Key Points
Ibrutinib may be associated with invasive fungal infections especially IA.
Most infections usually occur during the first months of treatment, often in patients with other risk factors for fungal infections.
Abstract
Ibrutinib has revolutionized the management of chronic lymphocytic leukemia and is now being increasingly used. Although considered to be less immunosuppressive than conventional immunochemotherapy, the observation of a few cases of invasive fungal infections in patients treated with ibrutinib prompted us to conduct a retrospective survey. We identified 33 cases of invasive fungal infections in patients receiving ibrutinib alone or in combination. Invasive aspergillosis (IA) was overrepresented (27/33) and was associated with cerebral localizations in 40% of the cases. Remarkably, most cases of invasive fungal infections occurred with a median of 3 months after starting ibrutinib. In 18/33 cases, other conditions that could have contributed to decreased antifungal responses, such as corticosteroids, neutropenia, or combined immunochemotherapy, were present. These observations indicate that ibrutinib may be associated with early-onset invasive fungal infections, in particular IA with frequent cerebral involvement, and that patients on ibrutinib should be closely monitored in particular when other risk factors of fungal infections are present.
Introduction
Infections still represent an important cause of morbidity and mortality in chronic lymphocytic leukemia (CLL). Impaired humoral and cellular immunity related either to CLL itself or to its treatments, corticosteroid use, and chronic neutropenia are risk factors. Invasive fungal infections (IFI) are rare in CLL, and most cases have been described in previously heavily pretreated patient.
Ibrutinib has revolutionized the therapeutic strategy of CLL.1-3 Clinical trials suggest ibrutinib is associated with fewer infectious complications than standard chemotherapy. Recently, 5 cases of Pneumocystis jirovecii pneumonia in a cohort of 96 CLL patients treated with single-agent ibrutinib have been reported.4 Four out of 5 patients were treatment naïve suggesting that ibrutinib per se may be a risk factor for Pneumocystis infection. Other IFIs, in particular invasive aspergillosis (IA), have been described in several case reports.5-7 Recently, an unexpected number of IA cases were observed in 2 randomized controlled trials in patients receiving ibrutinib for primary central nervous system (CNS) lymphoma.8,9 These data and several personal observations of IFI cases in patients receiving ibrutinib for CLL prompted us to address the question of a potential association of IFI with ibrutinib.
Here we report the results of a retrospective survey in France in which 33 cases of IFI were identified in patients receiving ibrutinib alone or in combination. Our data suggest that mold infections caused by Aspergillus species are the most frequently occurring IFI in patients treated with ibrutinib, with very frequent cerebral localizations, followed by disseminated cryptococcosis. Remarkably, the vast majority of IFI occurred within the first 3 months of treatment.
Study design
We conducted a multicenter survey aimed at identifying cases of IFI among centers of the French Innovative Leukemia Organization CLL group. This study was conducted in accordance with the Declaration of Helsinki and was approved by a local ethics committee. Anonymized data were collected retrospectively with the help of a standardized case report form. Between 2013 and 2017, 33 cases of IFI from 16 French centers were identified, including 2 previously published cases.6
Results and discussion
Patients’ characteristics are shown in Table 1. Thirty patients had CLL, including 15 with 17p deletion. One had mantle cell lymphoma and 2 Waldenström macroglobulinemia. Median number of previous treatments was 2 (range 0-4). Previous treatments consisted of immunochemotherapy. Three patients had received alemtuzumab >3 years before ibrutinib. None had undergone stem cell transplantation. All but 1 patient received ibrutinib for a relapsed/refractory disease. Median time between last treatment and ibrutinib was 10.5 months (range 1-96). Additional predisposing factors were present in the majority of patients. IA accounted for the majority of IFIs (27/33), including 11 cases (40.7%) with CNS localizations (Figure 1). Using European Organisation for Research and Treatment of Cancer criteria, 17 cases were proven, 9 probable and only 1 possible. We also observed 4 cases of disseminated cryptococcosis, 1 mucormycosis, and 1 Pneumocystis pneumonia. Median time between ibrutinib initiation and IFI diagnosis was 3 months (range 1-30). Twenty-eight cases were diagnosed within 6 months with 20 cases occurring ≤3 months. Only 2 patients had very late-onset pulmonary aspergillosis, occurring after 15 and 30 months, respectively. The diagnosis of IFI resulted in ibrutinib discontinuation in 21 patients. In the others, ibrutinib was either continued at a lower dose because of interactions between ibrutinib and voriconazole or resumed after resolution of the IFI. At last follow-up, 17 patients had died either from IFI (9), CLL (5), or unrelated causes (3).
Characteristics and outcome of patients with IFI
| Characteristics of patients . | Number of patients (n = 33) . |
|---|---|
| Gender | |
| Male | 22 |
| Female | 11 |
| Age, years | |
| Median [range] | 70 [31-82] |
| Number of previous lines of treatment | |
| Median [range] | 2 [0-4] |
| 17p deletions | 15/30 CLL |
| Interval between last line and ibrutinib (months) | |
| Median [range] | 10.5 [1-96] |
| Hypogammaglobulinemia | |
| Yes [intravenous immunoglobulins substitution] | 31 [8] |
| No | 2 |
| Additional predisposing factors for IFI | |
| Neutropenia [grade 4] | 5 [2] |
| Corticosteroids | 4 |
| Rituximab + corticosteroids | 3 |
| Rituximab | 2 |
| Concomitant immunochemotherapy | 3 |
| Chemotherapy ≤6 mo | 10 |
| Diabetes mellitus | 2 |
| Cirrhosis | 1 |
| Azathioprine | 1 |
| HIV | 1 |
| Characteristics of patients . | Number of patients (n = 33) . |
|---|---|
| Gender | |
| Male | 22 |
| Female | 11 |
| Age, years | |
| Median [range] | 70 [31-82] |
| Number of previous lines of treatment | |
| Median [range] | 2 [0-4] |
| 17p deletions | 15/30 CLL |
| Interval between last line and ibrutinib (months) | |
| Median [range] | 10.5 [1-96] |
| Hypogammaglobulinemia | |
| Yes [intravenous immunoglobulins substitution] | 31 [8] |
| No | 2 |
| Additional predisposing factors for IFI | |
| Neutropenia [grade 4] | 5 [2] |
| Corticosteroids | 4 |
| Rituximab + corticosteroids | 3 |
| Rituximab | 2 |
| Concomitant immunochemotherapy | 3 |
| Chemotherapy ≤6 mo | 10 |
| Diabetes mellitus | 2 |
| Cirrhosis | 1 |
| Azathioprine | 1 |
| HIV | 1 |
| Characteristics of invasive fungal infection . | |
|---|---|
| Interval between start of ibrutinib and diagnosis of IFI (months) | |
| Median [range] | 3 [1-30] |
| Ibrutinib dose at diagnosis of IFI (mg/d) | |
| 280 | 3 |
| 420 | 29 |
| 560 | 1 |
| Type of infection | |
| IA | 27 |
| Category | |
| Proven | 17 |
| Probable | 9 |
| Possible | 1 |
| Localization | |
| Pulmonary | 15 |
| Pulmonary + CNS | 10 |
| CNS + muscle abscess | 1 |
| Sinus | 1 |
| Cryptococcosis | 4 |
| Pneumocystis pneumonia | 1 |
| Mucormycosis | 1 |
| Isolated microorganism | |
| Aspergillus fumigatus | 16 |
| Aspergillus nidulans | 1 |
| Zygomycetes (Lichtheimia corymbifera) | 1 |
| Cryptococcus neoformans | 3 |
| Pneumocystis jirovecii | 1 |
| Outcome | |
| Alive at last follow-up | 16 |
| Death | 17 |
| Because of IFI | 9 |
| Because of CLL | 5 |
| Other causes | 3 |
| Characteristics of invasive fungal infection . | |
|---|---|
| Interval between start of ibrutinib and diagnosis of IFI (months) | |
| Median [range] | 3 [1-30] |
| Ibrutinib dose at diagnosis of IFI (mg/d) | |
| 280 | 3 |
| 420 | 29 |
| 560 | 1 |
| Type of infection | |
| IA | 27 |
| Category | |
| Proven | 17 |
| Probable | 9 |
| Possible | 1 |
| Localization | |
| Pulmonary | 15 |
| Pulmonary + CNS | 10 |
| CNS + muscle abscess | 1 |
| Sinus | 1 |
| Cryptococcosis | 4 |
| Pneumocystis pneumonia | 1 |
| Mucormycosis | 1 |
| Isolated microorganism | |
| Aspergillus fumigatus | 16 |
| Aspergillus nidulans | 1 |
| Zygomycetes (Lichtheimia corymbifera) | 1 |
| Cryptococcus neoformans | 3 |
| Pneumocystis jirovecii | 1 |
| Outcome | |
| Alive at last follow-up | 16 |
| Death | 17 |
| Because of IFI | 9 |
| Because of CLL | 5 |
| Other causes | 3 |
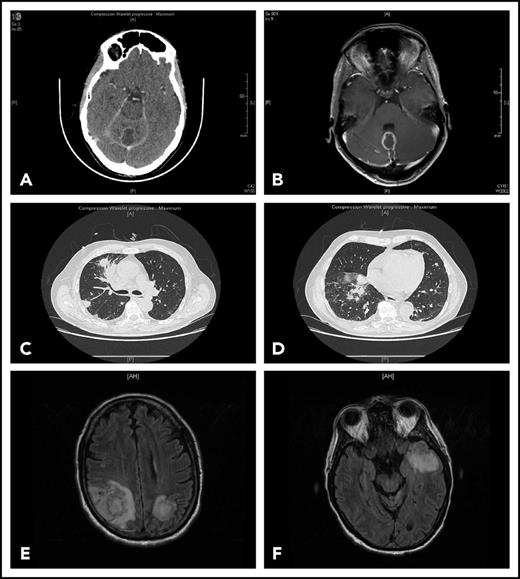
Representative findings in 2 patients with IFI. Patient 1 had been receiving ibrutinib 420 mg/d for 4 months for CLL associated with grade 3 autoimmune neutropenia when she complained of gait disorder. Computed tomography scan (A) and T1-weighted magnetic resonance imaging with gadolinium injection (B) revealed a solitary abscess of the vermis. Neurosurgical drainage disclosed Aspergillus fumigatus. Patient 2 was hospitalized after a third cycle of bendamustine, rituximab, ibrutinib for fever, cough, and confusion. Chest computed tomography scan revealed multiple pulmonary nodules (C-D) and T2-weighted fluid attenuation inversion recovery (FLAIR) magnetic resonance imaging multiple cerebral abscesses (E-F). Aspergillus antigenemia was strongly positive, and Aspergillus fumigatus was isolated in the bronchoalveolar lavage.
Representative findings in 2 patients with IFI. Patient 1 had been receiving ibrutinib 420 mg/d for 4 months for CLL associated with grade 3 autoimmune neutropenia when she complained of gait disorder. Computed tomography scan (A) and T1-weighted magnetic resonance imaging with gadolinium injection (B) revealed a solitary abscess of the vermis. Neurosurgical drainage disclosed Aspergillus fumigatus. Patient 2 was hospitalized after a third cycle of bendamustine, rituximab, ibrutinib for fever, cough, and confusion. Chest computed tomography scan revealed multiple pulmonary nodules (C-D) and T2-weighted fluid attenuation inversion recovery (FLAIR) magnetic resonance imaging multiple cerebral abscesses (E-F). Aspergillus antigenemia was strongly positive, and Aspergillus fumigatus was isolated in the bronchoalveolar lavage.
Epidemiological data on fungal infections in CLL are scarce and usually predate the advent of targeted therapies. IFIs seem rare in newly diagnosed CLL patients.10 In the prospective 2005 to 2007 French study, CLL accounted for 6.6% of the 393 probable or proven cases of IA.11 In the Prospective Antifungal Therapy Alliance registry, CLL patients accounted for 7.1% of the entire hematologic cohort with aspergillosis.12 In a large monocentric study in 1191 adult patients with B-cell malignancies, the rates of IFI and IA in the 305 patients with CLL were much lower, 1.3% and 0.3%, respectively.13 The recent Mayo Clinic CLL database analysis (2005-2016) reported only 30 cases of IA including 5 on ibrutinib.14
Sporadic cases of IA or cryptococcosis occurring in CLL patients during ibrutinib therapy have been reported.5-7 In addition, an unexpectedly high incidence of invasive pulmonary and cerebral aspergillosis was observed in patients enrolled in 2 clinical trials treated with ibrutinib for relapsing/refractory primary CNS lymphoma.8,9 These cases raised concerns about the imputability of ibrutinib15 inasmuch as IA was considered to be a very rare complication in patients with primary CNS lymphoma despite frequent exposure to high-dose corticosteroids.16 A similar warning was also issued by Chamilos et al, who reviewed all cases of IFI reported in patients receiving ibrutinib or other small-molecule kinase inhibitors.17
Interestingly, most of our cases appear to follow a common pattern, which closely resembles the recent series of Ruchlemer et al.18 First, most IFIs had an early onset, suggesting that impairment of antifungal responses occurs rapidly before the immune reconstitution that has been observed with prolonged ibrutinib treatment.19 Second, IA was overrepresented (80%) with a strikingly high incidence of CNS involvement. Finally, other factors that may have contributed to IFI were frequently associated. This has also been observed in other reports.7,9,18 Altogether, this suggests ibrutinib alone may not in most cases be sufficient for IFI development, but its effect can be unmasked when an underlying immune deficiency is associated. This might explain in part why nearly all cases were relapsed/refractory disease, as these patients are potentially more immunosuppressed than first-line patients.
Increased risk of IFI was not apparent in the results of the initial clinical studies on ibrutinib in CLL.20 Our results and that of others confirm that ibrutinib may be associated with IFI in real life. Although our study was not designed to determine an incidence, IFI seem infrequent considering the number of patients who received ibrutinib for the last 4 years in France. This is consistent with data from Ohio State University.21 Opportunistic infections were identified in 23/566 ibrutinib-exposed patients (4.1%), IFI representing nearly half the cases.
How ibrutinib may decrease antifungal immunity remains to be clarified. Multiple mechanisms may be involved (supplemental Figure 1; available on the Blood Web site). In mice, Btk plays an important role in neutrophil differentiation and function.22 Upon phagocytosis, Aspergillus fumigatus activates a TLR9-BTK-calcineurin-NFAT pathway.23 Btk−/− mice exposed to Aspergillus fumigatus have a higher mortality rate than mice with wild-type Btk.9 However, humans with X-linked agammaglobulinemia, caused by inactivating Btk mutations, are not susceptible to fungal infections.24 An off-target effect outside Btk inhibition may also be considered. For example, ibrutinib strongly inhibits platelet degranulation, which has been shown to attenuate the virulence of Aspergillus species in vitro.25
In conclusion, our data confirm that ibrutinib may be associated with the development of IFI, in particular IA. IFIs tend to occur within the first months of treatment and are infrequent thereafter. Although it seems difficult at this point to advocate for systematic antifungal prophylaxis in all patients, an increased awareness about the potential risk of IFI after initiating ibrutinib is warranted, especially when other predisposing factors are associated. Further prospective epidemiological studies will help determine the actual risk of IFI in patients treated with ibrutinib or other Btk inhibitors.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors would like to thank Julie Bonhomme (Caen, France), Renaud Verdon (Caen, France), Pierre Tattevin (Rennes, France), Jean-Phillippe Talarmin (Quimper, France), Sophie Raynaud (Nice, France), Benjamin Wyplosz (Le Kremlin-Bicêtre, France), Véronique Leblond (Paris, France), and Arnaud Fekkar (Paris, France) for their help in the realization of this study.
Authorship
Contribution: D.G. and L.Y. conceived the study and wrote the manuscript; and A.C., C.P., M.B., M.-P.L., G.D., C.H., M.D., B.D., E.F., K.L., R.L.C., M.M., F.P., L.S., M.T.-G., K.D., and C.D. took care of the patients and completed the data forms for the study.
Conflict-of-interest disclosure: D.G. is the recipient of a research grant from Janssen. L.Y. received consultancy fees from Janssen. The remaining authors declare no competing financial interests.
A list of additional members of the French Innovative Leukemia Organization (FILO) CLL group appears in “Appendix.”
Correspondence: David Ghez, Département d’Hématologie, Gustave Roussy, 114 rue Edouard Vaillant, 94805 Villejuif Cedex, France; e-mail: david.ghez@gustaveroussy.fr.
Appendix: study group members
Additional members of the French Innovative Leukemia Organization (FILO) CLL group: Alain Delmer (Reims, France); Thérèse Aurran-Schleinitz (Marseille, France); Marie-Christine Béné (Nantes, France); Annie Brion (Besançon, France); Guillaume Cartron (Montpellier, France); Aline Clavert (Angers, France); Florence Cymbalista (Bobigny, France); Sophie de Guibert (Rennes, France); Roseline Delépine (Tours, France); Marie-Sarah Dilhuydy (Bordeaux, France); Bernard Drenou (Mulhouse, France); Charles Dumontet (Lyon, France); Jehan Dupuis (Creteil, France); Pierre Feugier (Nancy, France); Fontanet Bijou (Bordeaux, France); Luc-Matthieu Fornecker (Strasbourg, France); Romain Guieze (Clermont-Ferrand, France); Katell Ledu (Le Mans, France); Magali Le Garff Tavernier (Paris, France); Véronique Leblond (Paris, France); Stephane Lepretre (Rouen, France); Rémi Letestu (Bobigny, France); Vincent Levy (Bobigny, France); Béatrice Mahé (Nantes, France); Karim Maloum (Paris, France); Marc Maynadié (Dijon, France); Fatiha Merabet (Versailles, France); Anne-Sophie Michallet (Lyon, France); Pierre Morel (Amiens, France); Florence Nguyen Khac (Paris, France); Delphine Nollet (Tour, France); Brigitte Pégourie (Grenoble, France); Bertrand Pollet (Boulogne sur Mer, France); Stéphanie Poulain (Valenciennes, France); Anne Quinquenel (Reims, France); Sophie Raynaud (Nice, France); Daniel Ré (Antibes, France); Philippe Rodon (Périgueux, France); Valérie Rouille (Montpellier, France); Laurence Sanhes (Perpignan, France); Cécile Tomowiak (Poitiers, France); Olivier Tournilhac (Clermont Ferrand, France); Xavier Troussard (Caen, France); Eric Van Den Neste (Bruxelles, France); Sandrine Vaudaux (Rouen, France); Marguerite Vignon (Paris, France); Jean-Pierre Vilque (Caen, France); Maud Voldoire (La Roche sur Yon, France); Lise Willems (Paris, France); and Jean-Marc Zini (Paris, France).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal