Overexpression of nuclear factor erythroid 2 (NFE2) is commonly observed in the myeloproliferative neoplasms (MPNs), especially polycythemia vera (PV) and primary myelofibrosis,1,2 but the mechanism that drives this feature has been unclear. In this issue of Blood, Peeken et al reveal a 2-pronged epigenetic pathway that promotes NFE2 overexpression and disease.3
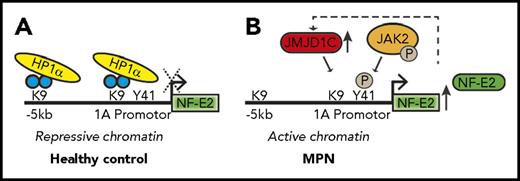
Two epigenetic pathways converge to control NFE2 expression in the MPNs. (A) In healthy hematopoietic cells, NFE2 expression is maintained at a low level by HP1α binding to unphosphorylated H3Y41 and by the presence of dimethylated H3K9. (B) In MPN cells, NFE2 expression is upregulated by both a reduction in HP1α binding, which is associated with increased phosphorylation of H3Y41 by JAK2V617F and by demethylation of H3K9. The figure has been adapted from Figure 6 in the article by Peeken et al that begins on page 2065.
Two epigenetic pathways converge to control NFE2 expression in the MPNs. (A) In healthy hematopoietic cells, NFE2 expression is maintained at a low level by HP1α binding to unphosphorylated H3Y41 and by the presence of dimethylated H3K9. (B) In MPN cells, NFE2 expression is upregulated by both a reduction in HP1α binding, which is associated with increased phosphorylation of H3Y41 by JAK2V617F and by demethylation of H3K9. The figure has been adapted from Figure 6 in the article by Peeken et al that begins on page 2065.
A hallmark of the MPNs is enhanced JAK/STAT activation, which is driven by mutations in JAK2, MPL, and CALR. These mutations are accompanied by dysregulated expression of many genes that modulate the disease phenotype. How enhanced JAK/STAT activation alters gene expression and how the subsequent gene dysregulation contributes to disease are important questions in the field. A notable example of a gene that is dysregulated in the MPNs is NFE2, whose overexpression was found to cause a myeloproliferative disease that resembles the MPNs in animal models.4,5 To study how increased levels of NFE2 contribute to the MPNs, Peeken et al analyzed published data regarding NFE2 chromatin immunoprecipitation sequencing and identified 60 epigenetic regulators as presumptive targets. Among these genes, they focused on the histone demethylase JMJD1C, which is reported to convert H3K9 from a mono- or dimethylated state to an unmethylated state, allowing for increased gene transcription.6 Consistent with it being a direct NFE2 target, JMJD1C expression was elevated in NFE2 transgenic mice, and its messenger RNA levels correlated with those of NFE2 in PV patients. Peeken et al further demonstrated that the increased levels of JMJD1C were associated with decreased H3K9 methylation along the NFE2 locus; this finding suggests that there is a positive feedback loop that augments NFE2 expression (see figure).
Prior studies demonstrated that, beyond enhancing STAT signaling, nuclear JAK2 phosphorylates Y41 on histone H37 ; this event impairs binding of the heterochromatin protein-1α (HP1α) to chromatin, which reduces heterochromatin formation and causes increased gene expression. Peeken et al found that HP1α binding along the NFE2 locus was reduced in PV patients. Reduction in HP1α binding and decreased methylation of H3K9 seem to cooperate to increase NFE2 activity. Further evidence for this dual epigenetic action to enhance NFE2 expression was obtained from studies with the epigenetic modulator decitabine. Treatment of MPN cell lines with decitabine reversed aberrant histone methylation at the NFE2 locus, increased HP1α binding, and shut down NFE2 expression. The levels of JMJD1C were similarly reduced. This effect was likely due in part to the ability of decitabine to suppress JAK2 activity.
JMJD1C has been shown to be essential for RUNX1-RUNX1T1 and MLL-AF9 leukemia,8,9 but what is its role in the MPNs? Peeken et al demonstrate that knockdown of JMJD1C preferentially suppresses cytokine-independent growth of JAK2V617F-expressing Baf/3 cells, suggesting that inhibiting this demethylase may provide therapeutic benefit.
The study by Peeken et al answers one important question about NFE2 regulation but raises several others. First, although JMJD1C knockdown suppressed the growth of Baf/3 cells, to what extent does the observed increase in JMJD1C contribute to disease progression in human MPNs? Second, is this suppression of cell growth upon JMJD1C knockdown a consequence of decreased NFE2 expression? Third, whereas Peeken at al demonstrated that decitabine reduced NFE2 expression, 5-azacitidine showed limited clinical activity in a phase 2 trial as a single agent.10 However, the degree of NFE2 knockdown was not examined in patients treated with 5-azacitidine. The results of Peeken et al raise a question: To what extent does increased NFE2 contribute to MPN pathogenesis, especially given the observation that it is sufficient to cause an MPN-like disease and progression of acute myeloid leukemia in animal models?4,5 Targeting NFE2 will prove challenging, but genetic studies in mice will shed light on this issue. Finally, 60 epigenetic regulators were identified as potential targets of NFE2; therefore, it is likely that there are other targets beyond JMJD1C that contribute to NFE2 dysregulation and the pathogenesis of the MPNs. Although JMJD1C is a putative demethylase for H3K9, inhibitors of the protein have not been developed; thus, an approach to targeting this gene in patients is not feasible at this time. Future studies to identify the other relevant NFE2 targets will increase our understanding of the disease and may reveal more tractable therapeutic targets.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal