the graft and, conversely, the capacity of donor lymphocytes to effectively target relapsed disease in the setting of posttransplant relapse. In addition, protection from relapse has been associated with the emergence of donor T cells targeting tumor-associated antigens. However, the lack of specificity of the alloreactive response results in significant toxicity due to targeting of normal tissues and the resultant graft-versus-host disease (GVHD).2 The development of strategies to effectively repair and harness host immunity to more selectively target malignant cells is predicated on reversing critical aspects of the immunosuppressive milieu that characterizes the tumor microenvironment.
Immune surveillance provides a critical barrier of protection against the development and progression of malignancies.3 Tumor cells bear unique antigens that may be recognized by the immune repertoire. However, disease evolution is supported by the development of an immunosuppressive milieu in the tumor microenvironment that favors tolerance resulting in immune escape.3,4 Immune editing of the malignant clones fosters the emergence of clonal populations with decreased immunogenicity. Dendritic cells (DCs) in the tumor bed exhibit functional deficiencies and a tolerizing phenotype through the blunting of maturation, the increased expression of negative costimulation and indoleamine 2,3-dioxygenase (IDO), and the secretion of cytokines that promote the expansion of inhibitory cells.5-7 Of note, in multiple myeloma, the interaction between DCs and plasma cells in the microenvironment results in a decrease in proteasome activity and reduced antigen presentation by the malignant plasma cell population.8 Regulatory T cells, M2 macrophages, and myeloid derived suppressor cells (MDSCs) in the bone marrow niche blunt the activation and expansion of functionally competent effector cells.9-11 Increased expression of CD47 is thought to disrupt macrophage-mediated phagocytosis and to facilitate the liberation of antigen in the context of inflammation. CD73 is a cell surface enzyme that catalyzes adenosine monophosphate (AMP) and whose increased expression in the tumor microenvironment by malignant hematopoietic cells and antigen-presenting cells is associated with immune suppression. Increased expression of negative checkpoints including PD-1, Tim3, and LAG3 mute potent antitumor immunity.12-14 The effective design of vaccination strategies for hematological malignancies is dependent on enhanced antigen presentation to induce the expansion of tumor-specific effector cells while overcoming the immunosuppressive milieu of the tumor microenvironment that blunts immune response.
Cancer vaccines represent a fundamental immunotherapeutic approach to harness the potency of immune effector cells in targeting malignant cells. The underlying principle is the enhanced presentation of tumor-associated epitopes to host immunity in a context of immune activation such that tumor-specific tolerance is broken while maintaining mechanisms of immune regulation against autoimmunity. Initial observations by Coley at the end of the 19th century noted anecdotal profound tumor regression in response to immune-mediated sequelae of inflammatory stimuli created by infection.15 In the modern era, efforts to induce antitumor immunity via vaccination were initially reliant on the introduction of individual peptide or protein antigens in the context of immune adjuvants in an effort to induce tumor-specific immunity. Although antigen-specific immune responses were often elicited, clinical efficacy was uncertain, blunting enthusiasm for this treatment approach. A growing appreciation emerged of the role of the immunosuppressive milieu of the tumor microenvironment in mediating tumor immune escape including the loss of functional competency of antigen-presenting and effector cells and the presence of soluble factors, negative costimulatory signals, and accessory cells that promote tolerance. Concurrently, there was increased understanding of the complexity of clonal diversity of hematologic malignancies, including the presence of critical subpopulations such as the leukemia stem cell that exhibit distinct patters of antigen expression that would need to be captured by immune therapy.
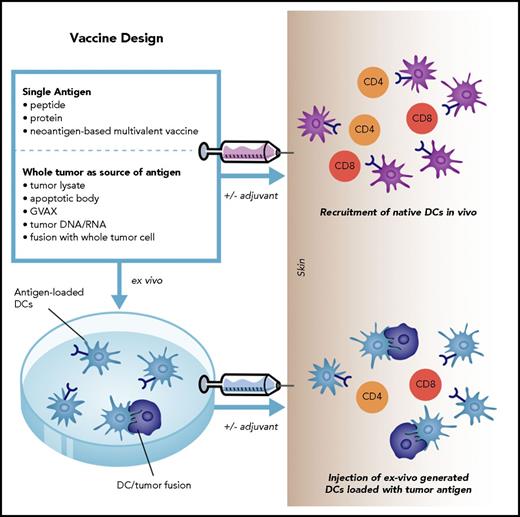
The development of an effective vaccine is predicated on the selective expansion of functionally competent tumor-specific lymphocytes with the capacity to migrate into the tumor bed, capturing the breadth of clonal diversity and evoking of a memory response protective against disease relapse. Efficacy of vaccination is dependent on the restoration of the competence of the native effector cell repertoire and reversing key elements of the immunosuppressive milieu. In this context, it is vital to provide an appropriate threshold for durable activation of tumor-specific lymphocytes while preserving the equilibrium between activation and tolerance such that immune-mediated damage to normal tissue is mitigated. As such, the fundamental aspects of vaccine design include (Figure 1):
Defining the optimal antigenic target(s)
Developing vaccine platforms for providing effective and durable costimulation
Determining the appropriate setting in which to vaccinate
Defining the immunomodulatory approaches to effectively target the tumor microenvironment to prevent extinguishing of T-cell activation and the reestablishment of tolerance
Fundamental aspects of vaccine design include defining the optimal antigenic target and providing effective and durable costimulation. Antigens may be administered in the form of individual peptides, proteins, or whole tumor cells derived DNA, RNA, cell lysate, administered alone or in conjunction with adjuvants. These vaccines rely on native antigen-presenting cells to uptake and present antigen to effector cell populations. Alternatively, vaccines can be generated by loading antigens onto ex vivo–generated DCs to overcome phenotypic and functional deficiencies of native antigen-presenting cells in patients with malignancy. GVAX, gene-transduced tumor cell vaccine.
Fundamental aspects of vaccine design include defining the optimal antigenic target and providing effective and durable costimulation. Antigens may be administered in the form of individual peptides, proteins, or whole tumor cells derived DNA, RNA, cell lysate, administered alone or in conjunction with adjuvants. These vaccines rely on native antigen-presenting cells to uptake and present antigen to effector cell populations. Alternatively, vaccines can be generated by loading antigens onto ex vivo–generated DCs to overcome phenotypic and functional deficiencies of native antigen-presenting cells in patients with malignancy. GVAX, gene-transduced tumor cell vaccine.
Identifying the optimal antigenic targets
Tumor-associated antigens have been identified for hematologic malignancies that serve as potential targets for vaccination. These include (1) antigens specific to the malignant clonal population (eg, idiotype derived peptides in lymphoma and multiple myeloma,16-18 bcr/abl fusion protein in chronic myelogenous leukemia [CML]19 ); (2) cancer testis antigens whose normal expression is restricted to fetal development (eg, NY-ESO, MAGE1, MAGE 3 in leukemia and multiple myeloma20-22 ); (3) antigens derived from aberrantly expressed by early hematopoiesis oncogenes (eg, WT1 MUC1 in leukemia23,24 ); and (4) antigens restricted to lineage-specific development (eg, CD138, BCMA, CD138, and CS1 in multiple myeloma20 ). An ideal antigen to immunize against would be one that is: uniformly expressed by the malignant clone; vital to the biology of the cell such that downregulation in the setting of immunologic pressure would not be readily seen; highly immunogenic; and specific to the malignant clone, limiting off-target toxicity. Vaccine strategies involving peptide-based antigens have been pursued in the setting of multiple myeloma, lymphoma, and acute myeloid leukemia (AML).
Single antigen peptide– and protein-based vaccines
Peptide-based approaches are highly feasible and preserve tumor selectivity, minimizing the risks for autoimmunity. Vaccines are administered in the context of immune adjuvants that recruit and activate native DCs to the vaccine bed, resulting in the crosspresentation of antigen by the infiltrating DC populations.25,26 This carries the potential advantage of a coordinated response between native and adaptive immune mechanisms with the appropriate tools for generating an immune synapse and chemotaxis both to site of stimulation and effector cell function. One potential concern with peptide-based vaccine approaches is the development of immune escape due to the downregulation of the target antigen. One strategy that has been evaluated to overcome this limitation is the use of multipeptide vaccines that would generate a polyclonal response.23,27 However, the list of known shared antigens is limited and their immunogenicity is uncertain. As tumor-associated antigens are self in origin, potentially reactive high-affinity T cells with greatest efficacy are deleted by the thymus during development by central tolerance.28
An alternative vaccination strategy involves the use of DNA or RNA encoding shared tumor antigens. Administration may be enhanced through the use of a gene gun in which DNA/RNA is loaded onto heavy metal–coated nanoparticles that facilitate entry into native antigen-presenting cells such as Langerhans cells.29 Vaccine potency may be augmented through the presence of bacterial plasmids or CPG or POLY IC sequences that ligate Toll-like receptors (TLRs) and facilitate immune activation.30,31
In myeloid malignancies, peptide-based vaccine strategies targeting BCR/ABL, WT1, and PR1 have been studied in clinical trials. Some of the earliest data evaluating peptide-based vaccine approaches in myeloid malignancies studied peptides derived from bcr/abl fusion proteins in CML. In 1 clinical study, a bcr/abl–derived fusion peptide vaccine was administered to 14 patients with CML, evoking a bcr/abl peptide-specific T-cell immune response.19 Several groups have evaluated peptide-based vaccine approaches in AML and myelodysplastic syndrome (MDS). In a review of data from 9 clinical trials published between 2004 and 2012 in which patients with AML or MDS were treated with WT1 peptide vaccination, vaccination was found to be well tolerated and resulted in the induction of WT1-specific T cells, as assessed by tetramer analysis in 4 clinical trials.24,32-35 Evidence of clinical effect was observed in a subset of patients, as demonstrated by a reduction in WT1 messenger RNA (mRNA) transcript levels, prolonged remission, and resolution of bone marrow blasts. Immune response to WT1-based vaccines is variable, as evidenced by results of a phase 1 clinical trial in which patients with AML and MDS were vaccinated with a polyvalent WT1 peptide vaccine without evidence of durable immune response following vaccination.36 In a recent report of 66 patients with AML (42 patients), CML, or MDS who were treated with a PR1 peptide vaccine, immune response, as defined by at least a twofold increase in the percentage of PR1-cytotoxic T lymphocytes in the blood, was observed in 53% of patients.37 Clinical response was observed in 23% of patients with active disease. Interestingly, clinical response was predominantly noted in patients with low disease burden (<10% blasts in the marrow), and correlated with immune response.37
In patients with lymphoma, antigen-based vaccine approaches targeting idiotype have demonstrated immune response, and promising clinical outcomes in several phase 2 clinical trials.17,38,39 In 1 study in which patients received anti-idiotype vaccination following second-line chemotherapy for relapsed disease, it was demonstrated that the duration of second remission following chemotherapy and vaccination was longer than the duration of first remission following upfront chemotherapy alone.17 However, results from 3 large randomized phase 3 randomized clinical trials did not demonstrate a survival advantage in vaccinated patients.40,41 In the Genitope study, 287 patients with newly diagnosed follicular lymphoma were randomized to receive a recombinant idiotype vaccine (MyVax) or observation following 8 cycles of chemotherapy. The primary end point of the study was progression-free survival (PFS), which was not improved with vaccination. It was noted, however, that patients who mounted an anti-idiotype immune response did demonstrate an improvement in PFS.40 The Favrille study evaluated the FavId idiotype-based vaccine or control following rituximab (Rituxan) treatment in patients with follicular lymphoma. The study did not meet its primary end point of improvement in time to progression with vaccination.41 A third large randomized trial of idiotype vaccination in patients with follicular lymphoma studied BiovaxId in 177 patients who achieved remission following chemotherapy.42 The study did not demonstrate an improvement in disease-free survival (DFS) in an intention to treat analysis. Notably, 60 of the patients randomized toccination did not receive vaccine. When the group of patients who received vaccine was analyzed and compared with the control group, an improvement in DFS was observed (44.2 months vs 30.6 months, P < .05).42 In multiple myeloma, approaches targeting MUC1, idiotype, and BCL2 family proteins have each been studied in phase 1 clinical trials demonstrating safety and immune response in a subset of patients.18,43,44
In summary, results from several clinical studies evaluating single antigen–based vaccines have demonstrated immune response and evidence of clinical effect in a subset of patients (Table 1). However, a clearly defined impact on disease outcome has not yet been documented. Potential limitations include lack of immunogenicity of the antigen, reliance on native antigen-presenting cells with functional deficiencies, the restriction to a particular HLA phenotype in the setting of peptide vaccination, and the possibility of immune evasion by the tumor`s ability to downregulate expression of a particular antigen. Despite these potential limitations, evidence of clinical potency of peptide-based vaccine approaches is emerging in AML patients, particularly in those with low burden of disease.
Peptide- or protein-based vaccines, without exogenous antigen-presenting cell
| Disease . | Peptide/protein . | N . | Immune response . | Clinical response . | Reference . |
|---|---|---|---|---|---|
| AML/MDS | WT1 | 51 reviews of data from 9 clinical trials | 2.4-fold expansion of WT1-specific T cells. Immune response analyzed from 4 trials (n = 23) in which WT1-specific expansion was assessed without ex vivo stimulation. | In each of 9 clinical trials, clinical responses were observed, including a patient who remained in CR for >8 y in 1 study. | 24 |
| AML/ MDS | WT1 | 16 | Significant expansion of WT1 tetramer-positive CD8 T cells was not observed. | 1 of 2 MDS patients demonstrated decrease in transfusion requirement and a transient reduction in blasts. | 36 |
| Median time to recurrence in 14 AML patients was 244 d. | |||||
| AML/MDS CML | PR1 | 66 | Doubling of PR1 tetramer-positive T cells in 53% of patients | 24% clinical response (8 patients CR; 1 patient PR; 3 patients demonstrated hematologic improvement) | 37 |
| Multiple myeloma | Idiotype DNA vaccine linked to FrC of tetanus toxoid | 15 | 71% immune response to FrC | Median TTP 38 mo | 18 |
| 29% immune response to idiotype | OS 64% after median follow-up of 85.6 mo | ||||
| Multiple myeloma | Peptides derived from Bcl-2 family proteins with montanide | 7 | Immune response noted in all 6 patients who received more than two vaccines | No disease regression | 43 |
| Follicular lymphoma | Idiotype MyVax, randomized trial | 287 patients | Anti-idiotype immune response was associated with longer PFS | Median PFS 19.1 mo, not a statistically significant difference from control group | 40 |
| Follicular lymphoma | Idiotype vaccine Favrille study, randomized trial | 349 patients | Immune response was not assessed | Median TTP was 9 mo in vaccine arm vs 12.6 mo in control (P = .019). The shorter TTP in the vaccine arm was speculated to relate to differences in FLIPI scores. | 41 |
| Follicular lymphoma | Idiotype Biovaxid | 234 patients | Immune response was not reported | Median DFS was significantly higher in vaccinated patients (44.2 mo) compared with control (30.6 mo; P = .05) | 42 |
| Disease . | Peptide/protein . | N . | Immune response . | Clinical response . | Reference . |
|---|---|---|---|---|---|
| AML/MDS | WT1 | 51 reviews of data from 9 clinical trials | 2.4-fold expansion of WT1-specific T cells. Immune response analyzed from 4 trials (n = 23) in which WT1-specific expansion was assessed without ex vivo stimulation. | In each of 9 clinical trials, clinical responses were observed, including a patient who remained in CR for >8 y in 1 study. | 24 |
| AML/ MDS | WT1 | 16 | Significant expansion of WT1 tetramer-positive CD8 T cells was not observed. | 1 of 2 MDS patients demonstrated decrease in transfusion requirement and a transient reduction in blasts. | 36 |
| Median time to recurrence in 14 AML patients was 244 d. | |||||
| AML/MDS CML | PR1 | 66 | Doubling of PR1 tetramer-positive T cells in 53% of patients | 24% clinical response (8 patients CR; 1 patient PR; 3 patients demonstrated hematologic improvement) | 37 |
| Multiple myeloma | Idiotype DNA vaccine linked to FrC of tetanus toxoid | 15 | 71% immune response to FrC | Median TTP 38 mo | 18 |
| 29% immune response to idiotype | OS 64% after median follow-up of 85.6 mo | ||||
| Multiple myeloma | Peptides derived from Bcl-2 family proteins with montanide | 7 | Immune response noted in all 6 patients who received more than two vaccines | No disease regression | 43 |
| Follicular lymphoma | Idiotype MyVax, randomized trial | 287 patients | Anti-idiotype immune response was associated with longer PFS | Median PFS 19.1 mo, not a statistically significant difference from control group | 40 |
| Follicular lymphoma | Idiotype vaccine Favrille study, randomized trial | 349 patients | Immune response was not assessed | Median TTP was 9 mo in vaccine arm vs 12.6 mo in control (P = .019). The shorter TTP in the vaccine arm was speculated to relate to differences in FLIPI scores. | 41 |
| Follicular lymphoma | Idiotype Biovaxid | 234 patients | Immune response was not reported | Median DFS was significantly higher in vaccinated patients (44.2 mo) compared with control (30.6 mo; P = .05) | 42 |
CR, complete response; DFS, disease-free survival; FLIPI, follicular lymphoma international prognostic index; FrC, fragment C; OS, overall survival; PR, prognostic index; TTP, time to progression.
Neoantigen-based vaccines
Tumor cells exhibit mutational events generated during the process of oncogenesis that encode potential antigenic targets that are considered non-self and are potentially targetable by high-affinity immune effector cells.45 Computational algorithms have been developed that identify potential peptide epitopes expressed as a result of specific mutational patterns found on whole genome or RNA sequencing that can be further refined to apply to those antigens expressed in the context of a particular HLA restriction.46 Investigators have identified those epitopes identified by the host immune repertoire and in this setting generated a multivalent vaccine. Early clinical studies in melanoma have shown evidence of immune response, and clinical efficacy is currently being studied.47 Although this approach has potential for generating functionally competent effector cells, the process of vaccine generation is logistically complex and the immunogenicity of the neoantigens may be as similarly variable as self-antigens. In addition, hematologic malignancies have lower mutational burden as compared with solid tumors such as melanoma and lung cancer, consistent with a lower volume of neoantigen epitopes. Conversely, the oncogenesis in hematologic malignancies is often associated with the presence of chromosomal translocations that may generate unique targets through the creation of fusion proteins.
Whole tumor cells as a source of vaccine antigen
An alternative approach has been the use of whole tumor cells as a source of antigens (Table 2). Preclinical studies have evaluated using apoptotic bodies, tumor lysate, whole tumor DNA and RNA, and cellular derivatives such as tumor exosomes as a source of tumor antigens.48-53 One strategy has been the differentiation of leukemia precursors into DCs such that tumor-associated antigens are presented in the context of costimulation.54 One potential concern is that DC differentiation would result in loss of antigen expression restricted to primitive hematopoietic precursors. Another strategy has been the use of the gene-transduced tumor cell vaccine (GVAX) platform in which cell lines or primary tumor cells are manipulated to express granulocyte macrophage–colony-stimulating factor (GM-CSF) or comingled with GM-CSF–expressing cell lines such that native DCs are recruited to the site of vaccination with the potential for crosspresentation of multiple tumor-derived antigens. Administration of GVAX following allogeneic transplantation in patients with AML/MDS was shown to be safe and elicited immune response in a clinical trial55 ; a randomized clinical trial is ongoing (NCT01773395). Similarly, in chronic lymphocytic leukemia (CLL), vaccination with GVAX following reduced-intensity transplant was safe, induced potent immune response, and demonstrated a 2-year PFS of 82%.56 A myeloma GVAX has been studied,57 generated using 2 allogenic myeloma cell lines (H929 and U266) and K562/GM as a GM-CSF–secreting cell line. In a clinical trial, 15 immunofixation electrophoresis–positive patients received myeloma GVAX vaccine in conjunction with lenalidomide. Median PFS has not been reached with a median follow-up of 36 months, supporting interest in further study in a randomized trial.20,57
Whole cell vaccine, without exogenous antigen-presenting cell
| Disease . | Vaccine . | N . | Immune response . | Clinical response . | Reference . |
|---|---|---|---|---|---|
| AML/MDS | GVAX administered following allogeneic transplantation | 15 patients vaccinated | Local vaccine site reaction, biopsy demonstrating T-cell infiltrate. | 2-y OS for patients who received at least 1 vaccine was 57% ± 14% | 55 |
| Decrease in soluble MICA and MICB in clinical responders. | |||||
| CLL | GVAX administered following allogeneic transplantation | 18 patients vaccinated | Interferon γ secretion by CD8 T cells isolated from vaccinated patients after exposure to autologous tumor. | Estimated 2-y PFS in vaccinated patients: 82% | 56 |
| In 4 patients, it was shown that 17% of CD8+ T-cell clones reacted against CLL antigens. | Estimated 2-y OS in vaccinated patients: 88% | ||||
| Multiple myeloma | GVAX administered following autologous transplant | 28 patients vaccinated | Injection site reactions in all patients 41% of patients who received at least 4 posttransplant vaccines demonstrated increase in T-cell secretion of interferon γ secretion after ex vivo stimulation. | 3-y PFS in 28 patients treated with vaccine was 61.8%; 3-y OS was 73.7% | 57 |
| Disease . | Vaccine . | N . | Immune response . | Clinical response . | Reference . |
|---|---|---|---|---|---|
| AML/MDS | GVAX administered following allogeneic transplantation | 15 patients vaccinated | Local vaccine site reaction, biopsy demonstrating T-cell infiltrate. | 2-y OS for patients who received at least 1 vaccine was 57% ± 14% | 55 |
| Decrease in soluble MICA and MICB in clinical responders. | |||||
| CLL | GVAX administered following allogeneic transplantation | 18 patients vaccinated | Interferon γ secretion by CD8 T cells isolated from vaccinated patients after exposure to autologous tumor. | Estimated 2-y PFS in vaccinated patients: 82% | 56 |
| In 4 patients, it was shown that 17% of CD8+ T-cell clones reacted against CLL antigens. | Estimated 2-y OS in vaccinated patients: 88% | ||||
| Multiple myeloma | GVAX administered following autologous transplant | 28 patients vaccinated | Injection site reactions in all patients 41% of patients who received at least 4 posttransplant vaccines demonstrated increase in T-cell secretion of interferon γ secretion after ex vivo stimulation. | 3-y PFS in 28 patients treated with vaccine was 61.8%; 3-y OS was 73.7% | 57 |
CLL, chronic lymphocytic leukemia; MICA, MHC class I chain-related protein A; MICB, MHC class I chain-related protein B. Other abbreviations are explained in Table 1.
Use of ex vivo–generated antigen-presenting cells as a vaccine platform to provide effective costimulation
A potential concern of vaccines consisting of antigens administered with adjuvant is the reliance on native antigen-presenting cells to uptake and present antigen. Native DCs in patients with malignancy exhibit both phenotypic and functional deficiencies that may limit their potential for effective T-cell stimulation. Alternatively, DCs may be generated ex vivo from precursor populations cultured with cytokines that constitutively express positive costimulation and may more effectively induce primary immune responses.58 Table 3 provides a summary of clinical trials using ex vivo–generated antigen-presenting cells loaded with tumor antigen. In a phase 1 trial evaluating a vaccine generated by pulsing idiotype-based DNA onto antigen-presenting cells and administered following autologous transplantation for multiple myeloma, immune response was observed in 71% of patients, and overall survival was 64% with a median follow-up of 85.6 months.59 DCs pulsed with idiotype protein have also been studied in patients with lymphoma. In 1 study, 35 patents with follicular lymphoma underwent vaccination with DCs pulsed with idiotype protein. Of 10 initial patients treated with established disease, 8 demonstrated T-cell anti-idiotype proliferative responses and 4 demonstrated clinical disease regression of which 2 had complete responses. Of 25 patients undergoing vaccination after induction chemotherapy, 70% remained without disease progression at 43 months after completion of chemotherapy.60 In a phase 2 clinical trial, an autologous DC vaccine generated by pulsing autologous DCs with WT1 mRNA was assessed in 30 AML patients.61 WT1 mRNA DC vaccine was administered to high-risk AML patients who achieved a remission following chemotherapy. Clinical response rate was 43%, including 30% of patients who demonstrated molecular remission. A relapse reduction rate of 25% compared with historic controls, and clinical response correlated with immune response to vaccination.61 The results suggest that vaccination with WT1 mRNA-electroporated DCs provides a promising strategy to evoke immune response, eradicate residual disease, and prevent relapse. Similarly, promising results were demonstrated in a clinical trial in which 22 AML patients in remission following chemotherapy were vaccinated with autologous DCs pulsed with human telomerase reverse transcriptase.62 T-cell responses targeting t-HERT were observed in 58% of patients, and 58% of patients remained in remission with a median follow-up of 52 months.62
Vaccine generated by loading tumor antigen onto antigen-presenting cells ex vivo
| Disease . | Peptide/protein or whole cell as antigen source . | N . | Immune response . | Clinical response . | Reference . |
|---|---|---|---|---|---|
| Myeloma | Idiotype-loaded antigen-presenting cells (Mylovenge) following autologous transplantation | 27 patients vaccinated; compared with historical control group of 124 patients | Not assessed | Median PFS 1.5 y both arms.Median OS vaccine group: 5.3 y vs 3.4 y in controls.P = .02 | 59 |
| Follicular lymphoma | Idiotype-pulsed DC | 10 patients with active disease | T-cell response in 8/10 patients | 4/10 patients demonstrate response, including 2 CR | 60 |
| 25 patients in remission after chemotherapy | 23 evaluable patients, T-cell or humoral anti-Id response in 65% | 70% remained without disease progression at 43 mo after completion of chemotherapy | |||
| AML | DC electroporated with WT1 mRNA | 30 AML patients, in remission after chemotherapy | At least a 1.5-fold increase in WT1 tetramer-positive CD8+ T cells in 6 of 12 evaluable patients | Clinical response rate 43% | 61 |
| AML | DCs pulsed with hTERT | 24 AML patients, in remission | T-cell responses targeting t-HERT were observed in 58% of patients | 58% of patients remained in remission with a median follow-up of 52 mo | 62 |
| Myeloma | Whole cell DC/myeloma fusion | 17 patients | Vaccination resulted in the expansion of circulating CD4 and CD8 lymphocytes reactive with autologous myeloma cells in 11/15 evaluable patients | Disease stabilization in 66% of patients | 66 |
| AML | Whole cell DC/AML fusion | 17 patients, vaccinated in CR1 | Durable expansion of leukemia-reactive T cells in the blood and bone marrow | 71% of patients alive with median follow up of 57 mo | 67 |
| Myeloma | Whole cell DC/myeloma fusion | 36 patients vaccinated | All evaluable patients had at least a twofold expansion of myeloma-specific CD4+ and/or CD8+ T cells | 31% achieved a CR/nCR in the early posttransplant period, whereas an additional 17% achieved CR/nCR only after day 100 posttransplant after undergoing vaccination | 69 |
| Disease . | Peptide/protein or whole cell as antigen source . | N . | Immune response . | Clinical response . | Reference . |
|---|---|---|---|---|---|
| Myeloma | Idiotype-loaded antigen-presenting cells (Mylovenge) following autologous transplantation | 27 patients vaccinated; compared with historical control group of 124 patients | Not assessed | Median PFS 1.5 y both arms.Median OS vaccine group: 5.3 y vs 3.4 y in controls.P = .02 | 59 |
| Follicular lymphoma | Idiotype-pulsed DC | 10 patients with active disease | T-cell response in 8/10 patients | 4/10 patients demonstrate response, including 2 CR | 60 |
| 25 patients in remission after chemotherapy | 23 evaluable patients, T-cell or humoral anti-Id response in 65% | 70% remained without disease progression at 43 mo after completion of chemotherapy | |||
| AML | DC electroporated with WT1 mRNA | 30 AML patients, in remission after chemotherapy | At least a 1.5-fold increase in WT1 tetramer-positive CD8+ T cells in 6 of 12 evaluable patients | Clinical response rate 43% | 61 |
| AML | DCs pulsed with hTERT | 24 AML patients, in remission | T-cell responses targeting t-HERT were observed in 58% of patients | 58% of patients remained in remission with a median follow-up of 52 mo | 62 |
| Myeloma | Whole cell DC/myeloma fusion | 17 patients | Vaccination resulted in the expansion of circulating CD4 and CD8 lymphocytes reactive with autologous myeloma cells in 11/15 evaluable patients | Disease stabilization in 66% of patients | 66 |
| AML | Whole cell DC/AML fusion | 17 patients, vaccinated in CR1 | Durable expansion of leukemia-reactive T cells in the blood and bone marrow | 71% of patients alive with median follow up of 57 mo | 67 |
| Myeloma | Whole cell DC/myeloma fusion | 36 patients vaccinated | All evaluable patients had at least a twofold expansion of myeloma-specific CD4+ and/or CD8+ T cells | 31% achieved a CR/nCR in the early posttransplant period, whereas an additional 17% achieved CR/nCR only after day 100 posttransplant after undergoing vaccination | 69 |
hTERT, human telomerase reverse transcriptase; Id, idiotype; nCR, near complete response; t-HERT, telomerase reverse transcriptase. Other abbreviations are explained in Table 1.
We have developed a tumor vaccine in which patient-derived tumor cells are fused with autologous DCs such that a broad array of tumor antigens, including shared and neoantigens and those derived from the diverse clonal populations found in the primary tumor are presented in the context of DC-mediated costimulation.63-65 In a phase 1 study, vaccination with patients with advanced myeloma (median of 4 prior regimens) was well tolerated with feasibility of the planned dose escalation to a dose of 4 × 106 fusion cells.66 Despite the setting of recurrent disease, vaccination resulted in a mean 10-fold expansion of circulating tumor reactive CD4 and CD8 T cells and disease stabilization in 66% of patients.66 In a recently reported study, 17 patients with AML underwent vaccination serial vaccination with DC/AML fusions after achieving chemotherapy-induced remission.67 Vaccination was associated with marked inflammatory responses at the site of administration and the durable expansion of leukemia-specific T cells in the circulation and the bone marrow ongoing at 6 months following therapy as determined by intracellular expression of interferon γ by intracellular flow cytometric analysis. Of note, antigen-specific responses were noted against multiple leukemia-associated antigens including WT1, survivin, and MUC1 notable for their presence on leukemia progenitor cells. Remarkably, despite a median age of 63 years with an anticipated PFS of <20% at 3 years of follow-up, 71% of treated patients remained without evidence of disease at nearly 5 years of follow-up. A randomized trial to compare vaccination to best supportive care in this setting is now under way (NCT03059485).
Determining the appropriate setting in which to vaccinate
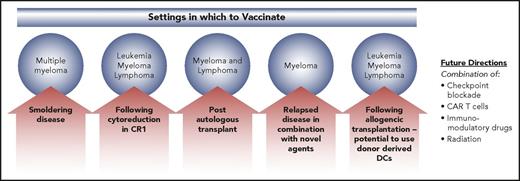
A critical issue regarding vaccine therapy is identifying the optimal setting in which to vaccinate, in order to maximize the potential for T-cell activation and generation of a memory response, mitigating the expansion of inhibitory populations (Figure 2). Vaccine induction of T-cell response appears to peak after at least 1 booster vaccination.67 As such, vaccine efficacy is unlikely to be observed in rapidly progressive disease in which the kinetics of tumor proliferation outpace expected time to response. In addition, tumor-mediated immune suppression is likely to be more pronounced in the setting of advanced disease compromising the capacity of the effector cell population to respond to vaccination. Several studies have examined vaccine efficacy following chemotherapy-induced cytoreduction, with early studies suggestive of improved duration of remission now being evaluated in a randomized trial. In multiple myeloma, smoldering disease offers an opportunity to incorporate vaccination into a milieu where disease-related immune suppression is limited, maximizing potential to evoke a potent immune response. A peptide-based vaccine approach is being assessed in patients with smoldering multiple myeloma (NCT02886065).
Settings in which to vaccinate. Low disease burden states are optimal settings for vaccination, minimizing the immunosuppressive milieu of the microenvironment that characterizes advanced malignancy. In the setting of more advanced disease states, combination therapies are critical, in order to overcome tumor-mediated immunosuppression. CART, chimeric antigen receptor T; CR1, complement receptor type 1.
Settings in which to vaccinate. Low disease burden states are optimal settings for vaccination, minimizing the immunosuppressive milieu of the microenvironment that characterizes advanced malignancy. In the setting of more advanced disease states, combination therapies are critical, in order to overcome tumor-mediated immunosuppression. CART, chimeric antigen receptor T; CR1, complement receptor type 1.
A unique setting for vaccination is during the period of posttransplant immune reconstitution. Despite suppression of general measures of cellular immunity, preclinical models suggest that transient depletion of regulatory T cells during the posttransplant period may enhance vaccine response.68 In vitro studies demonstrated the capacity of T cells isolated during this period to respond to DC-based vaccination. In a phase 2 study, vaccination with idiotype-pulsed antigen-presenting cells (Mylovenge) following autologous transplant for myeloma was well tolerated, and suggested an improvement in survival compared with historical controls.59 In a phase 2 study of vaccination with DC/multiple myeloma fusions following autologous transplantation, patients exhibited a rise in myeloma reactive T cells during the early period that was further durably expanded after exposure to the vaccine.69 Patients demonstrated a near doubling of CR/nCR in the period rom day 100 to 1 year posttransplant in the absence of other maintenance therapy, suggesting a correlation between expansion of tumor-reactive T cells and consolidation of the postchemotherapy response. In a first-of-its-kind collaborative effort of personalized cellular therapy involving 17 cancer centers under the auspices of the National Heart, Lung, and Blood Institute (NHLBI) Clinical Trials Network, a national randomized trial comparing DC/myeloma fusion cell vaccination in conjunction with maintenance lenalidomide compared with maintenance lenalidomide alone following autologous transplant is now under way (NCT02728102).
The allogeneic transplant period offers a unique setting for vaccination. In a recent review, unique aspects of the early period following allogeneic transplant that may allow for enhanced response to vaccination were summarized.70 Conditioning chemotherapy and/or radiation (i) provide a state of low disease burden and (ii) result in inflammatory conditions that activate antigen-presenting cells, both optimizing the potential for response to cancer vaccines. It has been demonstrated that the period of lymphopenia following allogeneic transplant supports the activation of antigen-specific T cells, and provides a platform for skewing of oligoclonal T cells toward tumor-reactive T-cell clones during the time of lymphoid reconstitution. Notably, unlike T cells isolated from patients with malignancy, donor-derived T cells have not been anergized to tumor and are not exhausted. As such, cancer vaccines administered in the postallogeneic transplant setting have the unique potential to activate tumor-specific immunity. In a recent study, 33 patients with AML (25 patients) or MDS (8 patients) were vaccinated with autologous myeloblasts mixed with GM-CSF–secreting K562 cells in the early period following allogeneic transplantation.71 Vaccination was well tolerated, with injection site reactions being the most common vaccine-associated toxicity. One patient died of eosinophilic myocarditis. Incidence of grade 2-4 acute and chronic GVHD were 24% and 33%, respectively. PFS at 5 years was 39%.71 In CLL, vaccination with GVAX following reduced-intensity transplant evoked immune response and demonstrated a 2-year PFS of 82%.56 Administration of GVAX following allogeneic transplantation in patients with AML/MDS is being evaluated in a randomized clinical trial (NCT01773395). Notably, the allogeneic transplant setting offers the opportunity to explore DCs generated using donor-derived cells. In a pilot study, the safety of a WT1 peptide vaccine generated using donor-derived DCs was assessed following allogeneic transplantation.72 The vaccine was administered in conjunction with donor lymphocyte infusion in 5 patients with relapsed disease posttransplant, demonstrating safety and stimulation of immune response. We are initiating a trial in which AML patients will be vaccinated following allogeneic transplant with donor-derived DC fused to patient-derived leukemia cells.
Critical factors to consider in the allogeneic transplant setting are potential toxicities, most importantly, the potential to evoke GVHD. In addition, timing of vaccination is critical, in particular with respect to the timing of taper of immune suppression. Whether immune suppression administered to prevent GVHD will preclude response to vaccination is an area that requires further study. Further studies to treat posttransplant relapse, and, importantly, to prevent relapse using vaccines generated from donor-derived DCs, are planned.
Defining the immunomodulatory approaches to effectively target the tumor microenvironment to prevent extinguishing of T-cell activation and the reestablishment of tolerance
Combination therapy with immunomodulatory therapy has been explored in an effort to reverse critical aspects of the immunosuppressive milieu of the tumor microenvironment and augment vaccine efficacy. Hypomethylating agents have been shown to augment antigen processing and presentation of tumor cells potentially due to stimulation of endogenous retroviral signaling resulting in the release of inflammatory cytokines and are being explored as adjuvants to vaccination in patients with acute leukemia.73-75 Lenalidomide is an immunomodulatory agent with potent antimyeloma activity that has been shown to enhance T-cell and NK-cell function.76,77 The ability of lenalidomide to enhance response to vaccination has been demonstrated preclinically78,79 and is currently being evaluated in clinical trials. Checkpoint blockade has demonstrated great promise in solid tumors and Hodgkin disease, whereas single-agent efficacy of immune checkpoint blockade in non-Hodgkin lymphoma and myeloma has been limited. Combining checkpoint blockade with vaccinations offers an opportunity to enhance response to vaccination and prevent the reestablishment of tolerance, and is currently being evaluated in clinical trials. Chimeric antigen receptor (CAR) T cells have demonstrated dramatic clinical effects in acute lymphoblastic leukemia and a subset of patients with lymphoma. However, durability of responses is variable due to lack of T-cell persistence in some patients, and antigen loss resulting in disease relapse remains a potential limitation of CAR T-cell therapy. Strategies that combine vaccination with CAR T cells, in order to stimulate the native T-cell receptor (TCR) and potentially result in epitope spreading, have demonstrated potent activity in preclinical models.80-82 Combination of vaccines and CAR T cells in hematologic malignancies holds great promise and merits investigation both preclinically and in clinical trials. Similarly, vaccine therapy may synergize with other strategies to generate effector cells with tumor specificity. Vaccine-mediated stimulation of tumor-infiltrating lymphocytes or T cells engineered to express a tumor antigen-specific TCR may facilitate epitope spreading for adoptive immunotherapy. Recent data demonstrating vaccine-mediated NK-cell activation suggest potential synergy with adoptive immunotherapy with NK cells.83
Conclusion
Cancer vaccines have the potential to generate potent immune responses that translate into clinically meaningful improvements in outcomes for patients with hematological malignancies. The ability to selectively target the malignant cells, capture tumor heterogeneity, provide an effective platform of costimulation, and evoke a memory T-cell response is critical to development of a clinically potent tumor vaccine. Vaccine efficacy is likely dependent on the presence of functionally competent effector cells with tumor specificity in the immune repertoire and disease kinetics that allow for a period of expansion and activation of T cells. As such, incorporating vaccination in settings of low disease burden, in order to eradicate residual disease and protect from relapse is likely to be the setting wherein vaccine approaches will have the greatest clinical impact. Combining this approach during lymphopoietic reconstitution following transplant-mediated tumor cytoreduction is potentially a powerful strategy. Partnering vaccination with adoptive T-cell transfer, and with immunomodulatory therapies that overcome the immunosuppressive milieu of the microenvironment, is an important area of investigation.
Authorship
Contribution: D.A. and J.R. wrote the manuscript.
Conflict-of-interest disclosure: D.A. receives research funding from Genus Oncology and Pharmacyclics and serves on advisory boards for Celgene and Bristol-Myers Squibb. J.R. receives research funding from Bristol-Myers Squibb and Celgene and has participated in scientific input engagements with Merck and an advisory board with Bristol-Myers Squibb.
Correspondence: David Avigan, Beth Israel Deaconess Medical Center, 330 Brookline Ave, Kirstein 136, Boston, MA 02215; e-mail: davigan@bidmc.harvard.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal