Key Points
The fVIII C1 domain contributes significantly to the immune response against fVIII in acquired and congenital hemophilia inhibitor patients.
B-cell epitopes identified for monoclonal murine and human C1 inhibitors are recognized by antibodies present in patients with hemophilia.
Abstract
Several studies showed that neutralizing anti–factor VIII (anti-fVIII) antibodies (inhibitors) in patients with acquired hemophilia A (AHA) and congenital hemophilia A (HA) are primarily directed to the A2 and C2 domains. In this study, the frequency and epitope specificity of anti-C1 antibodies were analyzed in acquired and congenital hemophilia inhibitor patients (n = 178). The domain specificity of antibodies was studied by homolog-scanning mutagenesis (HSM) with single human domain human/porcine fVIII proteins and antibody binding to human A2, C1, and C2 domains presented as human serum albumin (HSA) fusion proteins. The analysis with HSA-fVIII domain proteins confirmed the results of the HSM approach but resulted in higher detection levels. The higher detection levels with HSA-fVIII domain proteins are a result of antibody cross-reactivity with human and porcine fVIII leading to false-negative HSM results. Overall, A2-, C1-, and C2-specific antibodies were detected in 23%, 78%, and 68% of patients with AHA (n = 115) and in 52%, 57%, and 81% of HA inhibitor patients (n = 63). Competitive binding of the human monoclonal antibody (mAb) LE2E9 revealed overlapping epitopes with murine C1-specific group A mAbs including 2A9. Mutational analyses identified distinct crucial binding residues for LE2E9 (E2066) and 2A9 (F2068) that are also recognized by anti-C1 antibodies present in patients with hemophilia. A strong contribution of LE2E9- and 2A9-like antibodies was particularly observed in patients with AHA. Overall, our study demonstrates that the C1 domain, in addition to the A2 and C2 domains, contributes significantly to the humoral anti-fVIII immune response in acquired and congenital hemophilia inhibitor patients.
Introduction
The formation of neutralizing anti–factor VIII (anti-fVIII) antibodies (also called inhibitors) is not only the most challenging treatment-related complication of fVIII therapy in patients with congenital hemophilia A (HA) disorder1,2 but also causes the autoimmune disease acquired hemophilia A (AHA).3,4 Inhibitors in patients with HA can be eliminated by so-called immune tolerance induction (ITI) based on regular administration of high doses of fVIII.5 Patients with AHA are treated with fVIII bypassing agents or porcine fVIII (pfVIII) to control acute bleeds and various immunosuppressive therapies based on glucocorticoids alone or in combination with other immunosuppressive or immunomodulatory agents.6-8 Earlier studies showed that antibodies in both AHA and HA inhibitor plasmas are primarily directed to the A2 and C2 domains.9-11 However, patients with AHA seem to have a more restricted antibody response than patients with HA, because most autoantibodies are more likely to be directed against either the A2 or C2 domain, but not both domains.10,12 The first hint that the C1 domain of fVIII might also be immunogenic derived from a patient with mild HA resulting from a R2150H missense mutation who had developed inhibitors to allogeneic but not autologous fVIII.13 Characterization of a monoclonal antibody (mAb) LE2E9 isolated from this patient eventually identified the C1 domain as a novel target for inhibitors.14 Comparison of the antigenicity of human, porcine, and human/porcine hybrid fVIII proteins also suggested the potential presence of C1 inhibitors in patients with HA and high-titer inhibitors.15 Recently, Batsuli et al identified 2 distinct B-cell epitopes designated groups A and B within the C1 domain and showed that anti-C1 antibodies were found in up to 60% (7/12) of patients with HA and inhibitors.16 In addition, studies in hemophilic mice showed that the C1 domain makes a major contribution to the overall humoral anti-fVIII immune response.17 The presence of immunodominant regions within the C1 domain was further supported by data showing that hemophilic mice developed a stronger immune response to human than porcine C1.18 Therefore, the aim of this study was to analyze the frequency and epitope specificity of anti-C1 antibodies in plasma from patients with acquired hemophilia or patients with congenital hemophilia and inhibitors.
Methods
Study population
A population of 178 patients with hemophilia with inhibitors (115 AHA and 63 HA patients) was studied. Analysis was performed from stored plasma that was collected at a single point before ITI or IST start. Plasma samples derived from 2 prospective studies, the GTH-AH 01/2010 study19 (92 AHA samples; AHA group II) and the International Immune Tolerance Study20 (30 HA samples; HA group II), as well as from mainly German hemophilia treatment centers (33 HA and 23 AHA; HA and AHA groups I).
Approval
Institutional review board approval was granted for the study, and all patients provided written informed consent before blood collection.
Plasmid construction
Plasmid constructs encoding human serum albumin (HSA) fused to human fVIII A2a2 (HSA-hA2), human fVIII C2 (HSA-hC2), and porcine fVIII C1 (HSA-pC1) were cloned as previously described for HSA-hC116 and detailed in the supplemental Data, available on the Blood Web site. Point mutations in HSA-hC1 were generated by site-directed mutagenesis according to manufacturer’s instructions (Agilent Technologies GmbH & Co. KG, Waldbronn, Germany) and splicing-by-overlap extension mutagenesis, as previously described for human porcine fVIII hybrids.21 Therefore, human C1 residues that are not conserved among human and porcine fVIII C1 sequences were substituted for porcine residues, whereas conserved residues were substituted for alanine.
Protein purification
Recombinant human, porcine, and single human domain human/porcine hybrid fVIII (SHD hpfVIII) proteins were purified from cell culture media of stable transfected BHK-M cell lines, as described before.22 HSA-fVIII domain fusion proteins were purified from cell culture media as previously described for histidine-tagged fVIII A2 or C2 proteins.23 The functional integrity of SHD hpfVIII proteins was confirmed by measuring fVIII activity. The conformational integrity of HSA-hA2, HSA-hC1, and HSA-hC2 fusion proteins was confirmed by binding to commercially available fVIII domain-specific antibodies. Purified proteins were additionally analyzed by reducing sodium dodecyl sulfate polyacrylamide gel electrophoresis, followed by Coomassie or silver staining (supplemental Figures 1B and 2).
FVIII domain mapping of plasma anti-fVIII IgG
FVIII domain mapping by homolog-scanning mutagenesis (HSM) with SHD hpfVIII proteins was performed as previously described.22 A net absorbance at 492 nm above 0.2 (cut off), corresponding to 3 standard deviations of the mean IgG binding of 30 healthy individuals to porcine fVIII (pfVIII), determined on 3 separate days, was used for domain assignment. Antibody binding to hC1pfVIII was additionally analyzed in the presence of 3.3 pmol/well mAb 2A9 (GMA-8011; Green Mountain Antibodies, Burlington, VT).
Binding of plasma anti-fVIII IgG to HSA-fVIII domain fusion proteins was analyzed as described in detail in the supplemental data. IgG binding was considered positive if net absorbance values at 492 nm were above the fVIII domain-specific cut points (0.150 for A2, 0.159 for C1, and 0.138 for C2), which corresponded to the 95th percentile of IgG binding of 30 healthy individuals to HSA-hA2, HSA-hC1, and HSA-hC2 domain proteins determined on 3 separate days (supplemental Figure 3).
Competition fVIII binding of anti-C1 mAbs
Approximately 0.42 pmol of rfVIII was coated for 12-14 hours at 4°C onto microtiter plates (Microlon 600; Greiner BioOne, Solingen, Germany). Plates were washed with phosphate-buffered saline (PBS; pH, 7.4, Sigma-Aldrich) containing 0.05% vol/vol Tween-20 (PBST; Carl Roth GmbH, Karlsruhe, Germany) and between each of the following incubation steps. Unspecific binding sites were blocked by incubation with 5% wt/vol skim milk powder (Sigma-Aldrich) in PBST (MPBST) for 2 hours at room temperature (RT). Afterward, human LE2E9 at a final concentration of 0.2 μg/mL was incubated in the absence and presence of different concentrations (0.05, 0.1, 0.2, 1, 5, and 10 μg/mL) of murine anti-C1 mAbs comprising 2A9, F156, I84, M6143 (group A mAbs), and B136 (group B mAb) for 2 hours at RT. FVIII-bound LE2E9 was detected using an HRP-conjugated goat anti-human IgG (H+L) antibody (Invitrogen, Darmstadt, Germany) diluted 1:5000 in MPBST. HRP substrate composed of (2 tablets) o-phenylenediamine (Sigma-Aldrich) in 100 mL phosphate-citrate buffer (Sigma-Aldrich) and 40 µL hydrogen peroxide (Carl Roth GmbH) were added. The reaction was stopped after 6 minutes by adding 100 µL of 1 N sulfuric acid (AppliChem GmbH, Darmstadt, Germany), and absorbance was measured immediately with a microplate absorbance reader (Sunrise, Tecan, Crailsheim, Germany) at 492 and 620 nm (the reference/control was read out at 620 nm; OD620 nm was substracted from obtained OD492 nm values to reduce nonspecific ODs).
Binding of anti-C1 mAbs to HSA-hC1 domain variants
Approximately 0.1 µg/well anti-C1 mAbs 2A9, LE2E9, B136, and anti-flag M1 antibody (Sigma-Aldrich) were coated for 12-14 hours at 4°C onto microtiter plates (Greiner BioOne). Plates were washed with PBST between each of the following incubation steps. Nonspecific binding sites were blocked by incubation with MPBST for 2 hours at RT. Afterward, 50 µL conditioned cell culture media containing comparable amounts of HSA-hC1 variants in 50 µL PBST were incubated for 2 hours at RT. Cell culture media from nontransfected cells was used as negative control for background correction. Binding of HSA-hC1 variants was detected by incubation with biotinylated anti-HSA antibody (0.1 µg/well) for 2 hours at RT. Afterward, bound biotinylated anti-HSA antibody was detected by incubation with HRP-conjugated streptavidin (Dianova, Hamburg, Germany) diluted 1:5000 in MPBST for 1 hour at RT. Subsequent color development and absorbance measurement were performed as described earlier.
Coagulation assays with hC1pfVIII protein variants
The capacity of mAbs LE2E9 and 2A9 to inhibit fVIII coagulant activity (FVIII:C) of the hC1pfVIII and its variants E2066D and F2068H was tested in a coagulation assay. Equal volumes (50 µL) of various concentrations of LE2E9 or mAb 2A9 diluted in 4% wt/vol bovine serum albumin in PBS and conditioned cell culture media containing the respective hC1pfVIII proteins were incubated for 2 hours at RT (LE2E9) or 37°C (mAb 2A9), respectively. The residual FVIII:C was measured in a 1-stage clotting assay as previously described, and also in detail in the supplemental data.22 The measured residual FVIII:C was expressed as the percentage of the activity obtained in the absence of antibodies (%residual fVIII:C).
Statistical analysis
Statistical analysis was performed using GraphPad Prism (GraphPad Software Inc., La Jolla, CA). ELISA curves were fitted to the 4-parameter logistic equation, and the antibody concentrations required to achieve 50% inhibition also were calculated using GraphPad Prism. Data are presented as means of duplicates ± standard deviation unless otherwise specified. Significance for the different inhibition of patient IgG binding in the presence of mAb 2A9 was determined by the nonparametric Mann-Whitney test. A value of P < .05 was considered statistically significant.
Results
Frequency of fVIII domain-specific IgG
The frequency of fVIII domain-specific IgG was studied in patient plasma by antibody binding to human A2, C1, and C2 domains presented as SHD hpfVIII and HSA-fVIII domain fusion proteins. The median inhibitor titer of the study population was 5.4 BU/mL (interquartile range, 1.9-20.0 BU/mL). HSM with SHD hpfVIII proteins identified A2-, C1-, and C2-specific IgG in 21 (18%), 34 (30%), and 61 (53%) of 115 patients with AHA and in 18 (29%), 4 (6%), and 32 (51%) of 63 patients with HA and inhibitors (Table 1). The binding studies with HSA-fVIII domain proteins confirmed the results of the HSM approach but resulted in generally higher detection levels of A2-, C1-, and C2-specific IgG: 27 (23%), 90 (78%), and 78 (68%) of 115 patients with AHA and 33 (52%), 36 (57%), and 51 (81%) of 63 patients with HA and inhibitors (Table 1). Major differences in the results of both mapping techniques were in particular observed for the frequency of C1-specific IgG, which were detectable in only 38 (21%) of 178 patients with hemophilia by HSM but in 126 (71%) using the HSA-hC1 protein (Table 1;supplemental Figure 4).
Frequency of fVIII domain-specific antibodies in acquired and congenital hemophilia inhibitor patients
| Study . | Number of patients . | Absolute and relative number of patients with IgG binding to . | |||||
|---|---|---|---|---|---|---|---|
| HSA-domain fusion proteins . | SHD hpfVIII proteins . | ||||||
| A2 . | C1 . | C2 . | A2 . | C1 . | C2 . | ||
| AHA group I, n (%) | 23 | 4 (17) | 12 (52) | 17 (74) | 1 (4) | 5 (22) | 12 (52) |
| AHA group II, n (%) | 92 | 23 (25) | 78 (85) | 61 (66) | 20 (22) | 29 (32) | 49 (53) |
| Total AHA, n (%) | 115 | 27 (23) | 90 (78) | 78 (68) | 21 (18) | 34 (30) | 61 (53) |
| HA group I, n (%) | 33 | 28 (85) | 23 (70) | 31 (94) | 13 (39) | 4 (12) | 18 (55) |
| HA group II, n (%) | 30 | 5 (17) | 13 (43) | 20 (67) | 5 (17) | 0 (0) | 14 (47) |
| Total HA, n (%) | 63 | 33 (52) | 36 (57) | 51 (81) | 18 (29) | 4 (6) | 32 (51) |
| AHA + HA, n (%) | 178 | 60 (34) | 126 (71) | 129 (72) | 39 (22) | 38 (21) | 93 (52) |
| Study . | Number of patients . | Absolute and relative number of patients with IgG binding to . | |||||
|---|---|---|---|---|---|---|---|
| HSA-domain fusion proteins . | SHD hpfVIII proteins . | ||||||
| A2 . | C1 . | C2 . | A2 . | C1 . | C2 . | ||
| AHA group I, n (%) | 23 | 4 (17) | 12 (52) | 17 (74) | 1 (4) | 5 (22) | 12 (52) |
| AHA group II, n (%) | 92 | 23 (25) | 78 (85) | 61 (66) | 20 (22) | 29 (32) | 49 (53) |
| Total AHA, n (%) | 115 | 27 (23) | 90 (78) | 78 (68) | 21 (18) | 34 (30) | 61 (53) |
| HA group I, n (%) | 33 | 28 (85) | 23 (70) | 31 (94) | 13 (39) | 4 (12) | 18 (55) |
| HA group II, n (%) | 30 | 5 (17) | 13 (43) | 20 (67) | 5 (17) | 0 (0) | 14 (47) |
| Total HA, n (%) | 63 | 33 (52) | 36 (57) | 51 (81) | 18 (29) | 4 (6) | 32 (51) |
| AHA + HA, n (%) | 178 | 60 (34) | 126 (71) | 129 (72) | 39 (22) | 38 (21) | 93 (52) |
The frequency of fVIII domain-specific IgG in patients with AHA and congenital HA and inhibitors to human A2, C1, or C2 domains was measured by ELISA using HSA-fVIII domain fusion proteins and SHD hpfVIII proteins as antigens. For details regarding patient groups and experimental settings, see “Methods”.
The higher detection level with HSA-hC1 protein could be explained by cross-reactive anti-C1 IgG that binds to regions that are conserved between human and porcine sequences, and therefore escape detection by HSM, leading to false-negative results. Consistent with this hypothesis, a significant correlation of the amounts of HSA-hC1-binding (C1-specific) IgG and pfVIII-binding (cross-reactive anti-fVIII) IgG was observed in patients with AHA (n = 90; r = 0.685; P < .0001), indicating that C1-specific IgG contribute significantly to the total population of cross-reactive anti-fVIII IgG in these patients (supplemental Figure 5).
Furthermore, IgG binding to HSA-pC1 and HSA-hC1 proteins was compared for patients who tested negative for C1-specific IgG by HSM but positive with HSA-hC1 (HSM C1 negative, n = 15), as well as for patients who tested positive for C1-specific IgG by HSM and with HSA-hC1 (HSM C1 positive, n = 15). A very strong relative binding of patient IgG to porcine C1 compared with human C1 was observed in all tested patients, providing direct evidence for cross-reactive C1-specific patient IgG (supplemental Figure 6). Consistent with the HSM results, the median IgG cross-reactivity was statistically significantly lower for HSM C1-positive patients compared with HSM C1-negative patients (supplemental Figure 6). However, some HSM C1-negative patients revealed similar cross-reactivity as HSM C1-positive patients. Therefore, C1-specific cross-reactivity alone does presumably not decide whether or not C1-specific patient IgG can be mapped by HSM. One can imagine that, particularly in patients revealing overall high porcine fVIII reactivity, domain mapping by HSM will hardly be possible because of strong assay background signals.
Overall, our findings demonstrate that the C1 domain, in addition to the A2 and C2 domains, contributes significantly to the immune response to fVIII in patients with hemophilia.
Characterization of human anti-C1 mAb LE2E9
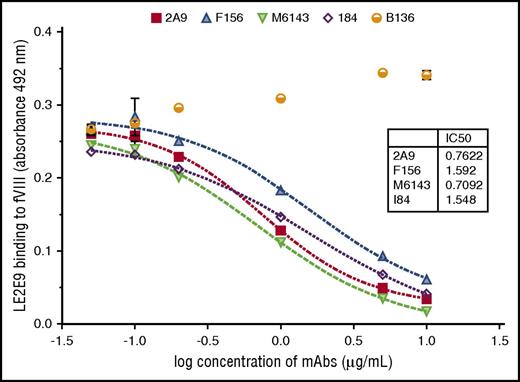
The human anti-C1 mAb LE2E9 was further characterized by comparative analyses with previously characterized murine anti-C1 mAbs, including the 4 group A mAbs 2A9, F156, I84, and M6143, as well as the group B mAb B136.16 First, the epitope group of human LE2E9 was determined by competitive ELISA with the murine anti-C1 mAbs. A dose-dependent inhibition of LE2E9 binding to fVIII was observed for all 4 group A mAbs (Figure 1). In contrast, the presence of the group B mAb B136 did not interfere with binding of LE2E9 to fVIII. On the basis of these results, LE2E9 was categorized as anti-C1 group A mAb. MAb 2A9 was selected from the panel of group A mAbs as lead molecule for further comparison. Thereby, it was shown that binding of LE2E9 and mAb 2A9 to heat-treated (56°C) fVIII and C1 domain was significantly reduced (supplemental Figure 7), indicating that both group A mAbs recognize a conformational C1 epitope.
The patient-derived anti-C1 mAb LE2E9 recognizes a group A B-cell epitope. The epitope of human mAb LE2E9 was mapped by competitive ELISA with murine anti-C1 mAbs including 4 group A mAbs (2A9, F156, I84, and M6143) and a single group B mAb (B136). For that, fVIII binding of LE2E9 used at a final concentration of 0.2 μg/mL was analyzed in the presence of increasing mAb concentrations (0.05, 0.1, 0.2, 1, 5, and 10 μg/mL). LE2E9 binding was detected using HRP-conjugated goat anti-human IgG (H+L) antibody. The experiments were repeated twice with similar results.
The patient-derived anti-C1 mAb LE2E9 recognizes a group A B-cell epitope. The epitope of human mAb LE2E9 was mapped by competitive ELISA with murine anti-C1 mAbs including 4 group A mAbs (2A9, F156, I84, and M6143) and a single group B mAb (B136). For that, fVIII binding of LE2E9 used at a final concentration of 0.2 μg/mL was analyzed in the presence of increasing mAb concentrations (0.05, 0.1, 0.2, 1, 5, and 10 μg/mL). LE2E9 binding was detected using HRP-conjugated goat anti-human IgG (H+L) antibody. The experiments were repeated twice with similar results.
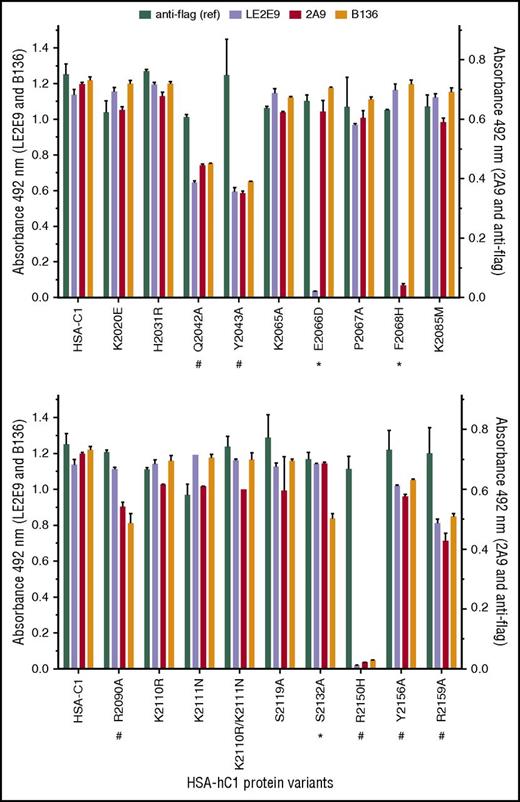
Next, solid-phase binding assays with HSA-hC1 variants were performed to elucidate which amino acid residues were crucial antigenic determinants for LE2E9 and mAb 2A9. As both mAbs inhibit the binding of fVIII to von Willebrand factor (VWF),14,16 surface-exposed C1 residues involved in VWF binding might be involved in binding of LE2E9 and mAb 2A9. Therefore, C1 residues known to impair VWF binding if mutated in patients24,25 (n = 8, rows 13-20), along with nonconserved C1 residues (n = 11, rows 1-12), were considered for mutational analysis (Table 2). Substitution of residues was performed on the basis of their predicted side-chain solvent accessibility (Table 2). In addition, we mutated residues Q2042, Y2043, and R2159 (Table 2, rows 20-22), which were identified by site-directed mutagenesis to be involved in VWF binding,26 as well as residues K2065, P2067, and Y2156 (Table 2, rows 23-25). Substitution E2066D prevented binding of LE2E9, but not of mAbs 2A9 and B136, whereas substitution F2068H strongly reduced binding of mAb 2A9, but not of mAbs LE2E9 and B136 (Figure 2). These results indicate that despite their competitive binding, distinct nonconserved C1 residues (F2068 and E2066) are essential for binding mAbs 2A9 and LE2E9 (supplemental Figure 8B). Moreover, substitution S2132A reduced binding of mAb B136, but not of mAbs 2A9 and LE2E9, suggesting a role of this residue for B136 binding (Figure 2; supplemental Figure 8D). The identification of epitope residues that are not conserved among human and porcine sequences for mAbs 2A9 and B136 is consistent with the fact that their C1 domain specificity was determined by HSM.17 Comparable loss or reduction in binding to HSA-hC1 variants Q2042A, Y2043A, R2090A, R2150H, Y2156A, and R2159A for anti-C1 group A (2A9 and/or LE2E9) and group B (B136) mAbs indicate that the mutation of these residues globally distorted the C1 domain in these variants, leading to the observed loss of antigenicity. Therefore, these amino acid residues were not considered as epitope determinants.
C1 constructs and predicted accessibility of C1 residues
| No. . | Construct name . | Residue . | Substitution . | Accessible surface area values . | |||
|---|---|---|---|---|---|---|---|
| hC1pfVIII . | HSA-C1 . | 2R7E . | 3CDZ . | 4BDV . | |||
| 1 | MHI-82 | MHI-128 | K2020 | E | 0.88 | 0.92 | 0.96 |
| 2 | T2023 | 0.16 | 0.29 | 0.19 | |||
| 3 | MHI-170 | MHI-171 | H2031 | R | 0.58 | 0.26 | 0.40 |
| 4 | MHI-83 | MHI-129 | E2066 | D | 0.80 | 0.51 | 0.42 |
| 5 | MHI-84 | MHI-130 | F2068 | H | 1.00 | 0.90 | 0.92 |
| 6 | MHI-85 | MHI-131 | K2085 | M | 0.29 | 0.31 | 0.26 |
| 7 | MHI-86 | MHI-132 | K2110 | R | 0.89 | 0.86 | 0.85 |
| 8 | MHI-87 | MHI-133 | K2111 | N | 0.64 | 0.76 | 0.69 |
| 9 | MHI-155 | MHI-172 | K2110/K2111 | R/N | — | — | — |
| 10 | T2114 | 0.49 | 0.17 | 0.22 | |||
| 11 | MHI-88 | MHI-134 | S2132 | A | 0.31 | 0.22 | 0.22 |
| 12 | I2145 | 0.30 | 0.32 | 0.25 | |||
| 13 | Q2087 | 0.02 | 0.06 | 0.02 | |||
| 14 | N/A | MHI-173 | R2090 | A | 0.62 | 0.69 | 0.76 |
| 15 | I2098 | 0.00 | 0.02 | 0.01 | |||
| 16 | N/A | MHI-174 | S2119 | A | 0.59 | 0.45 | 0.40 |
| 17 | N2129 | 0.14 | 0.06 | 0.03 | |||
| 18 | MHI-89 | MHI-135 | R2150 | H | 0.13 | 0.24 | 0.15 |
| 19 | P2153 | 0.11 | 0.16 | 0.10 | |||
| 20 | N/A | MHI-138 | R2159 | A | 0.21 | 0.05 | 0.08 |
| 21 | N/A | MHI-136 | Q2042 | A | 0.61 | 0.63 | 0.47 |
| 22 | N/A | MHI-137 | Y2043 | A | 0.30 | 0.39 | 0.46 |
| 23 | N/A | MHI-139 | K2065 | A | 0.27 | 0.67 | 0.63 |
| 24 | N/A | MHI-140 | P2067 | A | 0.47 | 0.61 | 0.64 |
| 25 | N/A | MHI-141 | Y2156 | A | 0.30 | 0.39 | 0.43 |
| No. . | Construct name . | Residue . | Substitution . | Accessible surface area values . | |||
|---|---|---|---|---|---|---|---|
| hC1pfVIII . | HSA-C1 . | 2R7E . | 3CDZ . | 4BDV . | |||
| 1 | MHI-82 | MHI-128 | K2020 | E | 0.88 | 0.92 | 0.96 |
| 2 | T2023 | 0.16 | 0.29 | 0.19 | |||
| 3 | MHI-170 | MHI-171 | H2031 | R | 0.58 | 0.26 | 0.40 |
| 4 | MHI-83 | MHI-129 | E2066 | D | 0.80 | 0.51 | 0.42 |
| 5 | MHI-84 | MHI-130 | F2068 | H | 1.00 | 0.90 | 0.92 |
| 6 | MHI-85 | MHI-131 | K2085 | M | 0.29 | 0.31 | 0.26 |
| 7 | MHI-86 | MHI-132 | K2110 | R | 0.89 | 0.86 | 0.85 |
| 8 | MHI-87 | MHI-133 | K2111 | N | 0.64 | 0.76 | 0.69 |
| 9 | MHI-155 | MHI-172 | K2110/K2111 | R/N | — | — | — |
| 10 | T2114 | 0.49 | 0.17 | 0.22 | |||
| 11 | MHI-88 | MHI-134 | S2132 | A | 0.31 | 0.22 | 0.22 |
| 12 | I2145 | 0.30 | 0.32 | 0.25 | |||
| 13 | Q2087 | 0.02 | 0.06 | 0.02 | |||
| 14 | N/A | MHI-173 | R2090 | A | 0.62 | 0.69 | 0.76 |
| 15 | I2098 | 0.00 | 0.02 | 0.01 | |||
| 16 | N/A | MHI-174 | S2119 | A | 0.59 | 0.45 | 0.40 |
| 17 | N2129 | 0.14 | 0.06 | 0.03 | |||
| 18 | MHI-89 | MHI-135 | R2150 | H | 0.13 | 0.24 | 0.15 |
| 19 | P2153 | 0.11 | 0.16 | 0.10 | |||
| 20 | N/A | MHI-138 | R2159 | A | 0.21 | 0.05 | 0.08 |
| 21 | N/A | MHI-136 | Q2042 | A | 0.61 | 0.63 | 0.47 |
| 22 | N/A | MHI-137 | Y2043 | A | 0.30 | 0.39 | 0.46 |
| 23 | N/A | MHI-139 | K2065 | A | 0.27 | 0.67 | 0.63 |
| 24 | N/A | MHI-140 | P2067 | A | 0.47 | 0.61 | 0.64 |
| 25 | N/A | MHI-141 | Y2156 | A | 0.30 | 0.39 | 0.43 |
Overview of plasmid constructs encoding HSA-fVIII C1 domain and hC1pfVIII variants. The names of the plasmid constructs, the position and substitution of respective amino acid residue in the C1 domain (2020-2172), and the values for solvent-accessible surface area (ASA) are listed. ASA values were calculated for the BDD-fVIII structures 2R7E,36 3CDZ,37 and 4BDV,38 using the program ASAView (www.abren.net/asaview/).39 Rows 1-12, nonconserved residues among human and porcine fVIII C1 domains; rows 13-20, HA missense mutations in the C1 domain associated with reduced VWF binding25 and identified in the FVIII Variant Database (www.factorviii-db.org) by Chiu et al24 ; rows 21-22, residues additionally identified by site-directed mutagenesis to be involved in VWF binding26 ; rows 23-25, residues identified by eye screening of fVIII structures to be in close proximity to the crucial antibody binding residues E2066 and F2068. All mutant constructs supported expression and secretion of the respective protein similar to the native hC1pfVIII and HSA-hC1 constructs. N/A, not available.
Identification of C1 domain residues involved in antibody binding using HSA-hC1 variants. Anti-C1 mAbs 2A9 and LE2E9 (group A), anti-C1 mAb B136 (group B), and anti-flag M1 antibody (used to control input) were immobilized onto microtiter plates. Binding of the indicated HSA-hC1 variants (for details, see Table 2) from cell culture media was detected with biotinylated anti-HSA antibody followed by incubation with HRP-conjugated streptavidin. Cell culture media from nontransfected cells were used as negative control for background correction. The experiments were repeated twice with similar results. Comparable changes in binding to HSA-hC1 variants for anti-C1 group A and group B mAbs are denoted by an octothorpe below the substituted residue and point to a global distortion of the C1 domain in these variants. Anti-C1 group A or group B mAb-specific binding defects are denoted by an asterisk below the substituted residue.
Identification of C1 domain residues involved in antibody binding using HSA-hC1 variants. Anti-C1 mAbs 2A9 and LE2E9 (group A), anti-C1 mAb B136 (group B), and anti-flag M1 antibody (used to control input) were immobilized onto microtiter plates. Binding of the indicated HSA-hC1 variants (for details, see Table 2) from cell culture media was detected with biotinylated anti-HSA antibody followed by incubation with HRP-conjugated streptavidin. Cell culture media from nontransfected cells were used as negative control for background correction. The experiments were repeated twice with similar results. Comparable changes in binding to HSA-hC1 variants for anti-C1 group A and group B mAbs are denoted by an octothorpe below the substituted residue and point to a global distortion of the C1 domain in these variants. Anti-C1 group A or group B mAb-specific binding defects are denoted by an asterisk below the substituted residue.
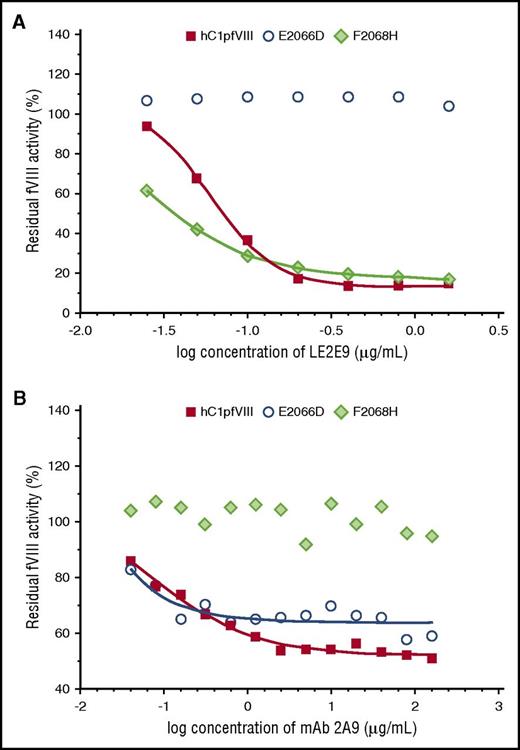
Eventually, in vitro coagulation assays were performed with the hC1pfVIII hybrid protein and its variants E2066D and F2068H in the presence of mAbs LE2E9 and 2A9. Consistent with the findings of the solid-phase ELISA, substitution E2066D exclusively prevented the inhibition of fVIII activity by LE2E9, but not by mAb 2A9, whereas substitution F2068H prevented mAb 2A9-mediated inhibition, but not the inhibition of fVIII activity by LE2E9 (Figure 3).
LE2E9- and mAb 2A9-mediated inhibition of the functional activity of hC1pfVIII variants in coagulation assays. The inhibition of the functional activity of hC1pfVIII and its variants E2066D and F2068H by LE2E9 (A) and mAb 2A9 (B) was measured in a 1-stage clotting assay (for experimental details, see “Methods”). LE2E9 and mAb 2A9 concentrations before mixing with hC1pfVIII variants are indicated.
LE2E9- and mAb 2A9-mediated inhibition of the functional activity of hC1pfVIII variants in coagulation assays. The inhibition of the functional activity of hC1pfVIII and its variants E2066D and F2068H by LE2E9 (A) and mAb 2A9 (B) was measured in a 1-stage clotting assay (for experimental details, see “Methods”). LE2E9 and mAb 2A9 concentrations before mixing with hC1pfVIII variants are indicated.
Epitope mapping of anti-C1 IgG in patients with hemophilia
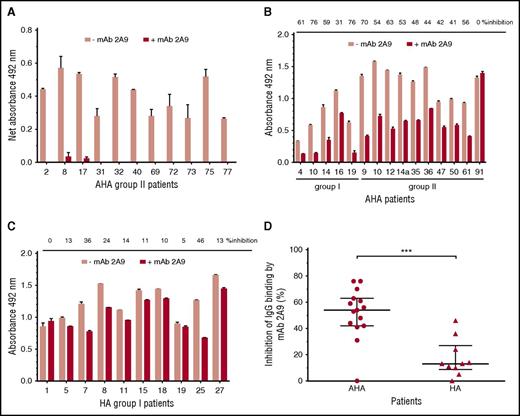
MAb 2A9 was used for indirect epitope mapping by competing with anti-C1 IgG from patients’ plasma with binding to hC1pfVIII and HSA-hC1 proteins. For competition, we selected plasma samples with the strongest binding signals to the human C1 domain. As binding signals of HA inhibitor, plasmas tested positive for anti-C1 IgG by HSM (n = 4; Table 1) were just above the cut-off value; only plasma samples from patients with AHA were used for this analysis. Binding of anti-C1 IgG from all tested AHA plasma samples (n = 11) to hC1pfVIII was almost completely blocked in the presence of mAb 2A9 (Figure 4A). In contrast, antibody binding to HSA-hC1 for AHA (n = 15) and HA inhibitor (n = 10) patients was only partially blocked by mAb 2A9 (Figure 4B-C). However, inhibition of IgG binding by mAb 2A9 was significantly stronger for patients with AHA than for HA inhibitor patients (Figure 4D).
Indirect epitope mapping of anti-C1 IgG in patients with hemophilia by competition binding with mAb 2A9. Binding of patient IgG to the human C1 domain presented as hC1pfVIII protein (A) and HSA-hC1 domain protein (B and C) was analyzed in the absence (pink bars) and presence (red bars) of mAb 2A9. Purified hC1pfVIII (A) and HSA-hC1 (B-C) proteins were immobilized on microtiter plates, and antibody binding from patient plasma was detected with an HRP-conjugated goat anti-human IgG. Antibody binding to immobilized pfVIII (A) and HSA alone (B and C) was subtracted to specifically analyze binding of non-cross-reactive (A) and cross-reactive and non-cross-reactive (B-C) anti-C1 IgG in patient plasma. The experiments were repeated twice with similar results. (D) Comparison of patients with AHA (B; n = 15) and patients with HA and inhibitors (C; n = 10) revealed a significant difference regarding the inhibition of IgG binding to HSA-hC1 by mAb 2A9 (P = .0009). Data are presented as median with interquartile range (*P < .05; **P < .01; ***P < .001. Mann-Whitney test).
Indirect epitope mapping of anti-C1 IgG in patients with hemophilia by competition binding with mAb 2A9. Binding of patient IgG to the human C1 domain presented as hC1pfVIII protein (A) and HSA-hC1 domain protein (B and C) was analyzed in the absence (pink bars) and presence (red bars) of mAb 2A9. Purified hC1pfVIII (A) and HSA-hC1 (B-C) proteins were immobilized on microtiter plates, and antibody binding from patient plasma was detected with an HRP-conjugated goat anti-human IgG. Antibody binding to immobilized pfVIII (A) and HSA alone (B and C) was subtracted to specifically analyze binding of non-cross-reactive (A) and cross-reactive and non-cross-reactive (B-C) anti-C1 IgG in patient plasma. The experiments were repeated twice with similar results. (D) Comparison of patients with AHA (B; n = 15) and patients with HA and inhibitors (C; n = 10) revealed a significant difference regarding the inhibition of IgG binding to HSA-hC1 by mAb 2A9 (P = .0009). Data are presented as median with interquartile range (*P < .05; **P < .01; ***P < .001. Mann-Whitney test).
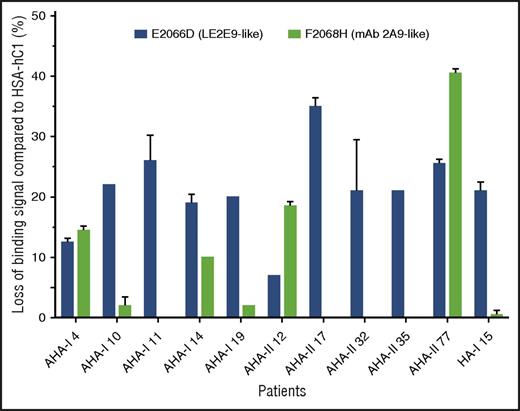
In addition, the loss-of-binding mutants for LE2E9 (E2066D) and mAb 2A9 (F2068H) expressed as HSA-hC1 proteins were used to elucidate whether epitopes identified for mAb 2A9 and LE2E9 were also recognized by antibodies present in patients with hemophilia. Our results show that in 9 (30%) of 30 patients with AHA and 1 (5%) of 20 HA inhibitor patients, IgG binding to the C1 domain variants was reduced 15% or more compared with native HSA-hC1 (Figure 5).
LE2E9- and mAb 2A9-like anti-C1 IgG contribute to patient’s total anti-C1 IgG population. Purified native HSA-hC1 domain and its variants E2066D and F2068H were immobilized on microtiter plates, and antibody binding from patient plasma was detected with an HRP-conjugated goat anti-human IgG. Antibody binding to immobilized HSA alone (background) was subtracted. The loss of IgG binding to the C1 domain variants E2066D (black bars) and F2068H (gray bars) compared with HSA-hC1 was calculated in percentage, and the results are depicted for the 9 of 30 patients with AHA and 1 of 20 patients with HA and inhibitors for which binding was reduced ≥15%. The experiments were repeated 2 times with similar results.
LE2E9- and mAb 2A9-like anti-C1 IgG contribute to patient’s total anti-C1 IgG population. Purified native HSA-hC1 domain and its variants E2066D and F2068H were immobilized on microtiter plates, and antibody binding from patient plasma was detected with an HRP-conjugated goat anti-human IgG. Antibody binding to immobilized HSA alone (background) was subtracted. The loss of IgG binding to the C1 domain variants E2066D (black bars) and F2068H (gray bars) compared with HSA-hC1 was calculated in percentage, and the results are depicted for the 9 of 30 patients with AHA and 1 of 20 patients with HA and inhibitors for which binding was reduced ≥15%. The experiments were repeated 2 times with similar results.
Overall, these results indicate that epitopes identified for mAb 2A9 and LE2E9 are also recognized by antibodies present in patients with hemophilia and suggest that LE2E9- and mAb 2A9-like anti-C1 IgG contribute to patient’s total anti-C1 IgG population.
Discussion
Previous studies showed that antibodies in acquired and congenital hemophilia inhibitor patients are mainly directed to either the A2 or the C2 domains, suggesting these 2 fVIII domains are the predominant immunogenic fVIII regions.9,10,21,27-30 In addition, a high frequency (78%) of autoantibodies directed against the A1a1 domain of fVIII was observed in 73 patients with AHA.30
In the present study, we determined the fVIII domain specificity of antibodies in acquired (n = 115) and congenital (n = 63) hemophilia inhibitor patients by antibody binding to human A2, C1, and C2 domains presented as SHD hpfVIII and HSA-fVIII domain fusion proteins. Previous attempts by our group and others14 to produce recombinant C1 domain in eukaryotic cells have failed because synthesized fragments were not secreted. N-terminal fusion of HSA to C1 prevents this cellular retention and allowed efficient recombinant protein production, as previously described, for expression of interleukins.31 The analysis with HSA-fVIII domain fusion proteins confirmed the results of the HSM approach with SHD hpfVIII proteins, but resulted in higher detection levels of anti-A2 (34% vs 22%), anti-C1 (71% vs 21%), and anti-C2 (72% vs 52%) IgG in patients. These results are in accordance with published data showing that fVIII domain mapping by HSM produces false-negative results as a result of antibody cross-reactivity with human and porcine fVIII.17 The C1 domain, exposing the highest degree of sequence conservation (92.8% identity and 96.7% similarity) between human and porcine fVIII (supplemental Figure 9), is thereby expected to produce the highest percentage of false-negative results. The results of our study showing a much lower detection level of C1-specifc IgG by HSM compared with the HSA-hC1 protein are consistent with this. Along the line, the results of the present study also showed that C1-specific IgG, in contrast to A2- and C2-specific IgG, contribute significantly to the overall amount of human porcine cross-reactive anti-fVIII IgG in patients and provided direct evidence for the presence of cross-reactive C1-specific IgG in patients. These results explain why the HSM approach produced false-negative results for many of these patients.
In general, the results of the domain mapping analyses confirmed the immunogenicity of A2 and C2 domains: anti-fVIII IgG against these 2 domains was found in 23% and 68% of patients with AHA and in 52% and 81% of HA inhibitor patients, respectively. In addition, a large number of acquired (78%) and congenital (57%) hemophilia inhibitor patients developed C1-specific IgG. The results presented in this study for congenital hemophilia inhibitor patients (n = 63) provide an important confirmation of the results recently presented by Batsuli et al in a smaller patient cohort (n = 13).16 The high prevalence of C1-specific IgG in patients with AHA has not been reported before. In summary, these results demonstrate that the C1 domain contributes significantly to the immunogenicity of fVIII in patients with hemophilia.
Interestingly, a previous study showed that the majority of inhibitors in 7 of 10 patients with HA before ITI were directed against fVIII regions other than A2 and A3-C1-C2 domains.32 Although these quantitative data cannot be directly compared with the qualitative data presented in our study, differences might be explained by the different detection methods applied. Our experimental setting prevented detection of A1-, B-, and A3-specific antibodies but allowed the analysis of nonneutralizing anti-fVIII antibodies, which escaped detection by inhibitor neutralization performed by van Helden et al.32 Anti-C1 antibodies that exclusively block binding of fVIII to VWF, but not to phospholipids, might be non- or low-inhibitory antibodies.
To determine the epitope specificity of anti-C1 IgG in patients with hemophilia, the human LE2E9 and the commercially available murine mAb 2A9 (GMA8011) were used after additional characterization. A panel of murine anti-C1 group A mAbs including 2A9 were shown to inhibit the binding of LE2E9 to fVIII in a dose-dependent manner indicating overlapping (group A) epitopes. Mutational analyses showed that different C1 residues that are not conserved among human and porcine C1 sequences are essential for binding mAb 2A9 (F2068) and LE2E9 (E2066), despite their competitive binding. These findings are in agreement with the different specific inhibitory activities of mAb 2A9 (97 BU/mg; https://greenmoab.com) and LE2E9 (10.000 BU/mg)14 and support the fact that antibodies that recognize partially or completely overlapping epitopes may bind different residues within these epitopes. Both crucial antibody binding residues are located within the primary group A binding epitope (S2063-I2071)16 and overlap with 1 of the 3 VWF-binding sites on the C1 domain (W2062-S2069).24 Therefore, inhibition of fVIII binding to VWF by these 2 anti-C1 mAbs might occur by direct competition. In contrast, studies with the deglycosylated LE2E9 variant N47Q (TB-402), which does not prevent binding of fVIII to VWF, rather suggest steric hindrance as mode of action.33 Regardless of the type of interference, given the importance of VWF for fVIII stability in vivo,34 fVIII levels might be strongly reduced in plasma of patients with hemophilia with these anti-C1 antibodies. Recent data from Batsuli et al showing that mAb 2A9 significantly reduced circulating fVIII antigen and activity in a hemophilia A mouse model support this assumption.16
We also addressed the question of whether anti-C1 antibodies in patients with hemophilia comprise mAb 2A9- and LE2E9-like group A antibodies. Nearly complete inhibition of IgG binding to hC1pfVIII by mAb 2A9 indicated that the majority of non-cross-reactive anti-C1 IgG in patients with AHA binds to group A epitopes. In contrast, binding of patients’ IgG to HSA-hC1 was only partially blocked by mAb 2A9, suggesting the presence of cross-reactive, nongroup A anti-C1 IgG in patients with hemophilia. The nature and epitope specificity of this particular anti-C1 IgG population requires further investigation. Interestingly, inhibition of IgG binding by mAb 2A9 was significantly stronger for patients with AHA compared with HA inhibitor patients indicating that group A anti-C1 IgG are more abundant in patients with AHA. These findings were supported by binding studies with mAb 2A9- and LE2E9-specific C1 domain variants showing that a significant proportion of mAb 2A9- and LE2E9-like antibodies were mainly found in patients with AHA. Thirty-five years ago, Maria S. Gawryl and Leon W. Hoyer already described that type 2 inhibitors found mainly in patients with AHA are binding to a site on fVIII that is also the binding site of VWF.35 MAb 2A9- and LE2E9-like patient IgG identified in the current study might represent such type 2 inhibitors.
Overall, this study demonstrates that the C1 domain, in addition to the A2 and C2 domains, contributes significantly to the immunogenicity of fVIII in patients with acquired and congenital hemophilia with inhibitors. As recent data point toward a functional role of the C1 domain for binding to membranes, fX, and VWF,24,26 the clinical relevance of anti-C1 antibodies in patients with hemophilia should be analyzed in further studies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the coprincipal investigators Charles R. M. Hay and Donna M. DiMichele and all other participating investigators of the International Immune Tolerance Study for contribution of data and samples, the Thrombosis and Hemostasis Society of the German-speaking countries (GTH e.V.) for conducting the GTH-AH 01/2010 study, all their participating investigators for contribution of data and samples, and the patients and their families for support. We are grateful to Sonja Neimanis for her comments on the manuscript and acknowledge Ruth Biller, Ingrid Stier-Brück, and Birga Zwinge for their technical assistance. The authors further acknowledge Bayer Healthcare (Leverkusen, Germany) for providing rfVIII (Kogenate FS) as material for laboratory use only.
This work was supported by National Institutes of Health, National Heart, Lung, and Blood Institute grant U54 HL112309 and Hemophilia of Georgia, Inc. (P.L.).
Authorship
Contribution: J.K. designed and performed research, analyzed data, and cowrote the paper; A.O. and D. Stichel performed research and analyzed data; J.F.H. and E.T.P. designed research and contributed plasmids encoding human/porcine fVIII proteins and murine anti-C1 antibodies; M.J. designed research, contributed the human anti-C1 domain antibody LE2E9, and edited the manuscript; M.K. and A.T. acquired patient plasma and clinical data and edited the manuscript; D. Schwabe designed research and edited the manuscript; P.L. designed research and cowrote the paper; and C.K. designed research, acquired patient plasma and clinical data, analyzed data, and cowrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Christoph Königs, Frankfurt University Hospital, Department of Pediatrics, Clinical and Molecular Hemostasis and Immunodeficiency, Theodor-Stern-Kai 7, 60596 Frankfurt am Main, Germany; e-mail: christoph.koenigs@kgu.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal