Abstract
Advances in the prevention of graft-versus-host disease (GVHD) and opportunistic infection have improved survival after allogeneic hematopoietic cell transplantation (allo-HCT) in the past decade. However, few inroads have been made into the treatment or prevention of relapse of the underlying malignancy for which allo-HCT is being performed. The introduction of US Food and Drug Administration–approved agents with significant activity in a variety of hematologic malignancies provides an opportunity to evaluate these interventions in the allo-HCT setting. Some of the most promising new agents include tyrosine kinase inhibitors (TKIs) directed at bcr-abl, kinase inhibitors targeting fms-like tyrosine kinase 3, and immune checkpoint inhibitors blocking both CTLA4 and PD-1. Data have emerged indicating potential efficacy of these agents in preventing or treating relapse, though definitive evidence remains elusive. However, potential toxicity can be considerable, highlighting the need for further clinical trials to define the therapeutic window. This review explores the immunologic and clinical consequence of treatment with both TKIs and checkpoint inhibitors in the peri- and post–allo-HCT setting.
Introduction
Outcomes for patients who relapse after allogeneic hematopoietic cell transplantation (allo-HCT) are dismal.1 Among 351 patients who relapsed among 1080 transplants performed between 2004 and 2008 at Dana Farber Cancer Institute, the 3-year postrelapse overall survival (OS) rate was only 19%, and it was even lower among patients with a diagnosis of acute myelogenous leukemia (AML).2 Patients who received some form of cellular therapy (either donor lymphocyte infusions [DLIs] or second allo-HCT) fared better than those who did not, with the caveat that the ability to achieve a disease response before receiving additional cellular therapy is at least partly responsible for this difference. Survival was superior for patients who developed graft-versus-host disease (GVHD) after relapse, no matter what their postrelapse intervention had been. Indeed, for many years, the primary strategy to address post-HCT relapse has been directed toward inducing GVHD either through DLI or accelerated withdrawal of immune suppression.3 Although conventional wisdom holds that relapse after transplant can only with dealt with by cellular intervention, that may not be true. In the past few decades, the development of new pharmacologic agents has provided novel avenues to treat and potentially even prevent relapse post-HCT. Two important classes of drugs among these are tyrosine kinase inhibitors (TKIs) and immune checkpoint inhibitors.
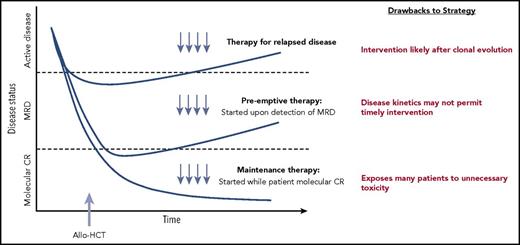
The timing of pharmacologic treatment around allo-HCT can vary based on the underlying disease and agent used. Interventions reserved for active relapse after transplant are clearly less than ideal. A maintenance or preemptive strategy to prevent disease relapse is more appealing. An ideal maintenance approach does not compromise graft integrity, induce significant GVHD, or interfere with metabolism of other essential pharmacologic agents such as calcineurin inhibitors. Although maintenance leads to treatment of all patients (many of whom may not be destined to relapse), a preemptive approach targets only patients with warning signs of impending relapse, such as those with minimal residual disease (MRD) detected by multiparameter flow cytometry, polymerase chain reaction, or more sensitive molecular techniques, or even those with falling donor hematopoietic chimerism. A preemptive strategy will only be effective if the kinetics of relapse allows sufficient time from MRD detection to initiate therapy before morphologic relapse and subsequent clinical consequences (Figure 1).4
Different approaches to posttransplantation therapies after allogeneic HCT, including treatment vs preemptive vs maintenance. Adapted from Defilipp and Chen4 with permission.
Different approaches to posttransplantation therapies after allogeneic HCT, including treatment vs preemptive vs maintenance. Adapted from Defilipp and Chen4 with permission.
Immunological impact of TKIs
Although TKIs exert their effects directly against the malignant clone itself, they also have an effect on the immune microenvironment. Patients with chronic myelogenous leukemia (CML) treated successfully with bcr-abl–directed TKIs demonstrate increased natural killer cell and effector T-cell cytolytic function, reduced T-cell PD-1 expression, and reduced numbers of monocytic-myeloid derived suppressor cells.5 Monocytic-myeloid derived suppressor cells derived from mature monocytes are significantly and dose-dependently reduced in the presence of dasatinib, nilotinib, and sorafenib. In functional analyses, myeloid cells treated with these agents display decreased suppressive capacity with regard to CD8+ T-cell proliferation.6 The mechanism of action of TKIs targeting FLT3 (fms-like tyrosine kinase 3), which is commonly mutated in acute myeloid leukemia, may extend beyond direct tumor cell killing. Certainly, nonspecific kinase inhibition may play a role, as the first-generation FLT3 TKIs used were not very specific; however, FLT3 inhibition itself may result in downstream immunomodulatory effects. It is noteworthy that the early sorafenib experience after allo-HCT has suggested a high incidence of skin rashes very much resembling acute GVHD yet responsive to drug dose lowering or discontinuation.7 Reports suggest that sorafenib may synergize with allogeneic T cells to improve survival in murine models. Sorafenib exposure induces interleukin-15 (IL-15) production in leukemic cells, rendering them more immunogenic. Sorafenib exposure reduces activating transcription factor 4 expression in leukemic cells, leading to activation of IRF7 (interferon regulatory factor 7), which in turn enhances IL-15 transcription. Sorafenib treatment–related IL-15 production caused an increase in CD8+ CD107a+ interferon-γ+ T cells, which eradicated leukemia in secondary recipients, indicating that recall immunity against the FLT3-ITD mutant leukemia had evolved. Human FLT3-ITD+AML cells from patients responding to sorafenib for relapse after allo-HCT demonstrated increased levels of IL-15, pIRF7, and a transcriptionally active IRF7-chromatin state, indicating that these leukemia cells were sensitive to sorafenib-induced IL-15 production. These effects were not observed in sorafenib nonresponders.8
TKIs targeting bcr-abl
When imatinib burst onto the scene at the beginning of this century, DLI was the standard approach to treating CML recurring post-HCT. Even with frank active disease, DLI is extremely effective, with high rates of durable response. However, DLI has its drawbacks. Dosing of DLI has not been standardized, and patients can develop significant GVHD. When the efficacy and safety of imatinib in nontransplant settings became apparent, reports appeared noting complete cytogenetic responses in over 70% and complete molecular responses in over 60% of CML patients who had relapsed post-HCT.9,10 Complete molecular remissions were durable in the majority of patients in the absence of additional DLI. In one study, multivariate analysis correlated the achievement of complete molecular remission with OS (odds ratio, 20.5; P = .007.10 Imatinib and its second-generation cousins, nilotinib and dasatinib, can readily induce durable cytogenetic and molecular remissions and promote conversion to full donor hematopoietic chimerism when used for relapse of CML after allo-HCT.11,12
Encouraged by this success in the relapsed setting post-HCT, practitioners began utilizing TKIs as maintenance therapy, both in advanced-phase CML and Philadelphia chromosome–positive (Ph+) acute lymphoblastic leukemia (ALL) early after allo-HCT. Most of the data supporting this practice came initially from single arm or retrospective experiences. Studies with imatinib revealed it could safely be given after allo-HCT without major myelosuppression or unmanageable interactions with calcineurin inhibitors.13 In one trial, imatinib was administered to 22 patients with Ph+ leukemia from the time of engraftment to 1 year after allo-HCT, with 77% maintaining major molecular remission.14 In a separate study of 22 patients with CML in which imatinib was administered for 1 year post-HCT, 95% completed therapy and remained without cytogenetic or hematologic relapse, although 15 patients did experience disease relapse once imatinib was stopped.15 Second- and third-generation TKIs have been studied in this setting as well, and small retrospective series have reported successful maintenance therapy with dasatinib and nilotinib.16-19 A larger, prospective study by Carpenter and colleagues underscored some of the real-life barriers to completion of a prolonged maintenance strategy. They reported 57 patients with Ph+ ALL or CML were enrolled on a study to receive maintenance nilotinib at a dose of 400 mg a day from day +80 to day +445. Only 40 of 57 actually started therapy after transplant, and only 13 completed the planned course due to a variety of reasons.19
Larger registry experiences evaluating the efficacy post–allo-HCT maintenance TKI for Ph+ ALL have yielded conflicting results. The Center for International Blood and Marrow Transplant Research analyzed 197 patients with Ph+ ALL undergoing allo-HCT in first complete remission (CR1), 43 of whom received posttransplant TKI. There was no difference in 3-year cumulative incidence of relapse (27% with TKI vs 28% without TKI in recipients of myeloablative conditioning and 59% vs 45%, respectively, in recipients of reduced intensity conditioning).20 However, in an European Blood and Marrow Transplant registry study of 473 patients with Ph+ ALL in CR1, of which 60 patients received maintenance TKI, imatinib administration was independently associated with a lower relapse rate and improvements in both leukemia-free survival (hazard ratio, 0.44; P = .0002) and OS (hazard ratio, 0.42; P = .004).21 Given that patients initiated maintenance at different time points post-HCT and received differing doses and duration of therapy, interpretation of these registry studies is difficult. Despite the lack of definitive proof of benefit, maintenance therapy with a TKI after allo-HCT for Ph+ ALL has been adopted by many transplant centers as a component of standard care.
Rather than employing a blanket maintenance strategy, an alternative strategy could leverage monitoring with quantitative bcr-abl polymerase chain reaction (PCR) assays from peripheral blood as a pre-emptive, MRD-triggered approach. In one single arm study, pre-emptive imatinib at detection of MRD after allo-HCT was associated with prolonged DFS in approximately half of patients with Ph+ ALL.22 In a small prospective randomized phase II study of 55 patients comparing maintenance vs pre-emptive therapy, prophylactic imatinib significantly reduced the incidence of molecular recurrence compared with MRD-triggered imatinib (40% vs 69%; P = .046). In both arms, hematologic relapse was low and imatinib had to be discontinued prematurely in many patients because of poor tolerability. Although the maintenance strategy reduced molecular recurrence as compared with pre-emptive therapy, there was no significant difference in overall outcomes between the two arms.23 Unfortunately, a prospective randomized trial comparing maintenance TKI to placebo (even with a preemptive MRD triggered approach) after allo-HCT for Ph+ leukemias is unlikely to be conducted at this point. Therefore, ongoing larger registry analyses with longer follow-up and more accurate molecular data will hopefully be performed to formulate an optimal strategy.
TKIs targeting FLT3-ITD
Allo-HCT in CR1 appears to improve the prognosis of patients with FLT3-ITD AML24 ; however, disease relapse after allo-HCT remains significant. An European Blood and Marrow Transplant analysis of 206 patients reported a relapse rate of 30% vs 16% (P = .006) in patients without or without the FLT3-ITD.25 In a Center for International Blood and Marrow Transplant Research analysis of 511 patients (158 with FLT3 mutations), there was an increase in relapse rate in mutated patients (38% vs 28% [P = .04]; relative risk, 1.60; 95% confidence interval, 1.15-2.22) compared with patients without a FLT3-ITD mutation.26 A number of FLT3 TKIs are being investigated in various phases of therapy, including the post-HCT maintenance setting for FLT3-ITD AML, such as sorafenib, midostaurin, quizartinib, crenolanib, and gilteritinib. Midostaurin was the first FLT3 inhibitor approved for the upfront treatment of FLT3 mutated AML in conjunction with chemotherapy, with benefit particularly noted in patients who went on to receive allo-HCT.27 A phase 2 study evaluating midostaurin as post-HCT or postconsolidation maintenance has preliminarily reported a low relapse rate at 12 months (9.2%).28
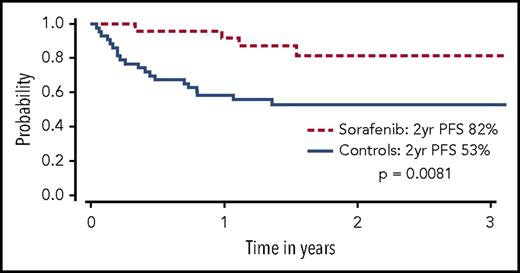
Sorafenib has been used for treatment of FLT3-ITD–mutated AML in several settings, partly because of its commercial availability from approval for use in other malignancies. In patients with FLT3-ITD AML who experienced relapse after allo-HCT, sorafenib was shown to reduce leukemic blasts and even bring about long-term survival in a minority of patients with or without DLI.29-32 In one follow-up report, 6 of 29 patients treated with sorafenib (21%) for relapse remain alive, 5 of whom (17%) achieved sustained complete remissions with a median treatment-free remission in excess of 4 years.33 These anecdotal reports led to a prospective phase 1 study of 22 patients with FLT3-ITD AML given sorafenib as maintenance therapy after allo-HCT. Sorafenib was relatively well tolerated, although dose reductions were common. Virtually no relapses were noted at a median follow-up of 16 months, and progression-free survival (PFS) and OS at 1 year were 85% and 95%, respectively.7 A follow-up retrospective landmark analysis compared 26 patients with FLT3-ITD AML treated with sorafenib maintenance after allo-HCT to a cohort of 43 contemporary matched controls with FLT3-ITD AML who were alive and in remission at day 65 after allo-HCT. Improved PFS (85% vs 52%, P = .0047) and OS (83% vs 58%, P = .019) was driven by a significant decrease in the rate of disease relapse (8.2% vs 37.7%, P = .0077) in patients receiving sorafenib maintenance (Figure 2).34 In another study, 25 of 27 patients who received maintenance sorafenib after allo-HCT remained alive and in remission at 1 year.35 Other studies have also reported encouraging results using sorafenib either as maintenance or preemptive therapy for relapsed disease post-HCT.36-39 Additional FLT3 TKIs under investigation as maintenance after HCT include quizartinib40 and crenolanib.41 A large prospective cooperative group international phase 3 placebo controlled randomized trial is underway to definitively determine the benefit of administering a FLT3 TKI (gilteritinib) as maintenance after allo-HCT for patients with FLT3-ITD AML (BMT Clinical Trials Network 1506, NCT02997202). This study will also seek to validate a PCR-based MRD assay for FLT3-ITD, possibly allowing future trials with FLT3 TKIs to focus on an MRD-triggered preemptive approach.
OS among sorafenib patients (dashed line) and controls (solid line). Only controls alive and without disease relapse at the median date of sorafenib initiation (+68) were included. Patients given sorafenib maintenance had a significantly higher OS than controls at 2 years (P = .029). Adapted from Brunner et al34 with permission.
OS among sorafenib patients (dashed line) and controls (solid line). Only controls alive and without disease relapse at the median date of sorafenib initiation (+68) were included. Patients given sorafenib maintenance had a significantly higher OS than controls at 2 years (P = .029). Adapted from Brunner et al34 with permission.
Checkpoint inhibition in hematologic malignancies
Immune escape (ie, tumor evasion of the donor immune system) plays a critical role in the survival of cancer cells. Agents that block immune checkpoints through either the CTLA4 or PD-1 pathways can stimulate endogenous antitumor immune responses and induce meaningful remissions in a variety of solid tumors.42,43 Immune checkpoint blockade has also demonstrated compelling efficacy in certain hematologic malignancies, most notably in classical Hodgkin lymphoma (HL) and primary mediastinal large cell B-cell lymphoma. The recognition of amplification at chromosome locus 9p24.1 and associated PD-1 ligand upregulation through JAK-STAT signaling in these cells laid the foundation for testing PD-1/PD-L1 inhibitors in these diseases.44,45 Both PD-1 inhibitors nivolumab and pembrolizumab showed significant activity in relapsed/refractory HL, leading to approval of both agents.46-48 In addition to HL, response to PD-1 blockade has been observed in primary mediastinal large cell B-cell lymphoma,49 primary central nervous system lymphoma,50 and T-cell lymphoma.51
Although the majority of clinical experiences using checkpoint blockade in hematologic malignancies have been in lymphoid malignancies, there are preclinical data that suggest it might be effective in myeloid diseases as well. CTLA4 is expressed on the surface and in the cytoplasm in malignant cells from patients with AML and CML.52 In MDS, PD-L1 is expressed at higher levels on blasts in patients with advanced disease and is upregulated in patients who progress on hypomethylating agents,53 yet studies of checkpoint blockade for therapy of AML thus far have been limited. A small series of pidilizumab (PD-1 blockade) yielded 1 partial response in 8 patients with AML.54 In patients with MDS, interim results of an ongoing phase 2 study assessing nivolumab or ipilimumab as monotherapy or in combination with 5-azacitidine have been presented. Responses were noted with single-agent ipilimumab, but not with nivolumab, though activity was noted when combined with hypomethylating agents.55
The rationale to use checkpoint inhibitors for relapsed disease after allo-HCT lies not only in the observed cytotoxicity mentioned but also in the intuitive potential to enhance the therapeutic graft-vs-malignancy effect. Some of this evidence comes from recent studies investigating the mechanism of action of DLI, which has been thought to be direct cell-mediated cytotoxicity from donor-derived effector T cells in the DLI product. However, more recent data suggest that DLI may act in part through stimulating effector cells already in residence in the tumor microenvironment. Utilizing antibody-based complementary DNA expression cloning and T-cell cloning techniques in patients who had received DLI, evidence has accumulated that long-lasting graft-versus-leukemia (GVL) responses are associated with coordinated adaptive and innate polyclonal immune responses directed against antigens highly expressed in leukemia progenitor cells.56-58 In addition, the activation state of marrow-infiltrating T cells before DLI appears to be predictive of clinical response. T cells bearing transcription signatures of T-cell exhaustion can be reversed after DLI administration, correlating with a clinical response.59 Therefore, agents that may reverse T-cell exhaustion signatures, such as immunological checkpoint inhibitors, might be particularly effective for patients who have relapsed after allo-HCT.
Checkpoint inhibition after autologous transplantation
One study of the PD-1 antibody pidilizumab as maintenance in 66 patients after auto-HCT for diffuse large B-cell lymphoma suggested excellent tolerability with promising long-term relapse-free survival. Treatment was associated with an increase in circulating lymphocyte subsets, including PD-L1–bearing lymphocytes.60 In a phase 2 study in 80 patients who had relapsed after autologous stem cell transplantation and brentuximab vedotin therapy, nivolumab produced a response rate of 66% (9% complete response).61 A study using the PD-L1 antibody pembrolizumab as maintenance therapy after autologous transplant is underway for patients with diffuse large B-cell lymphoma, classical HL, and T-cell non-HL (NCT02362997). There are also open studies of nivolumab after auto-HCT for multiple myeloma (NCT03292263): one evaluating durvalumab (anti-PD-L1; NCT03241017) for patients with diffuse large B-cell lymphoma and one giving durvalumab with tremelimumab (anti-CTLA4) for patients with multiple myeloma after auto-HCT (NCT02716805).
Checkpoint inhibition before allogeneic transplantation
A number of lymphoma patients with lymphoproliferative disease who have been treated with PD-1 inhibitors for relapsed disease have subsequently proceeded to undergo allo-HCT. An international retrospective analysis reported 39 patients with lymphoma who received prior treatment with a PD-1 inhibitor with the most recent dose at a median time of 62 days (7-260) before allo-HCT. After a median follow-up of 12 months, the 1-year cumulative incidences of grade 2-4 and grade 3-4 acute GVHD were 44% and 23%, respectively. There were 4 treatment-related deaths (1 from hepatic veno-occlusive disease and 3 from hyperacute GVHD). Seven patients developed a noninfectious febrile syndrome shortly after transplant, requiring prolonged courses of steroids. Despite early toxicities, the OS and PFS rates at 1 year were excellent (89% and 76%, respectively). Circulating lymphocyte subsets were analyzed in 17 patients. Compared with controls, patients previously treated with PD-1 blockade had significantly decreased PD-1+ T cells and decreased ratios of regulatory T cells to conventional CD4 and CD8 T cells.62 More study is certainly required to understand how prior PD-1 blockade influences allo-HCT outcomes and whether longer intervals between therapy and HCT can attenuate potential toxicities.
Checkpoint inhibition after allogeneic transplantation
For patients who relapse after allo-HCT, there has been understandable concern that checkpoint inhibition might lead to uncontrollable immune breakthrough events, specifically significant GVHD. In murine models, PD-1 blockade led to an increase in CD4+ and CD8+ T-cell alloimmune responses and enhanced GVHD associated with the release of inflammatory cytokines like interferon-γ and was independent of perforin/FasL-mediated cytolysis.63 There is evidence that blockade of PD-1/PD-L1, rather than PD-1/PD-L2, is more likely to induce GVHD.64 Moreover, PD-L1/CD80 interactions have been shown to augment alloreactive T-cell proliferation and induce GVHD.65 Although selective blockade of CD28/B7 interactions by CTLA4 monoclonal antibodies (mAb) can also accelerate GVHD early after transplant in murine models, enhanced T-cell expansion in mAb-treated mice could potentially significantly enhance GVL effects of DLI.66 Ongoing clinical trials have begun to investigate use of CTLA4 of PD-1 blockade after allo-HCT, yet caution remains.
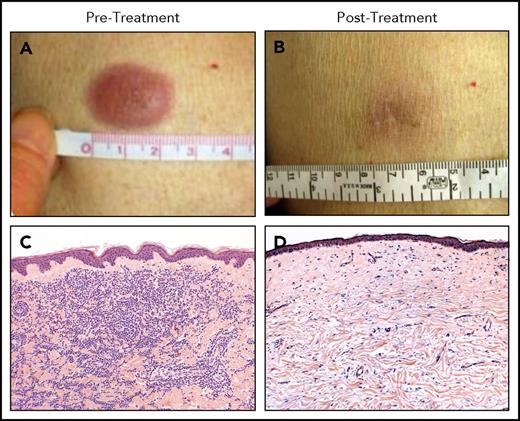
A phase 1 study of a single low-dose of ipilimumab (0.1 to 3.0 mg/kg) for patients who relapsed after allo-HCT did not seem to incite clinically significant GVHD and achieved responses in three patients with lymphoid malignancies.67 A subsequent phase 1/2 study was conducted in which patients were prescribed 4 doses of ipilimumab every 3 weeks. Patients with stable disease or better could then receive maintenance doses every 3 months for a year. Dosing started at 3 mg/kg but could escalate to 10 mg/kg. Although no formal objective responses were seen at 3 mg/kg, 7 of 22 patients at the 10 mg/kg dose responded, including 6 complete remissions and 1 partial remission. Responses were seen in patients with HL, multiple myeloma, myelodysplasia, and AML, including complete responses in 3 of 3 patients with leukemia cutis (Figure 3). Responders had a decreased number of CD4+ regulatory T cells with increased conventional T cells in peripheral blood as well as an increase in CD62L− effector memory T cells. Immunohistochemical and gene expression profiling revealed an influx of CD8+ T cells expressing perforin in responding leukemia cutis patients. GVHD responsive to corticosteroids developed in 4 of 28 patients. Immune-related adverse events typical of ipilimumab were observed in 6 patients, 1 of which was fatal (sepsis in the setting of corticosteroid treatment of pneumonitis). The median time from transplant to ipilimumab treatment was 675 days, so it is uncertain what the safety profile would be if administered early post-HCT as a maintenance or preemptive strategy.68 A subsequent intermediate-dosing cohort of patients treated at 5 mg/kg has been completed with 3 of evaluable 13 patients achieving partial remissions but also with 1 patient dying of fatal inflammatory myocarditis (NCT01822509).69 A recent report detailed the combination of ipilimumab and lenalidomide for relapse of lymphoid malignancies after allogeneic HCT. Patients received 10 mg oral lenalidomide daily for 21 days followed by IV ipilimumab at 3 mg/kg every 4 weeks for a total of 4 treatments. Seven of 10 patients responded (4 complete responses and 3 partial responses), with only 1 patient developing GVHD (occurring after lenalidomide alone).70
Clinical and histopathologic responses to ipilimumab therapy in a representative patient with leukemia cutis. Pretreatment (A-C) and posttreatment response (B-D) in a patient with leukemia cutis and corresponding histologic response. (C,D) Hematoxylin and eosin staining. Adapted from Davids et al68 with permission.
Clinical and histopathologic responses to ipilimumab therapy in a representative patient with leukemia cutis. Pretreatment (A-C) and posttreatment response (B-D) in a patient with leukemia cutis and corresponding histologic response. (C,D) Hematoxylin and eosin staining. Adapted from Davids et al68 with permission.
Given the success of PD-1 blockade in HL, anti-PD-1 mAbs are beginning to be used for relapsed disease following allo-HCT.71 Scattered reports about the efficacy of checkpoint inhibition with anti-PD-1 antibodies have appeared,72,73 yet others have reported significant GVHD as well. A case of fatal GVHD associated with pembrolizumab was reported in an HL patient who relapsed after allo-HCT,74 and another fatal case of GVHD was reported in a child with T-cell ALL after receiving PD-1 blockade post-HCT.75 A larger European multicenter series reported on the use of nivolumab in 20 patients with classical HL relapsed after allo-HCT. Response was noted in 95% of patients, with GVHD occurring in only 30% of patients (with 2 fatalities). Based on this report, the authors concluded that nivolumab is safe to use after allo-HCT.76 In contrast, a recent US multicenter retrospective analysis of 31 lymphoma patients receiving PD-1 blockade for relapsed disease after allo-HCT revealed a high response rate (77%), but GVHD developed in 17 out of 31 patients after a median of 1 or 2 doses. The response of GVHD to steroids alone was poor, and 8 patients died of complications due to GVHD.77 Recently, a report of 3 cases of relapsed AML after allo-HCT in which the patient was treated with nivolumab was described, reporting 1 complete response, one stable disease, and one case of GVHD.78 As mentioned, a prospective trial of PD-1 blockade for the treatment of relapsed hematologic malignancies after allo-HCT with lower doses of nivolumab is underway (NCT01822509), as is as a separate pilot study using pembrolizumab as well (NCT02981914). In addition, a phase 1 study is currently assessing the use of nivolumab in a preemptive fashion by administering it after allo-HCT to patients with evidence of MRD (NCT02985554). Moreover, another trial will formally investigate the use of ipilimumab, nivolumab, or combination therapy to prevent relapse after allo-HCT in patients with high-risk MDS and AML (NCT02846376). Given the toxicities observed to date, it would be difficult to recommend the routine use of PD-1 inhibitors after allo-HCT outside of the context of a clinical trial.
Future directions for checkpoint inhibitors
We are only beginning to scratch the surface in our understanding of whether we should be using checkpoint inhibition after allo-HCT, which agents to choose, what doses to use, and when they should be given. We need to discover whether there are particular aspects of immune reconstitution in the recipient that could tip the balance toward efficacy and away from toxicity. In addition, combinatorial strategies will need to be explored, whether it be with 2 distinct classes of checkpoint inhibitors, in conjunction with donor lymphocyte infusions, or with products that may upregulate ligand expression and potentiate GVL activity (eg, immunomodulatory or hypomethylating agents).70,79,80
Conclusions
The transplant community needs to continue its commitment to discovering new approaches to preventing and treating relapse after allo-HCT. We must discard the notion that allo-HCT by itself is definitive therapy and only manipulation of immune suppression or infusion of cellular therapy products will be sufficient to address disease relapse. Targeted therapies with TKIs or immune modulation with checkpoint inhibitors need to be carefully studied before such agents are routinely incorporated into the transplant paradigm. If shown to be safe, it will likely be preferable to use these agents upfront in a prophylactic or preemptive manner rather than wait for frank disease relapse. We must use tumor genomic profiling to better understand whose tumor is at greatest risk of relapse and which donor might provide heightened alloreactivity. Lastly, we must also convince pharmaceutical companies to engage with the transplant community to test novel agents earlier in the development cycle to better understand how they might be successfully applied to improve the outcome of a transplant recipient.
Acknowledgments
The authors thank Emily White for assistance in the preparation of this manuscript, as well as all patients (and their families) who participated in the clinical trials described.
Authorship
Contribution: R.J.S., M.S.D., and Y.-B.C. contributed equally to the generation of this review.
Conflict-of-interest disclosure: R.J.S. is on the supervisory board at Kiadis and DSMB-Juno and is a consultant with GSK, Merck, and Gilead. M.S.D. is on the consulting/advisory boards at Janssen, Celgene, Abbvie, Incyte, Merck, Astrazeneca, MEI, Genentech, Pharmacyclics, and TG Therapeutics and receives institutional research funding from BMS, Surface Oncology, Genentech, Pharmacyclics, and TG Therapeutics. Y.-B.C. is a consultant with Magenta and Takeda and on the advisory board at Incyte and Takeda.
Correspondence: Robert J. Soiffer, Dana Farber Cancer Institute, 450 Brookline Ave, Boston, MA 02215; e-mail: robert_soiffer@dfci.harvard.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal