Abstract
Context: Azacitidine (AZA) prolongs overall survival (OS) but does not cure higher-risk MDS and AML (MDS/AML) patients (pts). Conversely, response to AZA in MDS/AML has been linked with a slow reduction in the clonal burden of bone marrow nucleated cells (BMNCs, Uy, Leukemia 2016) and reduced frequency of phenotypic Leukemic stem/progenitor cells (LSPCs; Craddock, Leukemia 2013). MDS progenitor quiescence could also explain resistance to AZA (Unnikrishnan, Cell Rep 2017). Whether the clonal composition and quiescence of LSCPs can predict long-term response to AZA and herald the relapse clone has never been investigated.
Methods: We obtained bone marrow (BM) samples from higher-risk (IPSS int-2/high) or AML pts at onset of AZA, response evaluation, and relapse when available, and sorted LSCPs defined as GMPs (Lin-CD34+CD38+CD123+CD45RA+) or Lymphoid-primed multipotent progenitor (LMPPs, Lin-CD34+CD38-CD90-CD45RA+), as well as Hematopoietic Stem Cells (HSC, Lin-CD34+CD38-CD90+) when available, and performed sequencing of 57 genes recurrently mutated in MDS/AML (Agilent SureSelect, Illumina MiSeq) after whole genome amplification (WGA, Repli-G, Qiagen). We compared the resulting clonal composition to that of pre-AZA unamplified DNA from BM mononuclear cells (BMNCs). In an independent cohort of AML pts treated with AZA, we interrogated the cell cycle status (Ki67/Hoescht 33343) of GMPs and total blasts from thawed BMNCs collected prior to AZA.
Results: WGA did not alter the Variant Allele Frequency (VAF) of single nucleotide variants (R²=0.96) or small insertions/deletions (R²=0.99), enabling the clonal interrogation of as few as 300 cells. We studied a cohort of 11 pts (5 MDS, 6 AML) with a median of 6 mutations treated with AZA (median 9 cycles), including 7 responders (CR in 4, PR in 1, marrow CR in 2). We compared clonal burden of BMNCs, HSCs (mean 0.1% of BMNCs), LMPPs (mean 0.6%) and GMPs (mean 9.7%) in 4 pts prior to AZA. Of the 3 immature fractions, the GMP fraction had the highest abundance among BMNCs and the highest clonal burden (median VAF 24.2% vs 10.0% in BMNCs, P=0.001), representing an ideal leukemic population for the monitoring of clonal evolution with AZA.
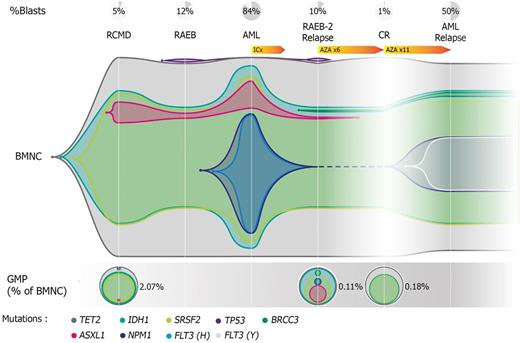
We next analyzed the clonal composition of GMPs at the time of AZA response evaluation in all 11 pts, after a median of 6 cycles. GMPs represented 0.78% of BMNCs at evaluation. While there was no change in VAFs in BMNCs at evaluation compared to pre-AZA samples (p=0.48), a lower clonal burden in GMPs was seen in responders (n=7) compared to non-responders (n=4, P=0.002). This difference reflected clearance of secondary lesions (eg. genes involved in signaling) in responders (median VAF 1.8% vs 38.2% in non-responders, p=0.007), while ancestral lesions (eg. genes involved in DNA methylation) retained high clonality irrespective of response (p=0.2). BMNCs at relapse were obtained in 5/7 responders (CR n=4, mCR n=1) and compared to samples collected before AZA or after 6 cycles. In two pts who relapsed after 9 and 12 cycles, GMP clonal architecture added no information compared to BMNC in predicting the composition of the relapse clone. Conversely, in 3 pts relapsing after 15, 16 and 23 total cycles, GMP clonal composition predicted the relapse clone composition better than BMNC because of contamination of BMNCs by a passenger clone (n=1), or over-representation of a FLT3 + relapse clone in GMPs compared to BMNCs (n=2). In pt SLS008, the relapse NPM1 +/ FLT3 + clone was detected only in GMPs before AZA and never in BMNCs with a detection limit of 1% (Figure).
We interrogated the cycling of GMPs compared to CD45dim/SSClow blasts in an independent cohort of 28 AML pts treated with AZA in first line (n=13) or in the relapsed/refractory setting (n=15). A lower proportion of quiescent (Ki67low) blasts predicted response (p=0.03) but had no impact on OS. Conversely, the proportion of quiescent GMP predicted progressive disease (p=0.04) and was also a prognostic factor for OS, with a median OS of 15.1 months in pts with <60% quiescent GMPs vs 4.2 months in those with ≥60% quiescent GMPs (p=0.04).
Conclusion: Our pilot study shows that clearance of secondary lesions in GMPs is associated with response to AZA and that GMP quiescence can predict long-term outcome of MDS/AML pts treated with AZA. Our results suggest that GMPs constitute key target cells for AZA in MDS/AML and warrant prospective validation of GMP-based biomarkers for hypomethylating agents.
Renneville: CELGENE: Research Funding. Pimanda: Celgene: Research Funding. Fenaux: Celgene: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Astex: Honoraria, Research Funding; Astex: Honoraria, Research Funding; Amgen: Honoraria, Research Funding. Itzykson: Novartis: Research Funding; Janssen: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal