Abstract
Therapeutic T-cell engineering is emerging as a powerful approach to treat refractory hematological malignancies. Its most successful embodiment to date is based on the use of second-generation chimeric antigen receptors (CARs) targeting CD19, a cell surface molecule found in most B-cell leukemias and lymphomas. Remarkable complete remissions have been obtained with autologous T cells expressing CD19 CARs in patients with relapsed, chemo-refractory B-cell acute lymphoblastic leukemia, chronic lymphocytic leukemia, and non-Hodgkin lymphoma. Allogeneic CAR T cells may also be harnessed to treat relapse after allogeneic hematopoietic stem cell transplantation. However, the use of donor T cells poses unique challenges owing to potential alloreactivity. We review different approaches to mitigate the risk of causing or aggravating graft-versus-host disease (GVHD), including CAR therapies based on donor leukocyte infusion, virus-specific T cells, T-cell receptor–deficient T cells, lymphoid progenitor cells, and regulatory T cells. Advances in CAR design, T-cell selection and gene editing are poised to enable the safe use of allogeneic CAR T cells without incurring GVHD.
Introduction
Therapeutic T-cell administration has been integral to bone marrow transplantation from its inception. Originally conceived as a hematopoietic rescue following intensive chemotherapy,1 allogeneic hematopoietic cell transplantation (allo-HCT) eventually evolved as a means to harness the immune system to treat hematologic malignancies in patients who failed to respond to standard chemotherapy.2 The biological foundation of this immunotherapy is the graft-versus-leukemia (GVL) effect, which is primarily mediated by donor T cells present in the graft.3 The combination of tumor burden reduction, immunosuppression, and provision of a diverse repertoire of alloreactive T cells can produce remarkable clinical responses, but this intervention comes with a severe risk, that of graft-versus-host disease (GVHD).4 The removal of donor T cells from the graft prevents GVHD, but at the cost of prolonged T-cell deficiency, increased viral infections, and increased tumor relapse.5,6 Thus, many patients with high-risk disease who receive an allo-HCT do not achieve a complete remission (CR) posttransplant and, for those who do, the risk of relapse remains high.7 Progressive disease is the leading cause of death following allo-HCT.8
These findings define the conundrum of relying on an uncontrolled T-cell repertoire to treat hematological malignancies and pose the fundamental challenge of allo-HCT: how to retain the benefit of graft-versus-tumor (GVT) effects while avoiding GVHD. Striking the right balance between tumor rejection and self-tolerance requires a deep understanding of the fundamental principles of tumor immunogenicity, the role of the tumor microenvironment, and the immunomodulatory effects of conditioning regimens and GVHD prophylaxis. Despite considerable progress in better appreciating the role of donor T-cell dose, tumor antigenicity and the effects of host conditioning,9-11 GVT, and GVHD continue to pose biological and clinical challenges.
In this review, we discuss T-cell engineering strategies that aim to accomplish a goal that cannot be reliably realized with heterogeneous natural T cells: to achieve complete and durable tumor responses without incurring the risk of GVHD. Targeted immune rejection of cancer cells requires the identification of suitable tumor antigens and cognate receptors that effectively direct T cells to identify and eliminate tumor cells. Two kinds of receptors may be used to this end, either physiological T-cell receptors (TCRs) or chimeric antigen receptors (CARs). CAR are synthetic receptors that retarget and reprogram T cells to target cell surface antigens independent of HLA.12 The costimulatory properties of second-generation CARs, typically mediated through their CD28 or 4-1BB signaling domain, determine the engineered T-cell’s function, metabolism, and persistence.12 CD19 was first proven to be a remarkable target in a range of B cell malignancies in murine models of CAR therapy.13,14 The adoptive transfer of autologous CD19 CAR T cells subsequently demonstrated the potency of CD19 CAR therapy in patients with refractory non-Hodgkin lymphoma, chronic lymphocytic leukemia, and acute lymphoblastic leukemia.15-24
We review here the potential of allogeneic CAR therapy in the posttransplant setting (the use of CAR T cells as a bridge to transplant is beyond the scope of this review). With the exception of a trial evaluating donor-derived CD123 CAR T cells for recurrent acute myeloid leukemia after allo-HCT (NCT03114670), donor CAR T-cell studies have centered on CD19. This review thus focuses on the use of donor CD19 CAR T cells in the posttransplant setting.
CARs in donor leukocyte infusions
One of the early approaches to mitigate the risk of relapse following allogeneic HCT is donor leukocyte infusion (DLI). In this paradigm, titrated amounts of donor T cells are administered to recipients of a T cell–depleted transplant to induce an immune-mediated remission.25 Kolb et al were the first to report on the use of DLI in patients with relapsed chronic myeloid leukemia (CML) given interferon-α in combination with donor buffy coats.26 Subsequent studies showed that donor mononuclear T cells could induce responses in Epstein-Barr virus–associated (EBV) B-cell lymphoproliferative disorder following a T cell–depleted allo-HCT for acute promyelocytic leukemia,27 acute myeloid leukemia, myelodysplasia, or CML.28 GVT was more effective against CML than acute leukemia. DLI is also effective against B-cell malignancies, including the more indolent lymphomas, mantle cell lymphoma, and Hodgkin lymphoma,7,29 but less so against acute lymphocytic leukemia, multiple myeloma, or aggressive phenotypes of non-Hodgkin lymphomas. Overall, DLI does not induce remission consistently and may cause acute GVHD in one-third of patients.30
Notwithstanding the risk of exacerbating GVHD, HLA-matched allogeneic CD19 CAR T cells have been infused to patients who relapsed or had not achieved a CR after allo-HCT. The donor CD19 CAR T cells were either harvested from the donor or from the recipient, two instances that should be examined separately (Table 1). Recipient-derived donor T cells may be expected to carry a lesser risk of acute and chronic GVHD if the CAR T cells are generated from tolerized cells.
Clinical outcomes in posttransplant recipients of allogeneic CAR T cells
| T cell and CAR types . | Clinical reports . | No. of patients . | Incidence of GVHD . | Efficacy of CAR therapy . |
|---|---|---|---|---|
| True-allo (donor-derived DLI)* | ||||
| CD19 CAR DLI (28z; γRV) | Brudno et al | 20 | Acute: 0% Chronic: 10% (2/20) | CR 30% (n = 6) PR 10% (n = 2) |
| CD19 CAR DLI (4-1BBz; LV) | Dai et al | 2 | Acute: 100% (2/2) Chronic: 0% | CR 50% (n = 1) |
| CD19 CAR DLI (28z; SB) | Kebriaei et al | 19 | Acute: 10% (2/19) Chronic: 6% (1/19) | 63% (9 CCR, 1 CR2, 2 DIR)† |
| CD19 CAR VST (28z; γRV) | Cruz et al | 8 | 0% | CR 38% (1 CR and 2 CCR)‡ PR 13% |
| Pseudo-allo (Recipient-derived DLI)§ | ||||
| CD19 CAR “DLI” (4-1BBz; LV) | Maude et al | 18 | Acute: 0% Chronic: 0% | EFS 67%, OS 78% (at 6 months)‖ |
| CD19 CAR “DLI” (28z; γRV) | Lee et al | 8 | Acute: 0% Chronic: 0% | MRD negative CR: 37% (n=3) |
| CD19 CAR “DLI” 1:1 (4-1BBz; LV) | Turtle et al | 11 | Acute: 0% Chronic: 9% (1/11) | BM remission: 93% |
| CD19 CAR “DLI” 1:1 (4-1BBz; LV) | Gardner et al | 27 | Acute: 3% (1/27) Chronic: 0% | MRD negative remission rate: 93% |
| CD19 CAR “DLI” (28z; γRV) | Park et al | 19 | Acute: 0% Chronic: 0% | MRD negative CR: 63%¶ |
| T cell and CAR types . | Clinical reports . | No. of patients . | Incidence of GVHD . | Efficacy of CAR therapy . |
|---|---|---|---|---|
| True-allo (donor-derived DLI)* | ||||
| CD19 CAR DLI (28z; γRV) | Brudno et al | 20 | Acute: 0% Chronic: 10% (2/20) | CR 30% (n = 6) PR 10% (n = 2) |
| CD19 CAR DLI (4-1BBz; LV) | Dai et al | 2 | Acute: 100% (2/2) Chronic: 0% | CR 50% (n = 1) |
| CD19 CAR DLI (28z; SB) | Kebriaei et al | 19 | Acute: 10% (2/19) Chronic: 6% (1/19) | 63% (9 CCR, 1 CR2, 2 DIR)† |
| CD19 CAR VST (28z; γRV) | Cruz et al | 8 | 0% | CR 38% (1 CR and 2 CCR)‡ PR 13% |
| Pseudo-allo (Recipient-derived DLI)§ | ||||
| CD19 CAR “DLI” (4-1BBz; LV) | Maude et al | 18 | Acute: 0% Chronic: 0% | EFS 67%, OS 78% (at 6 months)‖ |
| CD19 CAR “DLI” (28z; γRV) | Lee et al | 8 | Acute: 0% Chronic: 0% | MRD negative CR: 37% (n=3) |
| CD19 CAR “DLI” 1:1 (4-1BBz; LV) | Turtle et al | 11 | Acute: 0% Chronic: 9% (1/11) | BM remission: 93% |
| CD19 CAR “DLI” 1:1 (4-1BBz; LV) | Gardner et al | 27 | Acute: 3% (1/27) Chronic: 0% | MRD negative remission rate: 93% |
| CD19 CAR “DLI” (28z; γRV) | Park et al | 19 | Acute: 0% Chronic: 0% | MRD negative CR: 63%¶ |
To date, 132 patients with B cell malignancies who were infused with allogeneic CD19 CAR T cells have been have reported in the published literature. Four percent (n = 5) of the patients developed acute GVHD and 3 percent (n = 4) of the patients developed chronic GVHD. More specifically, in patients who received donor-derived DLI, the total incidence of GVHD was 14% (n = 7), with an incidence of acute and chronic GVHD of 8% (n = 4) and 6% (n = 3), respectively. In patients given recipient-derived CAR T cells, the total incidence of GVHD was 2% (n = 2), with an incidence of acute and chronic GVHD of 1% (n = 1) for both.
Forty-seven patients received donor-derived CD28-based CAR T cells, of which 10% developed GVHD (n = 5; 2 acute and 3 chronic). Two patients received donor-derived 4-1BB-based CAR T cells, of which 100% (n = 2) developed GVHD (both acute). In patients given recipient-derived CD19 CAR T cells, twenty-seven patients received cells with a CD28 costimulatory molecule and none developed GVHD. Fifty-six patients received recipient-derived 4-1BB-based CAR T cells, of which 3% developed GVHD (n = 2; 1 acute and 1 chronic). These outcomes are consistent with the findings of Ghosh et al. Overall, twenty-three (40%) patients were reported to have achieved a CR, which includes those who were reported as CR or those who were reported as a continuous complete remission (CCR). The thirty-eight patients reported by Maude et al, Turtle et al, Gardner et al, and Park et al are not included in this calculation as the clinical outcomes for these patients were reported as BM remission, EFS/OS, or cumulative MRD negative remission rather than CR and PR.
BM, bone marrow; CCR, continuous complete remission; CR2, second CR; DIR, died in remission; LV, lentiviral transduction; PR, partial remission; γRL, γ-retroviral transduction; SB, Sleeping Beauty transposon.
Donor T cells were collected from the allogeneic donor (donor-derived).
The authors report that 9 patients had a CCR since transplant. Additionally, 1 patient is reported to have had a CR2, while 2 other patients died in remission (DIR).
The authors report 1 CR and 2 CCRs.
Donor T cells were collected from the recipient posttransplant (recipient-derived).
The authors report that the “EFS and OS did not differ significantly” whether or not patients had received an allo-HCT. The did not report PR or CR data for the patients.
Sixteen of the 19 patients were MRD evaluable; so the MRD negative CR rate was calculated from this subset of patients.
Kochenderfer et al infused donor-derived leukocytes expressing a CD19 CAR to patients with persistent B-cell malignancies following allo-HCT.31 T cells were administered without additional chemotherapy or lymphodepleting conditioning. Three of 10 patients showed tumor regression without GVHD. In an update to this study, 8 of 20 patients with either B-cell acute lymphoblastic leukemia (B-ALL), chronic lymphocytic leukemia, or non-Hodgkin lymphoma, developed a remission, including 6 CRs and 2 partial remissions.32 14 of the 20 patients had previously developed GVHD after allo-HCT. No acute GVHD was reported after CAR T-cell infusion. Chronic GVHD occurred in 2 patients. One of the 2 developed mild chronic ocular GVHD about 2 years post–CAR T-cell therapy, whereas the other patient had slow progression of chronic GVHD symptoms following CD19 CAR therapy.31 In another clinical study, however, 2 patients with relapsed or refractory B-ALL who received allogeneic CD19 CAR T cells developed GVHD 3 to 4 weeks after CAR T-cell infusion. One patient presented with grade 2 liver GVHD, whereas the other developed grade 2 skin and liver GVHD.33 One of these patients died of relapse 8 weeks after T-cell infusion, whereas the other developed a hematologic CR as well as partial regression of extramedullary leukemic disease. In another study, Kebriaei et al reported on a phase 1 clinical trial in which allogeneic T cells were modified with the Sleeping Beauty transposon/transposase to express a second-generation CD19 CAR.34 Of the 19 patients, only 3 developed GVHD, presenting as acute skin grade 1, chronic skin, and acute liver GVHD, respectively. The authors reported that 11 of the 19 patients were in remission at a median follow-up of 7.5 months.34
These discrepant results in regard to GVHD may be explained by murine studies. In 3 different donor/host strain combinations, Ghosh et al found that GVHD could indeed be attenuated in recipients of allogeneic CD19 CAR T cells, depending on the CAR design.35 Using a CD28-based second-generation CAR,36 recipients of donor CD19 CAR T cells benefited from their antitumor effect without developing GVHD. This outcome was achieved by cumulative CAR and TCR signaling in alloreactive T cells (Figure 1A), resulting in activation-induced cell death or accelerated exhaustion, hence preventing or decreasing GVHD. Recognition of CD19+ in either B or tumor cells was required for protection from GVHD, consistent with the requirement for TCR and CAR coengagement at the clonal level. Nonalloreactive donor T cells, on the other hand, retained their full antitumor potential. In contrast, donor T cells expressing a 4-1BB–based CD19-specific CAR, which provides a weaker activation signal,36,37 did not protect from GVHD. These data potentially elucidate the discrepancy between the clinical results reported in studies in which donor-derived T cells expressed either a CD28- or 4-1BB–based CAR (Table 1).31-33 In another murine allogeneic model using an attenuated CD28-based CAR in which the first and third immunoreceptor tyrosine-based activation motifs of the CD3 ζ molecule were inactivated,38 mice treated with allogeneic CD4+ CD19 CAR T cells developed an inflammatory response with features similar to GVHD. Altogether, these reports suggest that different CAR designs providing different strengths of activation may determine whether GVHD develops or not.
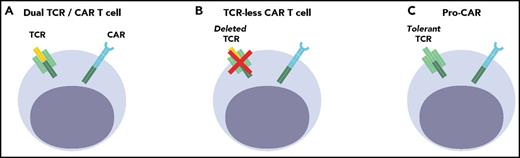
Cell engineering strategies to provide allogeneic CAR T cells in the posttransplant setting. (A) Dual TCR/CAR T cell: The TCR may be alloreactive (DLI) or virus-specific (VST). There is a risk of signaling overload in bispecific T cells. (B) TCR-less CAR T cell: The endogenous TCR can be disrupted in mature T cells to abrogate GVHD potential (UCART19, TRAC-CAR). (C) Pro-CAR: Mismatched lymphoid progenitors can yield host tolerant CAR T cells, but require successful engraftment, development, and selection (precursor T cells).
Cell engineering strategies to provide allogeneic CAR T cells in the posttransplant setting. (A) Dual TCR/CAR T cell: The TCR may be alloreactive (DLI) or virus-specific (VST). There is a risk of signaling overload in bispecific T cells. (B) TCR-less CAR T cell: The endogenous TCR can be disrupted in mature T cells to abrogate GVHD potential (UCART19, TRAC-CAR). (C) Pro-CAR: Mismatched lymphoid progenitors can yield host tolerant CAR T cells, but require successful engraftment, development, and selection (precursor T cells).
Other studies have made use of donor T cells harvested from the transplant recipient. Maude et al,21 Lee et al,23 and Park et al39 reported on the the administration of recipient-derived CAR T cells for patients with relapsed or refractory acute lymphoblastic leukemia or non-Hodgkin lymphoma. No GVHD was observed following CD19 CAR T infusion in either of these studies (Table 1).
Another variable that may affect the potential for GVHD is the T-cell subset composition of the CAR-DLI infusion product. Although most clinical studies have made use of an unselected cellular product comprising a variable mix of naïve, memory, and effector T cells, 2 trials used T cells of more defined composition.40-42 Turtle et al assessed the effect of manufacturing and infusing a 1:1 ratio of CD4+ and CD8+ CD19 CAR T cells for patients with refractory B-ALL.40 A ratio of 1:1 CD4+:CD8+ CAR T cells was administered in 27 patients, 11 of which were engineered from engrafted donor T cells. None developed acute GVHD after CAR T-cell therapy; 1 patient who had stage 1 acute skin GVHD before study enrollment developed chronic GVHD requiring corticosteroid therapy 3 months after CAR T-cell infusion.40 Gardner et al also administered defined ratios of 1:1 CD4+:CD8+ CD19 CAR T cells to patients with B cell ALL.42 Twenty-seven of the 45 patients in this study had previously undergone allo-HCT. One of these patients who had a prior history of GVHD and was tapered off immunesuppresion for GVHD more than a year before CAR T-cell treatment, developed grade 3 acute skin GVHD following CAR T-cell infusion. It is unclear whether the low incidence of GVHD in these 2 studies, both utilizing a 4-1BB-based CD19 CAR may be attributed to central memory T-cell purification or the use of tolerized donor T cells harvested from the transplant recipient (Table 1).
CARs in virus-specific T cells
Another approach to derive benefit from donor T cells while limiting the risk of GVHD is to select donor-derived antigen-specific T cells devoid of alloreactive potential. This technology requires the isolation and culture of highly specific T cells, which has been successfully deployed for viral antigens and used to fight posttransplant viral infections and virally induced tumors. Riddell et al initially reported on the administration of donor-derived cytomegalovirus (CMV)-specific T-cell clones to 3 immunodeficient patients who reconstituted T-cell immunity to CMV without GVHD.43 In patients who developed EBV-associated lymphoproliferative disease (EBV-LPD) after a T cell–depleted transplant, unirradiated donor lymphocytes were effective in treating EBV-LPD but carried a high risk of GVHD.44,45 The use of a virus-specific T-cell (VST) line, however, could afford the ability to mediate regression of an EBV immunoblastic lymphoma without causing GVHD.45 Donor VSTs specific for CMV, EBV, and adenovirus have since been extensively used as prophylaxis or posttransplant antiviral therapy.46-49 EBV-specific T cells have also been effective against nasopharyngeal carcinoma49 and other EBV-associated tumors.50
Interestingly, the donor T-cell lines that were established for a particular recipient can be used for alternative recipients.51 This “third-party” approach was found to be effective against biopsy-proven EBV-LPD in 19 patients without provoking acute or chronic GVHD or a flare of preexisting GVHD.52 No occurrence of acute GVHD was reported in another series of 153 transplant recipients who received allogeneic virus-specific CTLs, 73 of whom had an HLA mismatch.53 Although VSTs could cross-react with HLA-mismatched targets in vitro, GVHD reactivation was comparable for recipients of matched or mismatched CTLs.
Preclinical data with VSTs suggest that they may serve as an off-the-shelf resource for CAR therapy given that the defined TCR specificity mitigates alloreactivity that could result in GVHD (Figure 1A).54 In a clinical study in which 6 patients with relapsed B-cell leukemias were infused with donor-derived trispecific VSTs expressing a CD28-based CD19 CAR 3 months to 13 years after allo-HCT, 2 of them had an objective anti-tumor response, whereas the 2 patients who were treated in relapse remained free of disease.55 This approach merits further investigation of the biological requirements for the sustained persistence and function of engineered VSTs.
TCR-less CAR T cells
A more radical approach than relying on virus-specific, nonalloreactive T cells is to deprive T cells of their TCR (Figure 1B). This is possible by editing the genome of T cells, deleting genes required for expression of the TCR complex. TCR-less T cells are most commonly generated through the targeted disruption of the TCR α or β chain,56-60 using targeted nucleases such as zinc finger nucleases, transcription activator-like effector nucleases (TALEN), or the clustered regularly interspersed short palindromic repeat-Cas9 system (CRISPR/Cas9).61,62
This approach requires multiple T-cell manipulations that should be minimized to avoid exhausting the replicative or engraftment potential of primary T cells. Most T-cell editing studies initially proceeded by first deleting a TCR gene, then transducing a CAR (or defined TCR) using a retroviral or lentiviral vector.42,56-60 A recent report demonstrated that targeting the CAR complementary DNA to the TCR locus, coopting endogenous genomic elements to regulate expression of the CAR, yields CAR T cells with enhanced functionality.60 This “two-in-one” genetic deletion/insertion generates TCR-less CAR T cells (termed TRAC-CAR T cells) that not only avoid tonic signaling from overexpressed CAR molecules, but also delay functional exhaustion. Although TCR removal obviates the risk of T-cell exhaustion arising from cumulative TCR and CAR signaling,35 TRAC-encoded CARs offset hyperactive signaling through a distinct mechanism dependent on CAR internalization and kinetically optimal reexpression.60 These findings exemplify how genome editing will contribute to improving the efficacy and safety of next-generation CAR T cells.63
TCR-less CAR T cells have not yet been tried in the posttransplant setting, but a clinical study assessed their activity as part of a bridge-to-transplant protocol. In this trial, CAR T cells were generated using a TALEN to disrupt both the TCR α and CD52 loci.64 Disrupting CD52 expression enables the use of alemtuzumab for host T-cell depletion while sparing the infused CAR T cells. The CD19 CAR, bearing 4-1BB signaling elements, was randomly integrated using a lentiviral vector also encoding CD20 as a target for eventual antibody-mediated CAR T-cell depletion. To minimize the risk of GVHD, T cells that retain their TCR (ie, the fraction of T cells in which the TALEN process did not disrupt the TCR α locus) were depleted, yielding a final cell product with only 0.7% of TCR-positive T cells. These T cells, termed UCART19,64 were administered to 2 infants with relapsed CD19+CD52– ALL following intense conditioning regimens as compassionate therapy before allo-HCT. One of the 2 recipients of UCART19 cells developed grade 2 GVHD in the skin, which failed to respond to anti-CD20 antibody depletion with rituximab but was controlled by systemic steroids. Although the overall treatment was successful, this experience underscores the technical challenges to achieving stringent T-cell depletion and effective safety switches. The UCART19 trial is currently accruing pediatric (NCT02808442) and adult (NCT02746952) patients. A clinical trial to evaluate TRAC-CAR T cells is in preparation.
Lymphoid progenitor CAR therapy
Another approach to provide allogeneic CAR T cells without incurring the risk of GVHD is to provide mismatched allogeneic T-cell progenitors that will yield tolerized, host-restricted T cells. Hematopoietic progenitor cells can indeed develop into T cells in vitro in the presence of stromal cells and Notch ligands. The most widely used protocols to direct T-cell development make use of the OP9-DL1 coculture system.65,66 Murine bone marrow stroma OP9 cells67 expressing either DLL1 or DLL4 ligands have been harnessed to induce human T-lineage cells from bone marrow, cord blood, embryonic stem cells, or induced pluripotent stem cells.68-73 Stromal-free cell culture systems are emerging.74 Thus, Notch-based culture systems may offer an opportunity to generate naïve T cells for cell-based immunotherapies.
Several reports have demonstrated the efficient generation and therapeutic potential of T-cell precursors generated by Notch-based culture of hematopoietic progenitor cells.70,73,75,76 The concept of adoptive T-cell therapy was initially demonstrated by Zakrzewski et al, who reported that cotransplantation of allogeneic T-cell precursors significantly enhanced T-cell recovery after T cell–depleted allo-HCT.77 T-cell precursors engrafted into the recipient’s thymus and developed over several weeks into a single generation of naïve T cells that were fully functional. These cells were appropriately tolerized during thymic development and did not cause GVHD. Fully mismatched T-cell precursors can be further harnessed to provide host-restricted CAR T cells without risk for GVHD78 (Figure 1C). CAR T cells have been shown to mature in vivo in several models using first-generation CARs.78,79 The clinical implementation of this approach still requires proof of concept studies using human cells. It is encouraging that human T-cell precursors (pro-T cells) generated from cord blood–derived hematopoietic stem cells by OP9-DL1 coculture are able to engraft into the thymus of NOD.cg-PrkdcscidIL2rgtm/Wjl/Sz mice and establish human T-cell development in vivo.80
Negating the need for HLA matching opens the door for the use of third-party cells that could be produced (as unmodified or genetically engineered cells) and banked in large volumes. The ready-to-use frozen units of cells could be used universally to treat patients regardless of HLA disparity, enabling a cost-effective manufacturing model. Although tolerization of allogeneic T cells is a built-in benefit of T-cell precursor therapy, it comes at the expense of delayed potency because of the intrathymic “incubation time” of engrafted precursor cells. It further requires thymic conditioning, usually in the form of total body or thymic irradiation, to facilitate thymic engraftment of infused precursor cells. Tolerization depends on thymic function for optimal efficacy. However, adoptively transferred T-cell precursors have the ability to engraft and develop into mature T cells at extrathymic sites such as the mesenteric lymph nodes.81 This approach offers potential opportunities to expand current T-cell therapy platforms.
CAR Tregs
Another cellular therapeutic strategy to control GVHD involves the infusion of regulatory T cells (Tregs) in the peritransplant setting to establish dominant tolerance in GVHD-target tissues. Tregs are a subset of CD4+ T cells expressing the lineage determining transcription factor Foxp3 that may promote tolerance in a number of inflammatory settings, including autoimmunity and GVHD.82,83 Thus, CD4+ CD25+ Foxp3+ Treg can prevent development of colitis in immune-deficient mice84,85 and inhibit GVHD in mouse models of allo-HCT.86-89 Foxp3-transduced conventional T cells (Tconvs) are also able to mediate suppression of GVHD in mouse models.90 Recently, clinical studies have demonstrated the feasibility of the therapeutic infusion of donor-derived Tregs in the peritransplant setting as a form of GVHD prophylaxis.91,92 These trials have suggested a reduced incidence of GVHD in patients receiving haploidentical or third-party 4-6/6 HLA-matched cord blood Treg compared with historical controls, without an increased risk of disease relapse.
Tregs represent 1% to 5% of the population of human peripheral blood CD4+ T cells,93 necessitating ex vivo expansion92 or reduction in the number of Tconv in the stem cell graft91 in order to achieve the 1:1 Treg:Tconv ratio found to be suppressive in mouse models. Generating Foxp3-transduced Tconv for infusion in the peritransplant setting could potentially ameliorate the relative rarity of these cells.90,94 Alternatively, the use of antigen-specific Tregs may allow infusion of fewer cells to achieve inhibition of GVHD. A preclinical study using ovalbumin-specific TCR transgenic Tregs found that antigen-specific Tregs mediated significantly greater inhibition of GVHD when activated by ovalbumin in vivo, requiring infusion of a 10-fold lower Treg:Tconv ratio to prevent lethal GVHD compared with polyclonal Tregs.94 Similarly, alloreactive host-specific donor and third-party Tregs demonstrated greater GVHD suppressive capacity compared with host-derived95 or irrelevant anti–third-party Tregs.89
Genetic modification of polyclonal Tregs with CAR to promote antigen-specificity has demonstrated that CAR Tregs mediate enhanced suppression of inflammation compared with polyclonal Tregs in mouse models of TNP-induced colitis,96 experimental autoimmune encephalitis,97 anti-factor VIII autoantibody formation,98 and skin graft rejection.99 CARs may also redirect the function of human natural Tregs.100 HLA-A2–specific CAR Treg were recently shown to inhibit xenogeneic GVHD mediated by HLA-A2+ peripheral blood mononuclear cells in immune-deficient mice infused with Treg:HLA-A2+ peripheral blood mononuclear cell ratios of 1:1 to 1:2.101 A concern with this approach is the need to control GHVD without impairing GVL or the function of tumor-targeted CAR T cells.100,102
Perspectives
T-cell engineering strategies offer a fresh perspective to address the longstanding problem of separating the GVL and GVHD effects of allogeneic T cells. The use of allogeneic CAR T cells, which are not restricted by HLA and are thus independent of alloreactive T-cell responses, hold great promise to address the risk of relapse in the posttransplant setting. Several approaches, summarized in this review, are being developed to provide tumor-targeted nonautologous CAR T cells without increasing the risk of GVHD. These cell-engineering approaches rest on targeting T cells to malignant cells through a CAR on the one hand and abrogating TCR-mediated alloreactivity on the other. The current experience is limited to CD19 CARs but relevant in principle to an expanding array of hematological CAR targets (Table 2).
CAR targets in hematological malignancies
| Malignancy . | CAR targets . |
|---|---|
| B cell | CD19, CD20, CD22 |
| Myeloma | BCMA, CD38, CD56, CD138, CS-1, integrin β7, Κ light chain, Lewis Y |
| Myeloid leukemia | CD33, CD44v6, CD38, CD70, CD123, CLEC12A, EMR2, FOLR2, Lewis Y, Tim3 |
| T-ALL/ lymphoma | TRBC1, CCR4, CD7 |
| Malignancy . | CAR targets . |
|---|---|
| B cell | CD19, CD20, CD22 |
| Myeloma | BCMA, CD38, CD56, CD138, CS-1, integrin β7, Κ light chain, Lewis Y |
| Myeloid leukemia | CD33, CD44v6, CD38, CD70, CD123, CLEC12A, EMR2, FOLR2, Lewis Y, Tim3 |
| T-ALL/ lymphoma | TRBC1, CCR4, CD7 |
T-ALL, T-cell acute lymphoblastic leukemia.
Several challenges remain to be addressed. In approaches in which endogenous TCR are retained (Figure 1A), the latter’s potential alloreactivity needs to be strictly ascertained. Deletional approaches (Figure 1B) offer greater reassurance of abrogating GVHD potential, but technical challenges to manufacturing safe T-cell products in large amounts are yet to be solved. The transplantation of T-cell precursors that can be tolerized (Figure 1C) is yet another potential solution, supported by recent advances in progenitor T-cell expansion, but hinges on successful T-cell development in the recipient. In the event that GVHD cannot be prevented or contained, Treg engineering may offer a last-resort solution, provided that Treg can be engineered to quell GVHD without abating the antitumor response. Engineered Tregs could also be used prophylactically.
Significantly, advances in donor CAR T-cell therapy in the posttransplant setting will inform another major goal of CAR T cell-based medicines, the design of “off-the-shelf” CAR T cells for use beyond the realm of allotransplantation. In this circumstance, allogeneic CAR T cells will not only need to not induce GVHD but also escape allorejection by the recipient. Several initiatives to address these barriers are in progress, based on the use of mature T cells103 or induced pluripotent stem cells.73,104
The fields of CAR T-cell therapy and allogeneic transplantation thus intersect in many ways. It is plausible that CAR T-cell therapy will replace transplant for at least a subset of patients with hematological malignancies. Reciprocally, allo-HCT may sometimes consolidate an incomplete response to CAR therapy. CAR technologies can rescue posttransplant tumor relapse and may be used to harness Tregs to control GVHD. Encouragingly, preclinical models and some early clinical data on donor CD19 CAR T cells demonstrate a low incidence of GVHD with some CAR designs. GVHD incidence was lower when using recipient-derived rather than donor-derived CAR T cells, suggesting that recipient-derived donor T cells were tolerized. The infusion of selected T-cell subsets requires further investigation to assess whether defined subsets may contribute to attenuate GVHD. Nonetheless, the safest approaches will likely arise from the use of TCR-less CAR T cells or precursor T cells. In these scenarios, it may be at last possible to achieve effective GVT without GVHD.
Acknowledgments
Funding was received from the American Society for Blood and Marrow Transplantation New Investigator Award, the Burroughs Wellcome Fund Postdoctoral Enrichment Program, and the Damon Runyon Physician-Scientist Award (M. Smith).
Authorship
Contribution: M. Smith, M. Sadelain, J.Z., and S.J. composed the manuscript; and M. Smith and M. Sadelain edited and revised the manuscript and the figure.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Michel Sadelain, Center for Cell Engineering, Memorial Sloan Kettering Cancer Center, New York, NY 10065; e-mail: m-sadelain@ski.mskcc.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal