Abstract
The development of follicular lymphoma (FL) from a founder B cell with an upregulation of B-cell lymphoma 2 (BCL2), via the t(14;18) translocation, to a proliferating clone, poised to undergo further transformation to an aggressive lymphoma, illustrates the opportunistic Darwinian process of tumorigenesis. Protection against apoptosis allows an innocent cell to persist and divide, with dangerous accumulation of further mutational changes, commonly involving inactivation of chromatin-modifying genes. But this is not all. FL cells reflect normal B cells in relying on expression of surface immunoglobulin. In doing so, they add another supportive mechanism by exploiting the natural process of somatic hypermutation of the IGV genes. Positive selection of motifs for addition of glycan into the antigen-binding sites of virtually all cases, and the placement of unusual mannoses in those sites, reveals a posttranslational strategy to engage the microenvironment. A bridge between mannosylated surface immunoglobulin of FL cells and macrophage-expressed dendritic cell–specific ICAM-3–grabbing nonintegrin produces a persistent low-level signal that appears essential for life in the hostile germinal center. Early-stage FL therefore requires a triad of changes: protection from apoptosis, mutations in chromatin modifiers, and an ability to interact with lectin-expressing macrophages. These changes are common and persistent. Genetic/epigenetic analysis is providing important data but investigation of the posttranslational landscape is the next challenge. We have one glimpse of its operation via the influence of added glycan on the B-cell receptor of FL. The consequential interaction with environmental lectins illustrates how posttranslational modifications can be exploited by tumor cells, and could lead to new approaches to therapy.
Introduction
Follicular lymphoma (FL) is a paradigm of an indolent tumor where the steps from an early chromosomal translocation to a more developed clonal proliferation can be tracked. Understanding the biology involved in this process, whereby cells often have only limited additional genetic changes, but retain a complex and essential dependence on the microenvironment, is relevant for all B-cell malignancies and possibly for cancer in general. The theme of dependence of tumor cells on surrounding cells, and how it offers opportunities for intervention, is a major part of the 2018 American Society of Hematology (ASH) Meeting on Lymphoma Biology.
In this review, the focus is on FL and how it arises and continues to occupy the germinal center (GC), a site hostile to normal B cells unless selected by antigen. We will concentrate on this setting, while recognizing that transformation into a more aggressive high-grade lymphoma occurs in 2% to 3% of patients per year. Frustratingly, the factors involved in transformation remain unclear but appear not to be single genetic events but rather multiple hits within a varying molecular landscape.1
A striking feature of FL is the histological appearance which, at early stages, closely resembles that of a reactive lymph node (Figure 1). The distinction in FL lies mainly in a poorly defined mantle zone and a lack of the usual dark/light-zone polarity. Gene expression analysis indicates a similarity between FL cells and light-zone B cells.2 However, further probing reveals significant changes that have occurred in the FL cells.
Histological appearance of FL. (A) Hematoxylin-and-eosin staining of an FL grade 1 (original magnification ×40). Note the numerous follicles of the FL that lost the typical dark-zone/light-zone demarcation and are surrounded by thin mantle zones. (B) Ki-67 staining of an FL, revealing the typical low fraction of proliferating cells in an FL follicle (original magnification ×100). (C) BCL2 staining of an FL, showing strong BCL2 staining of the lymphoma cells (original magnification ×100). The center of the FL follicle is marked with an asterisk (*); the thin mantle zone surrounding the follicle is marked with a pound symbol (#). (D) BCL2 staining of a reactive lymph node with BCL2+ mantle-zone B cells and BCL2− GC B cells (original magnification ×100). For the BCL2 staining, a mouse anti-human monoclonal antibody (clone 124; DAKO Agilent, Hamburg, Germany) was used; for the Ki-67 staining, a mouse anti-human monoclonal antibody (clone MIB-1; DAKO). Stainings were kindly provided by S. Hartmann and M.-L. Hansmann (Senckenberg Center for Pathology, University of Frankfurt/Main, Frankfurt, Germany).
Histological appearance of FL. (A) Hematoxylin-and-eosin staining of an FL grade 1 (original magnification ×40). Note the numerous follicles of the FL that lost the typical dark-zone/light-zone demarcation and are surrounded by thin mantle zones. (B) Ki-67 staining of an FL, revealing the typical low fraction of proliferating cells in an FL follicle (original magnification ×100). (C) BCL2 staining of an FL, showing strong BCL2 staining of the lymphoma cells (original magnification ×100). The center of the FL follicle is marked with an asterisk (*); the thin mantle zone surrounding the follicle is marked with a pound symbol (#). (D) BCL2 staining of a reactive lymph node with BCL2+ mantle-zone B cells and BCL2− GC B cells (original magnification ×100). For the BCL2 staining, a mouse anti-human monoclonal antibody (clone 124; DAKO Agilent, Hamburg, Germany) was used; for the Ki-67 staining, a mouse anti-human monoclonal antibody (clone MIB-1; DAKO). Stainings were kindly provided by S. Hartmann and M.-L. Hansmann (Senckenberg Center for Pathology, University of Frankfurt/Main, Frankfurt, Germany).
Histogenesis and premalignant lesion
The histogenesis of FL is closely linked to key events of normal B-cell development and differentiation. During early B-cell development in the bone marrow, progenitor B cells undergo V(D)J recombination processes to assemble immunoglobulin heavy (IGH) and light chain V region genes that encode the variable parts of antibody molecules.3 This involves DNA double-strand breaks at specific recombination signal sequences (RSSs), located at the ends of the rearranging V, D, and J genes. First, in a pro-B cell, an IGHD gene is joined to an IGHJ gene, and in the next step, an IGHV gene is recombined to the DHJH joint.3 B-cell precursors expressing a heavy-chain gene as pre-B-cell receptor are pre-B cells. At the recombination sites, further diversity is generated by exonucleolytic removal of several bases and by addition of non–germ line–encoded bases, the N-nucleotides.3 Once a functional heavy chain is expressed, light-chain gene rearrangements are performed to generate a mature B-cell receptor (BCR).
In rare instances, mistakes happen during V(D)J recombination, and when the ends of the rearranging genes in 1 of the immunoglobulin loci are erroneously joined to DNA breaks in another chromosome, a reciprocal chromosomal translocation occurs.4 Such a translocation is likely the first event in the pathogenesis of FL. About 90% of FLs show a t(14;18)(q32;p21) in which the B-cell lymphoma 2 (BCL2) gene is joined to an IGHJ gene or a DHJH joint in the IGH locus.5,6 Specific characteristics of the translocations, in particular the presence of N-nucleotides at the joining sites of the 2 chromosomes and the location of the breakpoints in the IGH locus close to the RSS site of an IGHD or IGHJ gene, strongly argue for misguided V(D)J recombination at the pro-B-cell stage as the mechanism.4 As a consequence, the BCL2 gene is brought under control of the enhancers of the IGH locus, causing constitutive expression of the antiapoptotic BCL2 protein.4
BCL2 is not a strong proto-oncogene, and as naive B cells physiologically express BCL2, it seems that the presence of a t(14;18) IGH-BCL2 translocation does not cause a major disturbance of mature naive B-cell physiology. It unfolds its pathogenetic function when a naive B cell is driven into a T-cell–dependent immune response and becomes a GC B cell. In the dark zone of the GC, antigen-activated B cells undergo massive clonal expansion and activate the process of somatic hypermutation (SHM) to modify their IGV region genes.3,7 As somatic mutations are largely random, only few cells will acquire affinity-increasing mutations and are positively selected by T follicular helper (TFH) cells in the light zone.8 Most acquire disadvantageous mutations and are destined to die by apoptosis.8,9 The apoptosis proneness of GC B cells is also a tolerance mechanism to prevent survival of autoreactive B cells.10,11 Apparently, apoptosis is the default pathway for GC B cells, and an important factor for this intrinsic program is that they downregulate the antiapoptotic factor BCL2 (Figure 1).9,12 Only in positively selected light-zone GC B cells that undergo differentiation toward memory B cells or plasma cells is BCL2 expression reinduced.12,13 If a B cell with an IGH-BCL2 translocation is driven into a GC reaction, the normal apoptosis and selection process is disturbed, and such B cells have a survival advantage.14 This then increases the risk for acquisition of further genetic lesions and ultimately may lead to the development of a FL in some such cells. As will be discussed in more detail in the following sections, these additional genetic lesions and pathogenetic events cause differentiation arrest at the stage of a GC B cell.
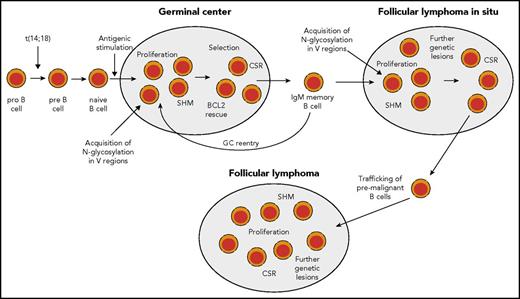
B cells with IGH-BCL2 translocations are also detectable in healthy human adults. The frequency of such cells is ∼1 per 105 peripheral blood B cells, with wide variation between individuals.15,16 The frequencies tend to rise with increasing age.17 Initially, it was thought that these BCL2 translocation-positive circulating cells are polyclonal naive B cells, stemming from numerous independent translocation events, and accumulating in this mature B-cell compartment. However, later elegant studies revealed that t(14;18)-carrying cells in healthy adults are mostly present among immunoglobulin M–positive (IgM+)IgD+CD27+ memory B cells with somatically mutated IGV genes, and that the pool of such cells is mostly dominated by 1 or a few clones.18,19 These clones often persist, and, in a few instances, it was shown that circulating clones present in peripheral blood several years before diagnosis of a FL already carried a number of genetic lesions in addition to the BCL2 translocation.20,21 Combined studies with human B cells and a murine model indicate that B cells constantly overexpressing BCL2 undergo several GC passages until they finally give rise to a FL (Figure 2).22
Scenario for FL pathogenesis. During early B-cell development, in rare instances, pro-B cells acquire a t(14;18) IGH-BCL2 translocation as a mistake of IGH recombination. This leads to constitutive expression of BCL2. t(14:18)-bearing B cells can develop further into mature naive B cells and may enter a GC reaction upon antigenic stimulation. In the GC, BCL2 is normally downregulated to promote apoptosis proneness of GC B cells. However, t(14;18)-carrying GC B cells have a survival advantage, and may clonally expand and become memory B cells. t(14;18)-positive B cells in the peripheral blood are mainly found among IgM memory B cells. Such cells may undergo repeated GC reactions and thereby acquire further genetic lesions. In some reactive lymph nodes, GCs dominated by monoclonal BCL2+ GC B cells can be found. These are called FLIS and the B-cell clones can be considered as premalignant as they often carry besides the t(14;18) further genetic lesions. From such structures, FLs can develop after additional gene mutations occurred. Furthermore, mutations promoting N-glycosylation of amino acids in the variable regions of the BCR have been detected in FLIS,28,61 so that chronic antigenic stimulation as an additional pathogenetic factor occurring through BCR stimulation by lectins on stromal cells can occur already in FLIS (and perhaps even earlier, as the mutations causing N-glycosylation may well occur in early GC passages, when SHM is highly active). Nearly half of FL cases express IgG, so that at some stage during FL pathogenesis, a considerable fraction of cases have undergone class-switch recombination (CSR).
Scenario for FL pathogenesis. During early B-cell development, in rare instances, pro-B cells acquire a t(14;18) IGH-BCL2 translocation as a mistake of IGH recombination. This leads to constitutive expression of BCL2. t(14:18)-bearing B cells can develop further into mature naive B cells and may enter a GC reaction upon antigenic stimulation. In the GC, BCL2 is normally downregulated to promote apoptosis proneness of GC B cells. However, t(14;18)-carrying GC B cells have a survival advantage, and may clonally expand and become memory B cells. t(14;18)-positive B cells in the peripheral blood are mainly found among IgM memory B cells. Such cells may undergo repeated GC reactions and thereby acquire further genetic lesions. In some reactive lymph nodes, GCs dominated by monoclonal BCL2+ GC B cells can be found. These are called FLIS and the B-cell clones can be considered as premalignant as they often carry besides the t(14;18) further genetic lesions. From such structures, FLs can develop after additional gene mutations occurred. Furthermore, mutations promoting N-glycosylation of amino acids in the variable regions of the BCR have been detected in FLIS,28,61 so that chronic antigenic stimulation as an additional pathogenetic factor occurring through BCR stimulation by lectins on stromal cells can occur already in FLIS (and perhaps even earlier, as the mutations causing N-glycosylation may well occur in early GC passages, when SHM is highly active). Nearly half of FL cases express IgG, so that at some stage during FL pathogenesis, a considerable fraction of cases have undergone class-switch recombination (CSR).
B cells carrying IGH-BCL2 translocations can also be been detected in the GC of ∼2% to 3% of reactive lymph nodes from healthy adults.23-25 Often, their numbers are rather small and they are scattered among normal GC B cells.26 In other instances, typically, several GCs are dominated by BCL2-expressing GC B cells with the t(14;18) translocation. This has been designated as FL in situ (FLIS).24,27 The cells express typical GC B-cell markers, such as CD10, are monoclonal, and show active hypermutation.24,28 However, their proliferation rate is lower than that of normal GCs, with an increased fraction of light-zone B cells.24 Progression of FLIS to FL is seen only in rare instances, but FLIS nevertheless seems to represent precursor lesions of FL and premalignant B-cell clonal expansions because, in a few instances, a clonal relationship between an FLIS and a subsequent FL was demonstrated, and FLIS frequently already carries genomic imbalances as further genetic lesions in addition to the t(14;18).29,30
The critical receptor of B cells: surface immunoglobulin
A key point for FL is that retention of expression of surface immunoglobulin is mandatory, in spite of the loss of 1 allele by the t(14;18) translocation. This indicates that, like normal B cells, the receptor has an important function in growth/survival of FL cells. The known functions of surface immunoglobulin are twofold: first, to provide a low-level “tonic“ signal essential for survival31 ; the exogenous drivers for normal B cells are not known but could involve low-affinity binding by environmental (auto)antigens.32 The second function is to mediate antigen-dependent responses in mature B cells. One distinction between “tonic“ and antigen-dependent signaling, derived from studies of developing B cells in knockout mice, appears to be a differential dependence of survival on the canonical NF-κB pathway. This pathway regulates NF-κB activation downstream of the BCR and appears essential for “tonic“ signal-mediated survival but not for short-term survival of follicular B cells. However, the differential does not hold for long-term survival or functional fitness of the latter,33 leaving no strict distinction between the 2 kinds of signals at this stage. This raises the question of what kind of signal might be involved in maintaining malignant B cells. There is no single answer to this because some tumors, such as chronic lymphocytic leukemia (CLL), do appear to be undergoing chronic antigen-mediated signaling, with consequent downregulation of surface immunoglobulin expression due to reversible endocytosis.34 This is not apparent in FL, suggesting a different kind of exogenous stimulus.
Because each FL case has a distinct receptor, and the original (foreign) antigen has probably long disappeared, some other ligand, likely a tissue-resident factor, might be operative. Autoantigen could be involved and, as for normal B cells, immunoglobulin rescued from a proportion of FL cells (∼26%) can bind to certain autoantigens.35,36 However, the spectrum of autoreactivity appears variable and is less common than for other B-cell tumors. To set the question in context, we will first consider the nature of the surface immunoglobulin in FL cells and then describe the distinctive feature that overcomes heterogeneity among FL immunoglobulins by conferring a novel universal ability to bind to a local autoligand.
Surface immunoglobulin generation in FL
The surface immunoglobulin of normal B cells has the clear function of antigen recognition via the immunoglobulin variable (IGV) regions (IGHV and IGLV), which are assembled and further modified by the process of SHM. The rather risky interference with DNA allows generation of a wide range of sequences able to cope with the vast array of invading pathogens. The price paid is generation of B-cell malignancies. These carry the immunoglobulin of the parental normal B cell, which indicates the point of differentiation reached prior to transformation. In the case of FL, that is the GC, as evidenced by the accumulation of IGV somatic mutations at levels which, although widely varying between cases, are comparable to the similarly variable levels in normal GC B cells.37 Another feature shared with normal GC B cells is expression of activation-induced cytidine deaminase (AID), an essential requirement for SHM and isotype switch. AID is detectable in ∼25% of FL cells and accompanies the ongoing SHM, which leads to intraclonal variation in IGV sequences.38 Although SHM evidently continues posttransformation, it may be limited and does not appear to lead to an overall accumulation of mutations, but rather to dynamic emergence of different subclones over time.39,40
About 40% to 50% of FL have undergone isotype switching and express IgG.41,42 Curiously, most IgM+ FLs show class-switch recombination on the translocated allele and IGHC deletions downstream of IgM on the expressed IGH allele.42 This indicates selection to retain IgM expression in IgM+ FLs, and links these cells to the t(14;18)-carrying B cells in healthy individuals, which are mostly IgM+ memory B cells. Retention of IgM expression, at least in the early phase of FL development, might be advantageous for the (pre)malignant cells, as there is indication that IgM+ memory B cells have a higher propensity to reenter GCs than class-switched memory B cells.43,44 However, during the pathogenetic process, this preference is apparently frequently lost, perhaps due to the particular combination of transforming events acquired by an FL clone, and many cases switch to IgG expression.
The IGHV gene repertoire used by normal B cells is well documented, and biased usage by B-cell tumors is an indicator of a drive on parental B cells, and possibly on tumor cells, by a superantigen and/or autoantigen.45 This is evident in some B-cell tumors such as CLL and some mucosa-associated lymphomas, but does not appear to be the case in FLs. Classical superantigens, often expressed by pathogens and some autoantigens, are therefore apparently not driving FL cells.
Modification of surface immunoglobulin in FL cells by glycan addition
During SHM, replacement mutations tend to concentrate in the complementarity-determining regions (CDRs).46 Amino acid sequence motifs consisting of Asn-X-Ser/Thr can arise, where X can be almost any amino acid except Pro. The motifs are cues for addition in the endoplasmic reticulum (ER) of a dolichol-linked oligosaccharide chain to the Asn residue, a process known as N-glycosylation. Although germ line–encoded motifs are present in the constant regions of normal immunoglobulin and in a few IGV sequences, motifs introduced into the IGV regions during SHM are rare in normal memory B cells, in mutated CLL or in myeloma. However, they are present in almost all cases of FL where they accumulate in the antigen-binding sites. They are found in most soluble IgM+ cases (90%) but there are slightly fewer (73.5%) in IgG+ cases.47 Motifs are also present in a subset of diffuse large B-cell lymphomas,48 and cell line studies indicate that, as might be anticipated, it is the GC B-cell–like subset that has the higher frequency.49,50
We have described a further curiosity in FL, which is that, after addition of the so-called “high-mannose“ glycan in the ER, the usual further processing of the oligosaccharides that occurs as the immunoglobulin passes through the Golgi stacks does not take place for the IGV glycan.51 Normal immunoglobulin has germ line–encoded glycan addition sites in the constant region and these are processed by addition of further sugars, trimming, and addition of terminal sugars such as fucose and sialic acid before reaching the cell surface.52 This also occurs for the constant region sites in the FL immunoglobulin.51 However, the introduced sites in the IGV regions appear to be protected from further processing so that the glycan remains highly mannosylated.53 The result of this is expression of unusual mannosylated glycan in the antigen-binding site of almost all cases of FL.
Microenvironmental interactions
In some ways, the mannoses resemble those expressed by pathogens, and we have found that FL surface immunoglobulin binds to the lectin dendritic cell–specific ICAM-3–grabbing nonintegrin (DC-SIGN) in a similar way.54 DC-SIGN also induces tumor-specific downstream signaling in FL cells.53,54 The lectin is expressed by macrophages in FL tissue, possibly upregulated by interleukin-4 (IL-4) produced by local TFH cells,55 indicating a potential interactive loop operating between FL cells and macrophages in the tissue. The association between macrophage infiltration and prognosis has been evident in several studies,56,57 although this broke down when anti-CD20 was part of the therapy, possibly because antibody-mediated attack requires macrophages.58 However, macrophages are clearly present in FL tissue sites, often sited close to proliferating tumor cells (Figure 3). Interestingly, binding of DC-SIGN to FL cells does not induce endocytosis, possibly explaining the maintenance of the relatively high expression of surface immunoglobulin in FL, and offering a persistent signaling mechanism for tumor survival/proliferation.54 We envisage the binding between the FL cell and the macrophage expressing tetrameric DC-SIGN to involve a persistent low-level signal, potentially important for survival (Figure 4).
Macrophages are located close to proliferating FL cells. Macrophage marker CD68 (PG-M1) (red) and proliferation marker Ki-67 (MIB-1) (brown) show colocalization of macrophages and proliferating FL cells (original magnification ×1000).
Macrophages are located close to proliferating FL cells. Macrophage marker CD68 (PG-M1) (red) and proliferation marker Ki-67 (MIB-1) (brown) show colocalization of macrophages and proliferating FL cells (original magnification ×1000).
Interaction between DC-SIGN expressed by macrophages and mannosylated surface immunoglobulin on the surface of FL cells. A diagrammatic view of a cell-cell interaction between a polarized macrophage expressing naturally tetrameric DC-SIGN and an FL cell carrying mannosylated motifs in the IGV region. Data in vitro show that DC-SIGN engagement leads to phosphorylation of intracellular kinases in FL cells.54 BLNK, B-cell linker protein; BTK, Bruton tyrosine kinase; P, phosphorylation; PDK, phosphoinositide-dependent protein kinase; PI3K, phosphatidylinositol 3-kinase; PIP3, phosphatidylinositol (3,4,5)-tris- phosphate; PLC, phospholipase C.
Interaction between DC-SIGN expressed by macrophages and mannosylated surface immunoglobulin on the surface of FL cells. A diagrammatic view of a cell-cell interaction between a polarized macrophage expressing naturally tetrameric DC-SIGN and an FL cell carrying mannosylated motifs in the IGV region. Data in vitro show that DC-SIGN engagement leads to phosphorylation of intracellular kinases in FL cells.54 BLNK, B-cell linker protein; BTK, Bruton tyrosine kinase; P, phosphorylation; PDK, phosphoinositide-dependent protein kinase; PI3K, phosphatidylinositol 3-kinase; PIP3, phosphatidylinositol (3,4,5)-tris- phosphate; PLC, phospholipase C.
Obviously, the microenvironment of FL includes not only macrophages, but also many interacting cell types, including TFH cells and follicular dendritic cells (FDCs). These have been extensively reviewed,59 and we have added the novel interaction between surface immunoglobulin and DC-SIGN–expressing tumor-associated IL-4–polarized macrophages to the mix (Figure 5).
Influences of the cellular microenvironment on FL cells. Potential interactions between FL cells and local FDCs and TFH cells are indicated. High levels of IL-4 are likely to polarize the tumor-associated macrophages (TAM) to M2 type with upregulated DC-SIGN expression and subsequent engagement of mannosylated surface immunoglobulin on FL cells. BAFF, B-cell–activating factor; BAFFR, BAFF receptor; ICOS, inducible costimulator; ICOSL, ICOS ligand; MHC, major histocompatibility complex; TCR, T-cell receptor.
Influences of the cellular microenvironment on FL cells. Potential interactions between FL cells and local FDCs and TFH cells are indicated. High levels of IL-4 are likely to polarize the tumor-associated macrophages (TAM) to M2 type with upregulated DC-SIGN expression and subsequent engagement of mannosylated surface immunoglobulin on FL cells. BAFF, B-cell–activating factor; BAFFR, BAFF receptor; ICOS, inducible costimulator; ICOSL, ICOS ligand; MHC, major histocompatibility complex; TCR, T-cell receptor.
Clinical relevance of modified surface immunoglobulin
Two distinctive early features of the majority of FL cases are: the t(14;18) translocation and mutations in 1 or more of the chromatin-modifying genes, with changes in KMT2D (MLL2) being the most frequent, but those in CREBBP apparently enriched in the earliest inferable progenitor.60 We can now add the glycan modification of surface immunoglobulin evident in 74% to 90% of cases to define a common 3-component setting for FL that then can accumulate further genetic change (Table 1). Interestingly, in 1 available case, the glycan addition motifs were also detected at the stage of FLIS, consistent with the timing of SHM.61 Although the correlative data indicate the importance of the glycan addition for the lifestyle of FL cells, functional proof is difficult to obtain. One reason for this is the lack of a mouse model. In our view, any attempt to model indolent human lymphomas is challenging, but in the case of the mannosylated immunoglobulin–DC-SIGN interaction there is the added problem that expression patterns and function of lectins in mice are different from human subjects.62 A possible step toward solving this is the availability of a transgenic mouse model expressing human DC-SIGN.62
Genetic changes found in earliest inferable progenitor of FL
| . | Genetic change . | Approximate frequency in FL, % . |
|---|---|---|
| 1 | Translocation of BCL2 | 80-90 |
| 2 | Mutations in 1 or more chromatin-modifier genes | 95 |
| 3 | Acquisition of N-glycosylation sites in IGV region of BCR | 75-90 |
| . | Genetic change . | Approximate frequency in FL, % . |
|---|---|---|
| 1 | Translocation of BCL2 | 80-90 |
| 2 | Mutations in 1 or more chromatin-modifier genes | 95 |
| 3 | Acquisition of N-glycosylation sites in IGV region of BCR | 75-90 |
The triad of foundational changes including t(14;18) translocation, mutations in chromatin-modifying genes, and the mannosylation of the surface immunoglobulin–binding sites could be used to assist diagnosis and, because they are clonal markers, for monitoring. For therapy, the apparently essential microenvironmental interaction with macrophage lectins could be blocked by mannose-specific antibodies, possibly derived from those developed for HIV,63 or by glycans in various formats such as galactomannans,64 star glycans,65 or mannose-expressing nanoparticles.66
Genetic lesions
With the development of novel sequencing technologies, a multitude of recurrent somatic mutations were identified that provided exciting new insights into the molecular pathogenesis of FL (Table 2).67-78 The products of several of the most frequently mutated genes, including KMT2D (MLL2), CREBBP, EP300, EZH2, and histone H1 variants, modify histones and hence play a role in epigenetic regulation (Table 2). KMT2D encodes a histone methyltransferase of H3K4 that is affected by inactivating mutations in 60% to 90% of FL.67,71,76 Mice studied revealed that KMT2D is indeed a tumor suppressor, and that its functional impairment causes an expansion of GC B cells and promotes lymphomagenesis.79,80 CREBBP and EP300 are histone acetyltransferases that are mutated in 60% to 70% and 10% to 20% of FL, respectively.71,75 The mutations are inactivating, but mostly affect only 1 allele, suggesting a haploinsufficiency. Importantly, these genes not only modify histones and thereby have genome-wide effects on gene regulation, but they also acetylate nonhistone proteins. Among the nonhistone targets in B cells are TP53 and BCL6, 2 key transcription factors for GC B-cell physiology.75 TP53 is normally activated by acetylation and BCL6 is inactivated. Hence, the impaired function of CREBBP and EP300 in GC B cells causes an inhibition of TP53-mediated apoptosis and leads to enhanced BCL6 function in the mutated GC B cells.75 EZH2 functions as a histone methyltransferase that methylates H3K27. EZH2 is monoallelically mutated at particular positions causing a gain of function in 20% to 30% of FL.70,81 It is the combined function of wild-type and mutated EZH2 that causes increased dimethylation and trimethylation of H3K27.81 Mutations were also identified in various members of the linker histone H1 family, together affecting 30% to 40% of cases.68,69 The linker histone functions in nucleosome spacing and gene regulation, but the specific consequences of the mutations in HIST1H genes remain to be clarified. Chromatin structure of FL is additionally affected by mutations in 2 genes that function in nucleosome remodeling, namely ARID1A and BCL7A, which are mutated in ∼10% to 15% and 5% to 15% of FL, respectively.68,69
Genetic lesions in FL
| Functional category . | Gene . | Approximate frequency of mutated cases, %* . | Proposed functional consequences . | Selected references . |
|---|---|---|---|---|
| Apoptosis | BCL2 | 80-90† | Rescue from apoptosis in the GC | 5,6 |
| Histone modification | KMT2D (MLL2) | 60-90 | Reduced H3K4 methylation, promotion of GC B-cell proliferation | 67,71,76 |
| CREBBP | 60-70 | Reduced histone acetylation, enhances BCL6 function, impaired TP53 function | 71,75 | |
| EP300 | 10-20 | Reduced histone acetylation | 71,75 | |
| EZH2 | 20-30 | Increased bi- and trimethylation of H3K27, reduced expression of target genes | 70,81 | |
| HIST1H1B-E and other HIST1 members | 30-40 | Unclear | 68,69 | |
| Nucleosome remodeling | ARID1A | 10-15 | Alteration of chromatin remodeling, specific consequences unclear | 69,82 |
| BCL7A | ∼10 | Alteration of chromatin remodeling, specific consequences unclear | 68 | |
| Signaling | CARD11 | 10-15 | Activation of NF-κB signaling | 68,82 |
| RRAGC | 15-20 | mTORC1 activation, promotes cellular metabolism and growth | 68,73 | |
| GNA13 | ∼10 | Inactivating mutations promote B-cell growth and lymphoma cell dissemination | 71 | |
| TNFRSF14 | 30-40 | Loss-of-function mutations may prevent inhibitory HVEM signaling | 87,88 | |
| SESTRIN1 | ∼20‡ | Inactivating mutations promote mTOR activity | 74 | |
| Transcription factors | MEF2B | 10-20 | Enforced activity of BCL6 | 82,83 |
| FOXO1 | ∼10 | Mutations cause nuclear retention, maintains dark-zone B-cell program, cooperates with BCL6 | 82,84 | |
| STAT6 | 10-15 | Hyperactivation of JAK/STAT signaling | 77 |
| Functional category . | Gene . | Approximate frequency of mutated cases, %* . | Proposed functional consequences . | Selected references . |
|---|---|---|---|---|
| Apoptosis | BCL2 | 80-90† | Rescue from apoptosis in the GC | 5,6 |
| Histone modification | KMT2D (MLL2) | 60-90 | Reduced H3K4 methylation, promotion of GC B-cell proliferation | 67,71,76 |
| CREBBP | 60-70 | Reduced histone acetylation, enhances BCL6 function, impaired TP53 function | 71,75 | |
| EP300 | 10-20 | Reduced histone acetylation | 71,75 | |
| EZH2 | 20-30 | Increased bi- and trimethylation of H3K27, reduced expression of target genes | 70,81 | |
| HIST1H1B-E and other HIST1 members | 30-40 | Unclear | 68,69 | |
| Nucleosome remodeling | ARID1A | 10-15 | Alteration of chromatin remodeling, specific consequences unclear | 69,82 |
| BCL7A | ∼10 | Alteration of chromatin remodeling, specific consequences unclear | 68 | |
| Signaling | CARD11 | 10-15 | Activation of NF-κB signaling | 68,82 |
| RRAGC | 15-20 | mTORC1 activation, promotes cellular metabolism and growth | 68,73 | |
| GNA13 | ∼10 | Inactivating mutations promote B-cell growth and lymphoma cell dissemination | 71 | |
| TNFRSF14 | 30-40 | Loss-of-function mutations may prevent inhibitory HVEM signaling | 87,88 | |
| SESTRIN1 | ∼20‡ | Inactivating mutations promote mTOR activity | 74 | |
| Transcription factors | MEF2B | 10-20 | Enforced activity of BCL6 | 82,83 |
| FOXO1 | ∼10 | Mutations cause nuclear retention, maintains dark-zone B-cell program, cooperates with BCL6 | 82,84 | |
| STAT6 | 10-15 | Hyperactivation of JAK/STAT signaling | 77 |
Adapted from Weigert and Weinstock.78
HVEM, herpesvirus entry mediator; mTOR, mammalian target of rapamycin.
The table is restricted to genetic lesions that are estimated to occur in at least 10% of cases of FL.
Frequency of IGH-BCL2 translocations.
Homozygous or heterozygous deletions at Chr6q21 that involve the SESTRIN1 gene.
Three transcription factors are affected by recurrent somatic mutations in FL: MEF2B (10%-20% of cases), STAT6 (10%-15%), and FOXO1 (5%-10%) (Table 2).71,77,82,83 The mutations in MEF2B lead to its enhanced activity and as a consequence mediate enforced activity of BCL6.83 Mutations in FOXO1 lead to a gain of function and cause its nuclear retention and consequently enforced activity.84 FOXO1 is critical to maintain the dark-zone program in GC B cells and cooperates with BCL6, the master regulator of the GC B-cell program.85,86 Mutations in STAT6 occur in hot-spot motifs in its DNA-binding domain and hyperactivate IL-4/JAK/STAT6 signaling.77 IL-4 levels are increased in the microenvironment of FL, so that here microenvironmental and a genetic factor cooperate in FL pathogenesis.
TNFRSF14 is affected by genetic lesions in ∼30% to 40% of adult FLs, and is even more frequently mutated in pediatric-type FL.87,88 TNFRSF14 is a member of the tumor necrosis factor receptor family, which can have both stimulatory and inhibitory functions. In a FL mouse model, loss of TNFRSF14 (HVEM) leads to cell-autonomous activation of B cells by preventing inhibitory TNFRSF14–B- and T-lymphocyte attenuator interactions with B cells, and inducing a tumor-supportive microenvironment.89 Recurrent mutations in RRAGC, ATP6V1B2, and ATP6AP1 sustain mammalian target of rapamycin complex 1 (mTORC1) signaling, which plays a major role in regulating cellular metabolism and growth.68,73 A further mechanism for sustained mTORC1 activity even under nutrient-deprivation conditions is mediated by deletions and/or epigenetic inactivation of SESTRIN1, which physiologically causes cell growth inhibition under genotoxic stress.74
Taken together, FLs show a wide variety of genetic lesions, in particular in factors involved in epigenetic and transcriptional regulation, microenvironmental interactions, and signaling. Importantly, several of the most frequently affected genes (EZH2, CREBBP, KMT2D, MEF2B) play major roles in sustaining the GC B-cell program. Hence, these genetic lesions cause a “freezing” of the FL cells at the proliferative GC stage of differentiation and prevent the lymphoma cells from differentiating into resting memory or plasma cells.
Epigenetic dysregulation
As several of the recurrently mutated genes in FL affect epigenetic regulators, epigenetic dysregulation seems to be a hallmark of FL pathogenesis. Globally, FLs show a hypomethylation of their DNA, but specific regions are hypermethylated in comparison with normal GC B cells.90 The DNA methylation pattern of FLs overall is more similar to the one of light-zone GC B cells than to dark-zone B cells, in line with the idea that FLs are frozen at the light-zone differentiation stage, which is also indicated from gene expression profiling studies.2,90,91 The finding that EZH2 target genes are enriched among hypermethylated genes in FLs fits to the frequent finding of EZH2-activating mutations in FL, as discussed earlier. Whereas the epigenetic profile of superenhancers that mediate B-cell identity is retained in FL cells, some aspects of B-cell functions that appear not to be essential or even detrimental for FL cells are epigenetically silenced, including gene sets regulating apoptosis and cell cycle checkpoints.91 Moreover, FL cells also acquired epigenetic features of other cell lineages that are frequently found in various types of cancers, indicating that they control genes involved in cellular transformation.91 Detailed epigenetic profiling also indicated existence of 2 subsets of FL, 1 with a GC-specific profile, and another subgroup that has acquired some features of plasmablasts.91 The relevance of this finding needs further investigation.
Conclusions
In several of its key pathogenetic characteristics, FLs can serve as a paradigm for lymphomagenesis. The presence of t(14;18)-carrying B cells in healthy individuals and the fact that the translocations happen in pro-/pre-B cells exemplify 2 hallmarks of the multistep transformation process in human cancers. One is that the transformation process frequently involves premalignant cells with first genetic lesions that may persist in the body for years or even decades before, in some individuals, they give rise to fully malignant tumor clones. The other hallmark is that initial genetic lesions may occur at an earlier differentiation stage than the one from which the malignant clone derives. Transforming events may even occur in hematopoietic stem cells in several types of tumor from mature lymphocytes.92-94 A further hallmark of FL is that many of the frequent genetic lesions identified contribute to stabilizing the GC differentiation program and, in particular, the activity of BCL6 as the master regulator of the program. This keeps the cells in a proliferative state and prevents differentiation into resting memory B or plasma cells. The contribution of mutations affecting epigenetic regulators to this process highlights the important role of epigenetic dysregulation in lymphomagenesis, which has also similarly emerged in other types of lymphomas.71,75 FL is also a prototype of a B-cell lymphoma that depends on BCR expression and on microenvironmental interactions in addition to the genetic lesions.
A key pathogenetic mechanism that appears specific for FL is that the lymphoma cells have acquired a peculiar way for constitutive BCR triggering by presenting mannosylated glycans on the IGV regions, which bind to receptors on macrophages. This unique modification of the BCR must occur during SHM in the GC and is found in the early stage of lymphoma development, FLIS. In FL, sites for mannosylation are retained during ongoing SHM95 and presumably confer a growth/survival advantage on emergent GC-located clones, which can then accumulate additional (epi)genomic modifications. BCR stimulation remains essential but has become independent from the presence of foreign antigens that may initially have triggered proliferation of the GC B-cell precursors of the lymphoma. This posttranslational modification appears vital for the lifestyle of FL and draws our attention to a new level of tumor cell opportunism that may offer possibilities for intervention.
Acknowledgments
The authors thank Sylvia Hartmann, Martin-Leo Hansmann (both Senckenberg Center for Pathology, University of Frankfurt/Main, Frankfurt, Germany), and Maurilio Ponzoni (Istituto di Ricovero e Cura a Carattere Scientifico [IRCCS] San Raffaele Scientific Institute, Milan, Italy) for providing histological pictures of FL. The authors thank D. Taylor for help with figures and Lynsey Block for administrative support.
This work was supported by the German Ministry of Science and Education (Bundesministerium für Bildung und Forschung [BMBF]) in the framework of the International Cancer Genome Consortium (ICGC) MMML-Seq (01KU1002A-J) Project; the Deutsche Krebshilfe; the Dr. Werner Jackstädt-Stiftung; Cancer Research UK; the National Institute for Health Research (NIHR) Experimental Cancer Medicine Centre; University of Southampton; Bloodwise; and the Gilead UK and Ireland Oncology Fellowship Programme 2016.
Authorship
Contribution: F.K.S. and R.K. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Freda K. Stevenson, Cancer Research UK Centre, Cancer Sciences Unit, University of Southampton Faculty of Medicine, Southampton General Hospital, Southampton, SO16 6YD, United Kingdom; e-mail: fs@soton.ac.uk.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal