Key Points
Our 5-year experience shows sustained single-agent efficacy of ibrutinib in CLL patients, with complete response rates increasing over time.
Long-term ibrutinib was well tolerated with no new safety signals; rates of grade ≥3 cytopenias decreased with continued therapy.
Abstract
We previously reported durable responses and manageable safety of ibrutinib from a 3-year follow-up of treatment-naïve (TN) older patients (≥65 years of age) and relapsed/refractory (R/R) patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL). We now report on long-term efficacy and safety with median follow-up of 5 years in this patient population with TN (N = 31) and R/R (N = 101) CLL/SLL. With the current 5-year follow-up, ibrutinib continues to yield a high overall response rate of 89%, with complete response rates increasing over time to 29% in TN patients and 10% in R/R patients. The median progression-free survival (PFS) was not reached in TN patients. The 5-year PFS rate was 92% in TN patients and 44% in R/R patients. Median PFS in R/R patients was 51 months; in those with del(11q), del(17p), and unmutated IGHV, it was 51, 26, and 43 months, respectively, demonstrating long-term efficacy of ibrutinib in some high-risk subgroups. Survival outcomes were less robust for R/R patients with del(17p) and those who received more prior therapies. The onset of grade ≥3 cytopenias, such as neutropenia and thrombocytopenia, decreased over time. Treatment--limiting adverse events were more frequent during the first year compared with subsequent periods. These results demonstrate sustained efficacy and acceptable tolerability of ibrutinib over an extended time, providing the longest experience for Bruton tyrosine kinase inhibitor treatment in patients with CLL/SLL. These trials were registered at www.clinicaltrials.gov as #NCT01105247 and #NCT01109069.
Introduction
Chemoimmunotherapy has improved treatment outcomes in chronic lymphocytic leukemia (CLL),1-7 one of the most common forms of leukemia in Western countries. However, relapse after chemoimmunotherapy is common, and shorter remissions have been associated with short survival, regardless of the salvage therapy administered.8 Furthermore, chemotherapeutic regimens are often associated with significant toxicities,3,6,7 limiting their use in older individuals with comorbidities that compose the majority of patients.
Genomic factors play a critical role in determining survival outcomes in CLL. The presence of an unmutated IGHV gene or del(17p), del(11q), or TP53 mutations in patients receiving chemoimmunotherapy, including fludarabine, cyclophosphamide, and rituximab (FCR), has been associated with inferior progression-free survival (PFS).3,4,9-12 Treatment-naïve (TN) patients with CLL and del(17p) who received FCR experienced shorter PFS3 and postprogression survival8 compared with that seen in other genetic subgroups. The presence of del(17p) was identified as the strongest negative prognostic factor for PFS and overall survival (OS).3,4 Similarly, TN patients with an unmutated IGHV gene experience shorter PFS compared with that seen in patients with mutated IGHV.3,4,11 Shorter remission durations in high-risk patients, poor survival following FCR relapse, and toxicity associated with chemoimmunotherapy warrant investigation of novel targeted therapies in CLL.
Ibrutinib, a first-in-class, once-daily inhibitor of Bruton tyrosine kinase, is approved in the United States for the treatment of patients with CLL/small lymphocytic lymphoma (SLL) and allows for treatment without chemotherapy. Results from a phase 1b/2 study (PCYC-1102) of ibrutinib in relapsed/refractory (R/R) and symptomatic TN patients ≥65 years of age with CLL indicated high overall response rates (ORRs), sustained remissions, and acceptable toxicity.13,14 Follow-up of patients from PCYC-1102, including patients who continued on an extension study, PCYC-1103, supported continued activity of ibrutinib with durable responses and manageable toxicity over an extended 3-year period.15 In that analysis, the ORR was 89% with 11% complete response (CR) in patients receiving ibrutinib 420 or 840 mg daily.15 Because ibrutinib is a continuously administered oral therapy that is routinely used in the clinic, we continue to follow patients from PCYC-1102/1103, focusing on high-risk subgroups, prognostic determinants of efficacy, quality of response, and extended safety data over time. Here, we report our 5-year experience with single-agent ibrutinib, the longest follow-up reported to date for a Bruton tyrosine kinase inhibitor, in 132 patients enrolled in PCYC-1102/1103.
Materials and methods
Study design and patients
Study methods of PCYC-1102/1103 have been previously described.15 Briefly, patients received 420 or 840 mg ibrutinib orally daily until progressive disease or unacceptable toxicity. Although not mandatory, antimicrobial prophylaxis was allowed. Strong CYP3A4/5 inhibitors were avoided where possible. Following an amendment, warfarin use was prohibited but alternative anticoagulant drugs were allowed. Similarly, a precaution was added to hold ibrutinib for 3 to 7 days pre- and postinvasive procedures to reduce the risk of bleeding.
All patients provided written informed consent. The study was approved by the institutional review board or ethics committee at each participating institution and conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Guidelines for Good Clinical Practice. Data analyses were performed by Pharmacyclics LLC, and all authors had full access to study data.
End points and assessments
The primary end point was safety, as previously described.15 Additional end points included ORR, CR, PFS, and OS. Response assessments were performed every 3 months for cycles 1 through 18 and every 6 months thereafter and included computed tomography scans of the chest, abdomen, and pelvis. Responses in patients with CLL were assessed according to the International Workshop on CLL 2008 guidelines.16 A partial response (PR) in all other parameters in the setting of persistent lymphocytosis was characterized as a PR with lymphocytosis (PR-L).17 Response in patients with SLL was assessed according to the International Working Group for non-Hodgkin Lymphoma 2007 criteria.18 CR was confirmed by bone marrow biopsy/aspirate, and responses maintained for at least 2 months were considered confirmed. Duration of response (DOR) was calculated from date of first response to progression or death in patients with PR-L or better. Evaluation of chromosomal abnormalities (detected by fluorescence in situ hybridization [FISH]) and complex karyotype (CK; by CpG stimulation and metaphase analyses) were performed by a central laboratory.
Statistical analysis
Analyses were performed with SAS 9.3 (SAS Institute, Cary, NC). Survival analysis by FISH cytogenetic subgroups was based on Döhner hierarchy categorization. Cox proportional hazard model and Kaplan-Meier methods were used for univariate and multivariate analyses of PFS and OS in R/R patients. The following variables were used for analyses: age (<65 vs ≥65 years), gender (male vs female), Rai stage (0-II vs III-IV), Eastern Cooperative Oncology Group performance status (≥1 vs 0), number of prior lines of therapy (1-3 vs ≥4), bulky disease (≥5 cm vs <5 cm), β2-microglobulin levels (>3.5 mg/L vs ≤3.5 mg/L), del(13q) (yes vs no), del(11q) (yes vs no), CK (yes vs no), trisomy 12 (yes vs no), IGHV mutation status (unmutated vs mutated), and del(17p) (yes vs no). Univariate analyses were performed first, and significant variables (P < .1) were included in the multivariate model, which was performed using a stepwise approach.
Results
Patient demographics and baseline characteristics
The PCYC-1102/1103 studies included 132 patients (31 TN, 101 R/R). The median age was 68 years (range, 37-84), with 43% of patients ≥70 years (Table 1). Bulky disease (>5 cm) at baseline was present in 46% of patients. High-risk genomic features were present in a large proportion of patients, including unmutated IGHV (71%), CK (31%), del(17p) (27%), and del(11q) (27%) (Table 1). R/R patients received a median of 4 prior therapies (range, 1-12 therapies).
Baseline characteristics of all treated patients
| . | TN ≥65 y (n = 31) . | R/R (n = 101) . | All patients (N = 132) . |
|---|---|---|---|
| Median age (range), y | 71 (65-84) | 64 (37-82) | 68 (37-84) |
| Age ≥70, n (%) | 23 (74) | 34 (34) | 57 (43) |
| ECOG performance status, n (%) | |||
| 0 | 23 (74) | 43 (43) | 66 (50) |
| 1 | 8 (26) | 54 (53) | 62 (47) |
| 2 | 0 (0) | 4 (4) | 4 (3) |
| Rai stage, n (%) | |||
| 0-II | 13 (42) | 38 (38) | 51 (39) |
| III-IV | 17 (55) | 58 (57) | 75 (57) |
| Unknown | 1 (3) | 5 (5) | 6 (5) |
| Bulky disease (lymph nodes), n (%) | |||
| ≥5 cm in diameter | 6 (19) | 55 (54) | 61 (46) |
| ≥10 cm in diameter | 0 | 15 (15) | 15 (11) |
| Unmutated IGHV gene, n (%) | 15 (48) | 79 (78) | 94 (71) |
| Cytogenetic abnormalities, n (%) | |||
| del(17p) | 2 (6) | 34 (34) | 36 (27) |
| del(11q) | 1 (3) | 35 (35) | 36 (27) |
| Trisomy 12 | 8 (26) | 12 (12) | 20 (15) |
| del(13q) | 17 (55) | 47 (47) | 64 (49) |
| Complex karyotype | 4 (13) | 37 (37) | 41 (31) |
| β2-microglobulin level >3.5 mg/L, n (%) | 4 (13) | 37 (37) | 41 (31) |
| Median CrCl (range), mL/min | 67 (31-156) | 81 (36-213) | 79 (31-213) |
| <60 mL/min, n (%) | 9 (29) | 20 (20) | 29 (22) |
| Median ANC (range), ×109/L | 3.9 (0-19.4) | 2.5 (0-19) | 2.6 (0-19.4) |
| ANC ≤1.5 × 109/L | 1 (3) | 34 (34) | 35 (27) |
| Median hemoglobin (range), g/L | 122 (77-157) | 115 (62-176) | 117 (62-176) |
| ≤11 g/dL, n (%) | 11 (35) | 42 (42) | 53 (40) |
| Median platelets (range), ×109/L | 113 (32-217) | 105 (2-310) | 105 (2-310) |
| ≤100 × 109/L, n (%) | 12 (39) | 49 (49) | 61 (46) |
| Hemoglobin ≤11 g/dL or platelets ≤100 × 109/L, n (%) | 20 (65) | 61 (60) | 81 (61) |
| Median ALC (range), ×109/L | 41.1 (0.3-240.2) | 8.9 (0.1-298.9) | 13 (0.1-299) |
| Median no. of prior therapies, n (range) | — | 4 (1-12) | — |
| 1-2, n (%) | — | 27 (27) | — |
| 3, n (%) | — | 14 (14) | — |
| ≥4, n (%) | — | 60 (59) | — |
| Types of prior systemic therapy, n (%) | |||
| Chemotherapy | — | 101 (100) | — |
| Nucleoside analog | — | 97 (96) | — |
| Alkylator (including bendamustine) | — | 92 (91) | — |
| Anti-CD20–based regimen | — | 99 (98) | — |
| Anti-CD20–based chemoimmunotherapy | — | 97 (96) | — |
| Alemtuzumab-based regimen | — | 23 (23) | — |
| Idelalisib | — | 6 (6) | — |
| . | TN ≥65 y (n = 31) . | R/R (n = 101) . | All patients (N = 132) . |
|---|---|---|---|
| Median age (range), y | 71 (65-84) | 64 (37-82) | 68 (37-84) |
| Age ≥70, n (%) | 23 (74) | 34 (34) | 57 (43) |
| ECOG performance status, n (%) | |||
| 0 | 23 (74) | 43 (43) | 66 (50) |
| 1 | 8 (26) | 54 (53) | 62 (47) |
| 2 | 0 (0) | 4 (4) | 4 (3) |
| Rai stage, n (%) | |||
| 0-II | 13 (42) | 38 (38) | 51 (39) |
| III-IV | 17 (55) | 58 (57) | 75 (57) |
| Unknown | 1 (3) | 5 (5) | 6 (5) |
| Bulky disease (lymph nodes), n (%) | |||
| ≥5 cm in diameter | 6 (19) | 55 (54) | 61 (46) |
| ≥10 cm in diameter | 0 | 15 (15) | 15 (11) |
| Unmutated IGHV gene, n (%) | 15 (48) | 79 (78) | 94 (71) |
| Cytogenetic abnormalities, n (%) | |||
| del(17p) | 2 (6) | 34 (34) | 36 (27) |
| del(11q) | 1 (3) | 35 (35) | 36 (27) |
| Trisomy 12 | 8 (26) | 12 (12) | 20 (15) |
| del(13q) | 17 (55) | 47 (47) | 64 (49) |
| Complex karyotype | 4 (13) | 37 (37) | 41 (31) |
| β2-microglobulin level >3.5 mg/L, n (%) | 4 (13) | 37 (37) | 41 (31) |
| Median CrCl (range), mL/min | 67 (31-156) | 81 (36-213) | 79 (31-213) |
| <60 mL/min, n (%) | 9 (29) | 20 (20) | 29 (22) |
| Median ANC (range), ×109/L | 3.9 (0-19.4) | 2.5 (0-19) | 2.6 (0-19.4) |
| ANC ≤1.5 × 109/L | 1 (3) | 34 (34) | 35 (27) |
| Median hemoglobin (range), g/L | 122 (77-157) | 115 (62-176) | 117 (62-176) |
| ≤11 g/dL, n (%) | 11 (35) | 42 (42) | 53 (40) |
| Median platelets (range), ×109/L | 113 (32-217) | 105 (2-310) | 105 (2-310) |
| ≤100 × 109/L, n (%) | 12 (39) | 49 (49) | 61 (46) |
| Hemoglobin ≤11 g/dL or platelets ≤100 × 109/L, n (%) | 20 (65) | 61 (60) | 81 (61) |
| Median ALC (range), ×109/L | 41.1 (0.3-240.2) | 8.9 (0.1-298.9) | 13 (0.1-299) |
| Median no. of prior therapies, n (range) | — | 4 (1-12) | — |
| 1-2, n (%) | — | 27 (27) | — |
| 3, n (%) | — | 14 (14) | — |
| ≥4, n (%) | — | 60 (59) | — |
| Types of prior systemic therapy, n (%) | |||
| Chemotherapy | — | 101 (100) | — |
| Nucleoside analog | — | 97 (96) | — |
| Alkylator (including bendamustine) | — | 92 (91) | — |
| Anti-CD20–based regimen | — | 99 (98) | — |
| Anti-CD20–based chemoimmunotherapy | — | 97 (96) | — |
| Alemtuzumab-based regimen | — | 23 (23) | — |
| Idelalisib | — | 6 (6) | — |
ALC, absolute lymphocyte count; ANC, absolute neutrophil count; CrCl, creatinine clearance; ECOG, Eastern Cooperative Oncology Group.
The baseline frequencies of CK, del(17p), del(11q), and unmutated IGHV were lower among TN patients than R/R patients (CK: 13% and 37%; del[17p]: 6% and 34%; del[11q]: 3% and 35%; unmutated IGHV: 48% and 78%). The presence of CK, del(17p), and del(11q) was observed in increasing proportions with greater numbers of prior therapies (supplemental Table 1 on the Blood Web site). The type of prior therapies did not differ substantially based on presence of high-risk features, although more R/R patients with CK received ≥4 prior therapies than those without CK (73% and 47%). The proportion of R/R patients who received ≥4 prior therapies was higher in patients with unmutated IGHV than in those with mutated IGHV (65% and 44%). The median number of therapies was higher in R/R patients with CK (4 vs 3 prior for patients without CK) and unmutated IGHV (4 vs 3 prior for mutated IGHV). A higher proportion of R/R patients with CK had baseline cytopenias (78% and 59%) and bulky disease (70% and 51%) compared with those without CK. Bulky disease was more common in R/R patients with unmutated IGHV than in those with mutated IGHV (58% and 38%). Cooccurrence of high-risk features was not uncommon; of 37 R/R patients with CK, 33 (89%) had unmutated IGHV and 22 (59%) had del(17p).
Patient disposition and safety
The median time on ibrutinib for TN patients was 65 months, with 77% treated with ibrutinib for >4 years. Forty-five percent of these patients discontinued treatment, with the most common reasons being adverse events (AEs; 19%) and disease progression (6%). AEs leading to treatment discontinuation in TN patients included 1 case each of fatigue, viral infection, malignant neoplasm, pruritic rash, skin lesion, and hypertension. AEs led to dose reductions in 13% of TN patients. After approximately 5 years of follow-up, 55% of TN patients remain on ibrutinib treatment (supplemental Table 2).
The median treatment duration in R/R patients was 39 months, with 39% remaining on ibrutinib for >4 years. Median time on treatment of the R/R subgroup with del(17p) was 24 months (range, 0.3-73). Overall, 72% of R/R patients discontinued treatment, most commonly because of disease progression (33%) and AEs (21%). Among R/R patients with del(17p), 15% discontinued because of AEs and 53% from disease progression. AEs leading to discontinuation in more than 1 R/R patient included sepsis (n = 3), diarrhea (n = 2), and subdural hematoma (n = 2). Atrial fibrillation (AF) led to treatment discontinuation in 1 R/R patient. After approximately 5 years of follow-up, 28% of R/R patients (18% of R/R patients with del[17p]) continue ibrutinib on study (supplemental Table 2).
Analysis of disposition in R/R patients by additional high-risk features indicated that more patients with unmutated IGHV (77% vs 63% with mutated IGHV) and with CK (84% vs 63% without CK) discontinued treatment. The most common reason for treatment discontinuation was disease progression in patients with unmutated IGHV (37%) or CK (49%), whereas AEs comprise the most common reason in those with mutated IGHV (38%) or without CK (22%). Discontinuation rates were similar in patients with or without del(11q) (74% and 72%).
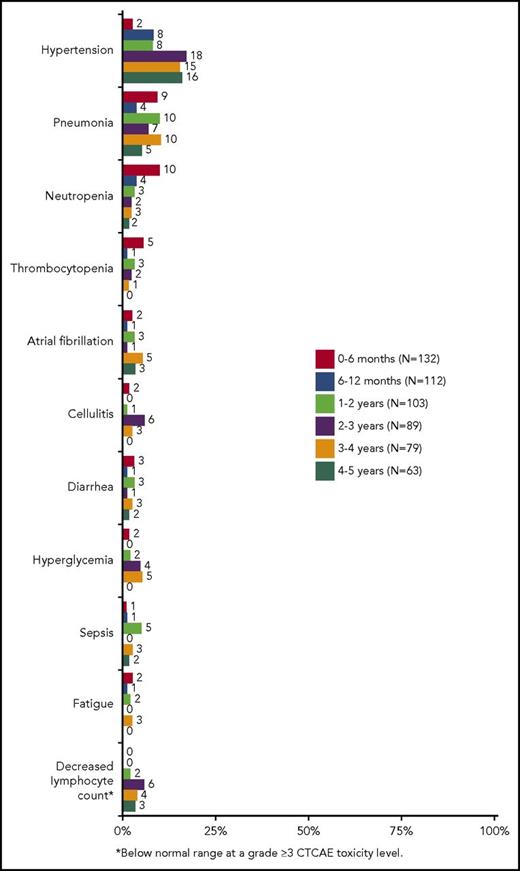
In TN and R/R patients, the most common grade ≥3 AEs over a median 65 and 39 months of therapy, respectively, included hypertension (32% TN, 25% R/R), pneumonia (10% TN, 27% R/R), neutropenia (3% TN, 21% R/R), thrombocytopenia (3% TN, 11% R/R), and AF (6% TN, 9% R/R). Of note, 70% (7/10) of TN patients and 68% (17/25) of R/R patients with grade ≥3 hypertension had preexisting hypertension at study entry. Other infections such as cellulitis, sepsis, bacteremia, and sinusitis also occurred more frequently in R/R patients than in TN patients (supplemental Table 3). The occurrence of major hemorrhage was similar between TN and R/R patients (10% and 9%, respectively). The onset of grade ≥3 AEs occurring in >3% of all treated patients is shown in Figure 1. The frequency of most common grade ≥3 cytopenias, including neutropenia and thrombocytopenia, was highest during the first year and subsequently decreased in later years, whereas the frequency of hypertension was relatively constant after the second year of follow-up and that of AF was relatively constant over the entire follow-up period.
Onset of common grade ≥3 adverse events (in >3% of all treated patients) over time.
Onset of common grade ≥3 adverse events (in >3% of all treated patients) over time.
Efficacy
Response and survival outcomes in the overall population
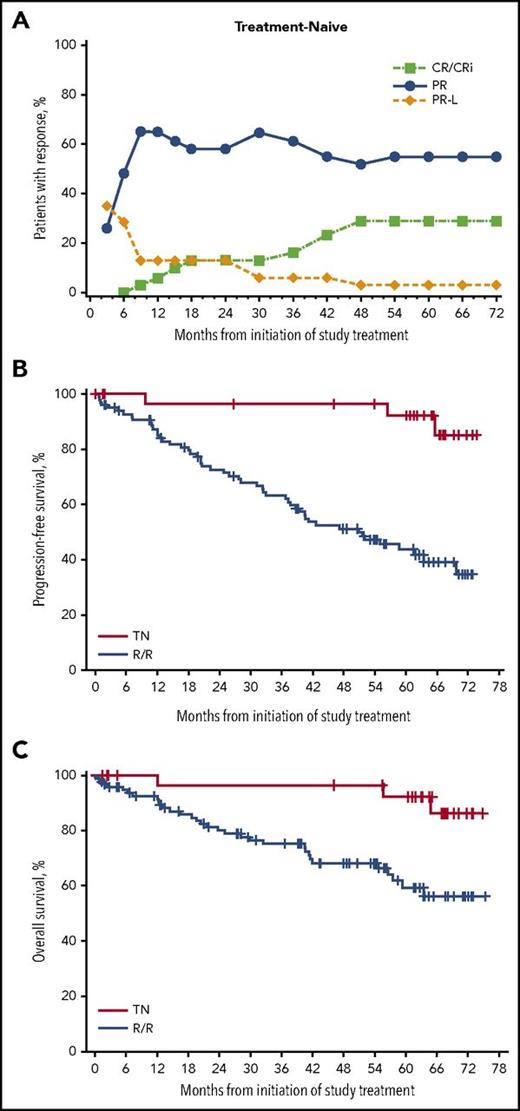
The ORR (including PR-L) by investigator assessment was 87% (95% confidence interval [CI], 70.2-96.4) for TN and 89% (95% CI, 81.3-94.4) for R/R patients (supplemental Figure 1A); the median DOR was 72.8 months (range, >0.0 to 72.8) for TN and 57 months (range, >0.0 to >71.0) for R/R patients. A higher CR rate was reported in TN patients (29% in TN and 10% in R/R). The CR rate improved with time in TN and R/R patients, with a larger increase in the CR rate observed in TN patients (Figure 2A; supplemental Figure 1B). Of the 77 (58%) patients who achieved PR-L as their initial response, 13 (17%) went on to achieve CR/CR with incomplete marrow recovery, and 60 (78%) went on to achieve PR.
Outcomes in TN and R/R populations. (A) Cumulative best response in TN patients over time. (B) PFS in TN and R/R patients. (C) OS in TN and R/R patients. One TN patient with disease progression on day 2274 was excluded because of the very limited numbers at risk at that time and to ensure appropriate calculation of median. The patient was censored at last response assessment before progression. CRi, CR with incomplete marrow recovery.
Outcomes in TN and R/R populations. (A) Cumulative best response in TN patients over time. (B) PFS in TN and R/R patients. (C) OS in TN and R/R patients. One TN patient with disease progression on day 2274 was excluded because of the very limited numbers at risk at that time and to ensure appropriate calculation of median. The patient was censored at last response assessment before progression. CRi, CR with incomplete marrow recovery.
At a median time on study of 61.5 months (range, >0.7 to 75.2), median PFS was not reached (NR) in TN patients and was 51 months in R/R patients (Figure 2B). At the end of the 5-year follow-up, 3% of TN patients had progressed compared with 36% of R/R patients. Among TN patients, 1 progression occurred within the first year and 1 progression occurred beyond 6 years of treatment; 2 other PFS events were deaths occurring beyond 4 years of treatment and were due to AEs unrelated to ibrutinib (normal pressure hydrocephalus and crypt cell carcinoma). The estimated 5-year PFS rate was 92% for TN patients and 44% for R/R patients. The median OS was NR for R/R and TN patients (Figure 2C). The 5-year OS rate was 92% in TN patients and 60% in R/R patients.
Predictors of outcomes in R/R patients
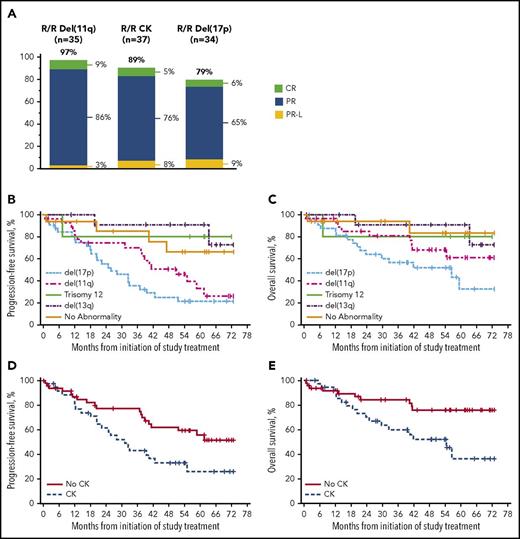
The ORR for R/R patients with del(17p) was 79%, with a median DOR of 31 months. Response rates exceeded 80% in all other R/R subgroups regardless of high-risk genetic features, including in patients with CK (89% ORR) (Figure 3A). Patients with del(11q) or CK tended to experience shorter DOR than patients without these abnormalities, with a median DOR of 39 months in patients with del(11q) (NR in patients without del[11q]) and 31 months in patients with CK (NR in patients without CK). Similar to results reported after 3-year follow-up, the median PFS and OS were shortest (26 and 57 months, respectively) in R/R patients with del(17p) compared with that seen in other high-risk cytogenetic groups; median PFS in patients with del(11q) was 51 months and median OS was NR (5-year OS rate, 61%) (Figure 3B-C). R/R patients with CK had a shorter median PFS (31 months and NR; Figure 3D) and median OS (54 months and NR; Figure 3E) than those without CK.
Outcomes in R/R patients with chromosomal abnormalities detected by FISH and with CK. (A) Best response by high-risk genetic features. (B) PFS by chromosomal abnormalities detected by FISH. (C) OS by chromosomal abnormalities detected by FISH. (D) PFS by CK. (E) OS by CK. Survival analysis by FISH cytogenetic subgroups was based on Döhner hierarchy categorization.
Outcomes in R/R patients with chromosomal abnormalities detected by FISH and with CK. (A) Best response by high-risk genetic features. (B) PFS by chromosomal abnormalities detected by FISH. (C) OS by chromosomal abnormalities detected by FISH. (D) PFS by CK. (E) OS by CK. Survival analysis by FISH cytogenetic subgroups was based on Döhner hierarchy categorization.
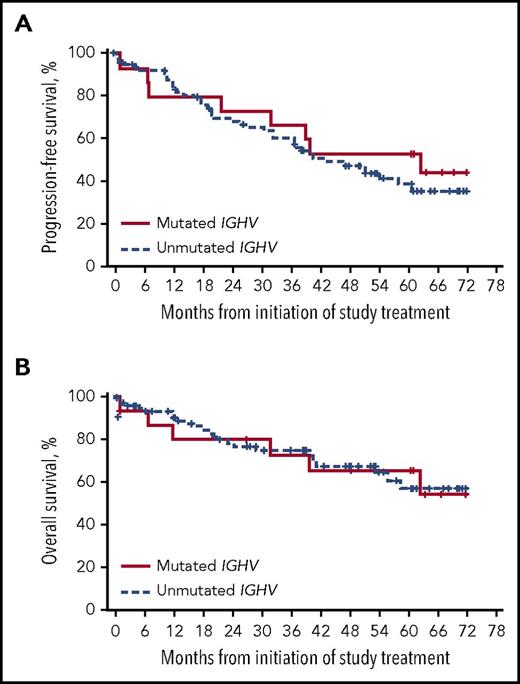
High ORR was observed regardless of IGHV mutation status (mutated, 81%; unmutated, 90%), with a CR rate of 9% for patients with unmutated IGHV. The median DOR in R/R patients with unmutated IGHV was 50 months and NR in patients with mutated IGHV. Median PFS was 43 months in patients with unmutated IGHV and 63 months in patients with mutated IGHV (5-year PFS: 39% [95% CI, 26-51] and 53% [95% CI, 26-74]; Figure 4A). Median OS was NR irrespective of IGHV mutation status, with a similar 5-year OS rate in patients with unmutated and mutated IGHV (57% and 66%; Figure 4B).
Outcomes in R/R patients by IGHV gene mutation status. (A) PFS by IGHV mutational status. (B) OS by IGHV mutational status.
Outcomes in R/R patients by IGHV gene mutation status. (A) PFS by IGHV mutational status. (B) OS by IGHV mutational status.
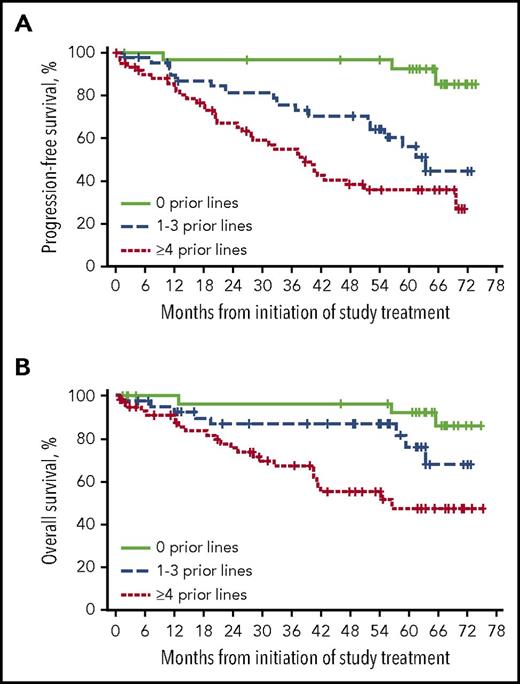
Patients who received 1 to 3 lines of prior therapy experienced a slightly higher ORR (93%) than patients who received ≥4 lines of prior therapy (87%); however, the median DOR was approximately doubled in patients with 1 to 3 lines compared with ≥4 lines of prior therapy (62 and 37 months). Median PFS was longer in R/R patients treated with fewer prior therapies (63 months for 1-3 and 39 months for ≥4 prior therapies; Figure 5A). Treatment with fewer prior therapies was also associated with longer median OS (Figure 5B). Among 5 patients who were treated with idelalisib before study entry and had at least 1 response assessment, all experienced a best response of PR with ibrutinib with DOR ranging from 0.03 to 23.1 months.
Outcomes by prior lines of therapy. (A) PFS by prior lines of therapy. (B) OS by prior lines of therapy.
Outcomes by prior lines of therapy. (A) PFS by prior lines of therapy. (B) OS by prior lines of therapy.
Patients with nonbulky disease (<5 cm) had longer PFS than patients with bulky disease (≥5 cm; median PFS, NR and 43 months; 5-year PFS, 67% and 44%; supplemental Figure 2A). Similarly, patients without bulky disease experienced longer OS than patients with bulky disease, with medians NR and 66 months, respectively (supplemental Figure 2B).
Analysis by del(17p) in R/R patients with CK
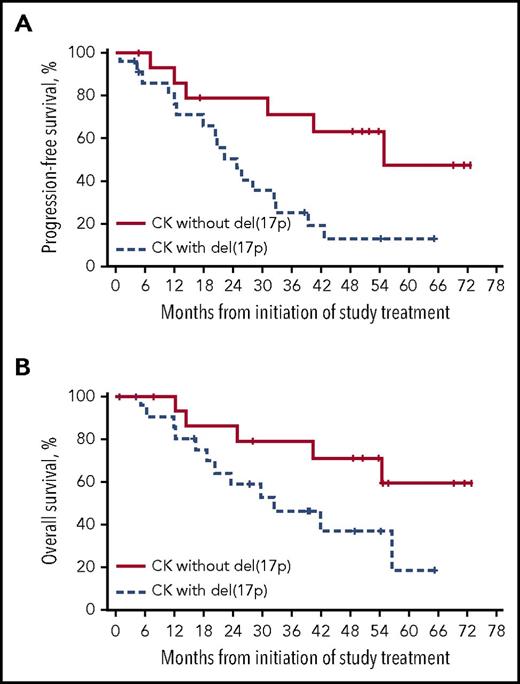
To evaluate the role of del(17p) in patients with CK, we further analyzed efficacy outcomes by coexistence of these features. The 15 patients who had CK without del(17p) exhibited a higher ORR and a longer DOR than the 22 patients with both CK and del(17p) (ORR, 100% and 82%; DOR, 53 and 23 months). The median PFS of patients with CK without del(17p) was more than twice the median PFS of those with del(17p) (55 and 25 months; Figure 6A). Further, patients with CK without del(17p) experienced a longer median OS of NR compared with 32 months in patients with CK and del(17p) (Figure 6B). Of the 15 patients with CK without del(17p), 14 had unmutated IGHV and 12 had abnormalities of chromosome 11 including del(11q) and/or loss of ATM; 5 were found to have 3 copies of MYC.
Outcomes in R/R patients with CK by presence of del(17p). (A) PFS by del(17p) in R/R patients with CK. (B) OS by del(17p) in R/R patients with CK.
Outcomes in R/R patients with CK by presence of del(17p). (A) PFS by del(17p) in R/R patients with CK. (B) OS by del(17p) in R/R patients with CK.
Multivariate analysis for PFS and OS in R/R group
The following variables were significantly associated with PFS in univariate analysis at a significance level of 0.1: β2-microglobulin >3.5 mg/L, bulky disease ≥5 cm, del(17p), more prior therapies (≥4 vs 1-3 prior), and CK. In multivariate analysis using a stepwise approach, del(17p) (hazard ratio [HR], 2.389; P = .008) and more prior therapies (HR, 2.095; P = .040) were the only independent factors significantly associated (P < .05) with decreased PFS in R/R patients.
In univariate analysis, bulky disease ≥5 cm, del(17p), greater lines of prior therapy (≥4 vs 1-3 prior), and CK were significantly associated with OS. Similar to PFS outcomes, multivariate analysis identified only del(17p) (HR, 2.439; P = .027) and greater lines of prior therapy (HR, 6.030; P = .004) as independent factors significantly associated with decreased OS.
Discussion
The results of this analysis demonstrate the long-term efficacy and durable responses with single-agent ibrutinib in patients with TN and R/R CLL, with sustained PFS and OS outcomes shown by 5-year PFS rates of 92% in TN and 44% in R/R patients; the median OS has not been reached in either group with the current follow-up. PFS with single-agent ibrutinib in TN patients appears particularly favorable because the median has not been reached. The 5-year PFS rate was 43% in TN patients ≥65 years treated with FCR chemoimmunotherapy.4 In our previous 3-year follow-up report from the same population, the ORR was 89% (11% CR, 74% PR, and 4% PR-L) at a median time on study of 35.2 months.15 With the current 5-year follow-up, the ORR remains at 89% and the CR rates improved over time to 29% in TN and 10% in R/R patients, demonstrating deepening of responses with continued ibrutinib therapy. Compared with R/R patients, the higher CR rates and 5-year PFS and OS rates in TN patients suggest that the best outcomes may be achieved with ibrutinib in earlier lines of therapy. This is further supported by results from both univariate and multivariate analyses showing that fewer lines of prior therapy were associated with significantly improved PFS and OS outcomes.
Efficacy with single-agent ibrutinib was observed in patients with high-risk genetic features, with high ORR (79% to 97%) regardless of genomic subgroups. R/R patients with del(11q) experienced PFS and OS outcomes similar to that of the overall R/R population in this study (median PFS, 51 months in the del[11q] subgroup and the overall population), with a median OS that was NR. In a recent integrated analysis of pooled data from 3 randomized trials of ibrutinib in CLL (N = 1210; data from A Phase 3 Study of Ibrutinib [PCI-32765] Versus Ofatumumab in Patients With Relapsed or Refractory Chronic Lymphocytic Leukemia, Study of Ibrutinib as Initial Therapy for Patients with Chronic Lymphocytic Leukemia, and A Study of Ibrutinib in Combination With Bendamustine and Rituximab in Patients With Relapsed or Refractory Chronic Lymphocytic Leukemia or Small Lymphocytic Lymphoma), the presence of del(11q) was associated with a trend toward prolonged PFS and OS (compared with absence of del[11q]), with a 24-month PFS of 82% and 30-month OS of 93% with ibrutinib, and was not an adverse prognostic factor for PFS in multivariate analysis.19,20 Consistent with this finding, we did not identify del(11q) as a significant factor affecting PFS or OS based on multivariate analysis. PFS outcomes in R/R patients with unmutated IGHV were not statistically different from that seen in patients with mutated IGHV, with similar 5-year OS rates and median OS that was NR regardless of IGHV status. In multivariate analysis, unmutated IGHV was not identified as an adverse prognostic factor for PFS or OS with ibrutinib therapy.
In this study, high ORR to ibrutinib were observed in R/R patients with del(17p) (79%) or CK (89%). The presence of del(17p) is recognized as a significant negative prognostic factor for survival in patients with CLL.3,4,11 Accordingly, del(17p) was identified as an independent prognostic factor for both PFS and OS in the current analysis. Nevertheless, an ORR of 79% and a DOR of 31 months represent promising single-agent efficacy for patients with del(17p) who had relapsed following multiple prior regimens (median, 4 prior therapies). Although PFS in our patients with del(17p) was less robust (median, 26 months) relative to that seen in other genetic subgroups, it compared favorably with that of R/R and even TN patients with del(17p) receiving FCR3 or alemtuzumab alone21 or in combination with steroids22 ; a median PFS of 11 to 18 months was reported for front-line therapy with these agents. Similarly, the efficacy of ibrutinib in R/R patients with CK (median PFS, 31 months) compared favorably with that of other salvage therapies in these patients, with a reported median PFS of 8 months.23 The frequency of high-risk genomic abnormalities including del(17p) and CK in patients in our study increased dramatically with increasing lines of chemotherapy. Thus, treatment with single-agent ibrutinib earlier in the disease course, before these abnormalities develop, may also improve patient outcomes.
The adverse impact of CK on patients with CLL treated with ibrutinib has been established in recent studies in which CK was independently associated with disease progression24 and inferior survival.25 Interestingly, the presence of CK was not identified as an independent prognostic factor for PFS or OS in our study, and survival in R/R patients with CK appeared to be largely influenced by the coexistence of del(17p). Patients with CK without del(17p) had prolonged PFS and OS compared with patients who had both CK and del(17p) (median PFS, 55 months and 25 months; median OS, NR and 32 months), indicating the adverse effect of del(17p) on survival in the R/R population. Patients with CK without del(17p) fared almost as well as patients without CK, suggesting that del(17p) may be a driver of shortened PFS within the CK subpopulation. This finding is in contrast to a previous report by Thompson et al, which suggested that CK is a stronger driver than del(17p) for inferior outcome.25 However, that study did not directly assess the effect of del(17p) within the subpopulation of R/R patients with CK.25 Furthermore, differences in the study populations may account for this discrepancy; the analysis by Thompson et al included only 56 patients receiving ibrutinib alone or in combination with rituximab or bendamustine and rituximab.25
The safety profile of ibrutinib over time remains acceptable and manageable, allowing almost one-half of the patients (48%) to be treated for more than 4 years and thus maximize response. The management of common toxicities such as infections, cytopenias, diarrhea, and bleeding has been previously described.13 Dose discontinuations and dose reductions resulting from AEs occurred more frequently during the first year and tended to decrease over time on ibrutinib therapy.
Overall, ibrutinib was well tolerated, with the onset of the majority of grade ≥3 AEs decreasing over time. Grade ≥3 hypertension and AF were reported in our previous 3-year follow-up, with cumulative rates of 20% and 6%, respectively.15 We report similar rates of grade ≥3 hypertension (26%) and AF (8%) in the current 5-year follow-up, the onset of which remained relatively constant throughout the course of follow-up. Treatment discontinuation because of hypertension or AF was infrequent (n = 1 each), suggesting that appropriate management of these events and a favorable benefit-risk profile can allow patients to continue ibrutinib therapy. An observational study of hypertension and AF in patients treated with ibrutinib-based regimens suggested that hypertension is unlikely to be a trigger for AF, because only 1 of 5 patients with new AF had new-onset hypertension in that study.26 Furthermore, the authors note that both conditions were generally manageable with medical intervention and did not require permanent discontinuation of ibrutinib.26 Similarly, there was no correlation between new-onset hypertension and AF in a recent analysis of AF reported in patients treated with ibrutinib from randomized studies.27 It is recommended that patients be periodically monitored for AF by clinical evaluation. The cumulative rate of major hemorrhage was 9% over the current 5-year follow-up period, which was only slightly increased relative to 8% seen with 3-year follow-up,15 suggesting that major hemorrhage is uncommon during long-term ibrutinib therapy. Nonetheless, low-grade bleeding events have occurred in approximately one-half of patients treated with ibrutinib, and precautions should be taken throughout the course of treatment to mitigate bleeding risks.
In this extended 5-year follow-up, the cumulative rate of other grade ≥3 AEs of interest such as diarrhea and neutropenia remain similar to that reported at the 3-year follow-up (grade ≥3 diarrhea, 7% and 6%; neutropenia, 17% and 14%). The cumulative rate of grade ≥3 infection has increased slightly from 42% at the 3-year follow-up to 48% at the present 5-year follow-up, and most cases were observed in R/R patients. Grade ≥3 cytopenias, such as neutropenia and thrombocytopenia, were infrequent overall and occurred more often among R/R than among TN patients; the onset of these cytopenias was highest during the first year and diminished in later years of follow-up. Given that the discontinuation rate is the highest during the first year of treatment, effective management of AEs (particularly grade 1-2 AEs) is critical through this initial year for patients to derive long-term benefit from ibrutinib therapy.
In summary, our comprehensive 5-year experience with single-agent ibrutinib in patients with TN and R/R CLL demonstrates the sustained efficacy of long-term treatment with ibrutinib despite the presence of high-risk genomic features in a large proportion of our patient population. Extended treatment with ibrutinib was well tolerated with no new safety signals, and the occurrence of severe AEs generally diminished over time with continued ibrutinib therapy.
Presented in part at the 58th annual meeting of the American Society of Hematology, San Diego, CA, 3-6 December 2016.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was sponsored by Pharmacyclics LLC, an AbbVie Company, and by grants from the National Institutes of Health, National Cancer Institute (R01 CA197870, R01 CA183444, R01 CA177292, and R35 CA197734) (J.C.B.), and the D. Warren Brown Foundation (J.C.B.). Medical writing support was provided by Allison Cherry, funded by Pharmacyclics LLC, an AbbVie Company.
Authorship
Contribution: S.O., J.C.B., R.R.F., A.J.J, and D.F.J. designed the study; S.O., R.R.F., S.C., I.W.F., J.A.B., K.B., J.S., W.W., J.J., W.Z., N.A.H., A.J.J., and J.C.B. collected the data; Y.L. performed statistical analyses; Y.L., D.F.J., and A.D.C. confirmed the accuracy of the data and compiled it for analysis; and all authors had access to the clinical trial data and were involved in the interpretation of data, contributed to the manuscript review and revisions, and approved the final version for submission.
Conflict-of-interest disclosure: S.O. holds a consultancy/advisory role and received honoraria from AbbVie, Janssen, and Pharmacyclics LLC, an AbbVie Company, and received research funding from Pharmacyclics LLC, an AbbVie Company. R.R.F. received honoraria, holds a consultancy/advisory role, and received travel accommodations and expenses from Pharmacyclics LLC, an AbbVie Company and AbbVie, and served on a speakers bureau for Pharmacyclics LLC, an AbbVie Company. S.C. holds a consultancy/advisory role for Pharmacyclics LLC, an AbbVie Company, AbbVie, Gilead, Novartis, Janssen, and Celgene, and received research funding from Gilead, Celgene, Novartis, AbbVie, and Pharmacyclics LLC, an AbbVie Company. I.W.F. received research funding from Genentech, Janssen, and Pharmacyclics LLC, an AbbVie Company. J.A.B. received honoraria, holds a consultancy/advisory role and received travel accommodations and expenses from Gilead, TG Therapeutics, Pharmacyclics LLC, an AbbVie Company, Novartis, and Janssen, and received research funding from Pharmacyclics LLC, an AbbVie Company. K.B. received research funding from Celgene, Novartis, Janssen, Pharmacyclics LLC, an AbbVie Company, Seattle Genetics, Millennium, Gilead, Morphosys, and Constellation Pharmaceutical. J.S. holds a consultancy/advisory role and received research funding from Gilead, Pharmacyclics LLC, an AbbVie Company, Celgene, AbbVie, Genentech, Acerta, and TG Therapeutics. W.W. received honoraria from Sanofi, Genentech/Roche, Pharmacyclics LLC, an AbbVie Company, Celgene, Gilead, GSK/Novartis, Genzyme, Merck, AbbVie, and Emergent; received research funding from GSK/Novartis, AbbVie, Genentech, Karyopharm, Pharmacyclics LLC, an AbbVie Company, Acerta, Gilead, Janssen, Emergent, Juno, and Kite; and holds a consulting/advisory role with Sanofi, Genentech/Roche, Pharmacyclics LLC, an AbbVie Company, Celgene, Gilead, GSK/Novartis, Genzyme, Merck, AbbVie, and Emergent. J.J. is employed with Celgene; received honoraria from Janssen and Acerta; holds a consultancy/advisory role with Pharmacyclics LLC, an AbbVie Company, Janssen, AbbVie, Morphosys, and Gilead; and received research funding from Pharmacyclics LLC, an AbbVie Company, AbbVie, Janssen, Genentech, Gilead, and Acerta. Y.L. is employed with Pharmacyclics LLC, an AbbVie Company; holds stock ownership with AbbVie; and has received travel accommodations and expenses from Pharmacyclics LLC, an AbbVie Company. D.F.J. is employed with Pharmacyclics LLC, an AbbVie Company; holds stock ownership with AbbVie; and has patents with AbbVie. A.D.C. is employed with Pharmacyclics LLC, an AbbVie Company, and holds stock ownership with AbbVie. J.C.B. received research funding from Genentech, Acerta, and Pharmacyclics LLC, an AbbVie Company. The remaining authors declare no competing financial interests.
Correspondence: Susan O’Brien, Chao Family Comprehensive Cancer Center, University of California Irvine, 101 The City Dr South, Orange, CA 92868; e-mail: obrien@uci.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal