Key Points
Downregulation of BACH2 increases MCL proliferation, dispersal, and drug resistance.
Distinct crosstalk between BACH2 and HIF-1α under different physiological conditions modifies MCL properties.
Abstract
BACH2, a B-cell–specific transcription factor, plays a critical role in oxidative stress–mediated drug resistance in mantle cell lymphoma (MCL); however, the biological functions of BACH2 and its regulation of B-cell malignancies in chronic hypoxic microenvironment have not been studied. Here, we found that silencing BACH2 led to not only increased tumor formation and colony formation but also increased tumor dispersal to spleen and bone marrow. Decreased BACH2 levels in patients were also correlated with bone marrow and gastrointestinal dispersal of MCL and blastoid subtypes of MCL. Unexpectedly, decreased BACH2 levels in dispersed MCL cells were due to direct transcriptional repression by hypoxia-induced factor 1α (HIF-1α) and increased heme-mediated protein degradation. In normoxic conditions, BACH2 was able to modulate HIF-1α degradation by suppressing prolyl hydroxylase 3 expression. Bifurcated BACH2 controls during hypoxia and normoxia coordinate not only MCL tumor dispersal but also drug resistance, including bortezomib resistance, via plasmacytic differentiation. Our data highlight an interactive relationship between tumor cells and local microenvironment and the mechanisms of B-cell transcription factor in the regulation of MCL dispersal.
Introduction
BACH2 (BTB and CNC homology 2) is a B-cell–specific transcription factor that regulates class switch recombination and somatic hypermutations of immunoglobulin genes.1 In mice, Bach2 plays a crucial role in germinal center formation during normal B-cell development and coordinates plasma cell differentiation by repressing PR domain–containing 1 (Prdm1; also known as Blimp1) and other target genes.2,3 Mutations in BACH2 are linked to numerous autoimmune and allergic diseases in humans such as type 1 diabetes,4 asthma,5 and multiple sclerosis.6 Despite its crucial role in regulating immune homeostasis and inflammatory responses, the functions of BACH2 in B-cell malignancies remain unclear.
Several lymphoma studies suggest that BACH2 may function as a tumor suppressor. Ectopic expression of BACH2 in Burkitt’s lymphoma cell lines markedly reduces cell proliferation and increases the cytotoxic effects of reactive oxygen species (ROS) produced by chemotherapeutic drugs.7 In diffuse large B-cell lymphoma (DLBCL), patients with higher BACH2 expression show a better prognosis.8 Loss of heterozygosity of BACH2 has been reported at a frequency of 20% in human B-cell lymphomas.9 A recent study showed that BACH2 is a key regulator of the pre-BCR checkpoint as well as a tumor suppressor in pre-B acute lymphoblastic leukemia.10 One mechanism of BACH2 downregulation in leukemias is the loss of the transcription factor PAX5, which is often mutated in B-cell acute lymphoblastic leukemia.10
Mantle cell lymphoma (MCL) accounts for ∼6% of all non-Hodgkin lymphomas. MCLs display cellular heterogeneity and are highly refractory to standard radiation and chemotherapy, thus contributing to one of the worst survival rates among non-Hodgkin lymphoma patients.11 A major genomic abnormality in MCL, which also distinguishes this subtype from low-grade B-cell lymphomas, is the t(11:14)(q13:q32) translocation that results in increased cyclin D1 (CCND1) expression. Although this translocation is a genetic hallmark of MCL, CCND1 overexpression in mouse models is insufficient to induce spontaneous tumors.12 Additionally, the t(11:14)(q13:q32) translocation exists in blood cells in ∼2% of healthy individuals without any evidence of disease,13 and some MCL patients lack this translocation.14,15 These findings suggest that other genetic or epigenetic events, possibly acting in cooperation with CCND1 overexpression, are required for the initiation and progression of MCL.
In the present study, silencing BACH2 in MCL cells resulted in increased proliferation and enhanced tumor dispersal in hypoxic microenvironments, suggesting a tumor suppressor–like role of BACH2. Notably, BACH2 levels can serve as a useful marker for tumor dispersal in either MCL patients or xenograft mice. The mechanisms of BACH2 regulation in chronic hypoxic microenvironments are the result of transcriptional repression of HIF-1α and heme-induced protein degradation. Under normoxic conditions, BACH2 modulates HIF-1α degradation by suppressing PHD3, suggesting an interconnected network between BACH2 and HIF-1α under different physiological conditions. Overall, our study provides novel insight of BACH2 activity in the pathogenesis of lymphomas. Targeting BACH2 and its network in human MCL may help in the development of new therapies in the near future.
Methods
Human MCL samples
Peripheral blood (PB), bone marrow (BM), and spleen (SP) samples from MCL patients were obtained after informed consent based on the protocol approved by the MD Anderson Cancer Center and the University of Texas Health Science Center (UT-HSC) institutional review boards.
Mice
Immunodeficient nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and maintained under barrier conditions at UT-HSC. All animal procedures were approved by the UT-HSC Animal Care Committee.
Intracellular BrdU incorporation assay
MCL cells were allowed to cycle for 6 days and intracellular 5-bromo-2′-deoxyuridine (BrdU) incorporation was analyzed using an APC BrdU Flow kit (BD Biosciences, San Jose, CA) according to the manufacturer’s protocol.
Luciferase activity assay
Luciferase activity was measured using a Dual-Luciferase Reporter Assay System kit (Promega, Madison, WI) and an Infinite M1000 (TECAN, Morrisville, NC) fluorescent plate reader. The data were normalized and presented as the ratio of firefly/Renilla luciferase activities.
Chromatin immunoprecipitation (ChIP)
ChIP assays were performed using a ChIP kit (Abcam, Cambridge, MA) according to the manufacturer’s protocol. Quantitative analyses of the ChIP assays were performed using real-time polymerase chain reaction (PCR).
Additional methods are in the supplemental Methods (available on the Blood Web site).
Results
BACH2 silencing enhances MCL cell survival and increases cell proliferation
To determine the role of BACH2 in MCL, we silenced BACH2 (BACH2KO) in human MCL cell lines using CRISPR-Cas9 genome editing. The knockout efficiency of BACH2 in MCL cells was evaluated by immunoblots, which showed complete silencing (supplemental Figure 1A). The expression levels of known genes that are repressed by BACH2, S100A2, and HMOX116 were increased in BACH2-silenced cells (supplemental Figure 1B). We then used BACH2KO and empty vector–transfected MCL cells (BACH2Con) to evaluate the effects of BACH2 on cell growth. Silencing BACH2 significantly increased cell numbers in MCL compared with the control cells (Figure 1A), while reintroducing BACH2 back to the knockout cells reversed the phenotype (supplemental Figure 1C-D), suggesting that BACH2 has a tumor suppressor–like function in MCL.
Silencing BACH2 in MCL increases cell proliferation and accelerates cell cycle progression. (A) Viable cells were counted using trypan blue staining in manipulated MCL cell lines. The data are presented the mean ± standard deviation (SD) from 3 independent experiments. Controls were mock-transfected cells (BACH2Con). (B) Representative cell-cycle distribution of BACH2KO or BACH2Con MCL cells. (C) Representative data showing an intracellular pulse staining of BrdU in BACH2KO or BACH2Con MCL cells (left). The population of BACH2KO cells in S phase was normalized to the control cells. Data are shown as the mean ± SD from 2 independent experiments (right). (D) mRNA levels of cell-cycle–related factors in BACH2KO or BACH2Con MCL cells. Each value from quantitative reverse-transcription PCR (qRT-PCR) was normalized to GAPDH and is presented as the mean ± SD. from 3 independent experiments. NS, not significant; *P < .05; **P < .01 (vs control cells; Student t test).
Silencing BACH2 in MCL increases cell proliferation and accelerates cell cycle progression. (A) Viable cells were counted using trypan blue staining in manipulated MCL cell lines. The data are presented the mean ± standard deviation (SD) from 3 independent experiments. Controls were mock-transfected cells (BACH2Con). (B) Representative cell-cycle distribution of BACH2KO or BACH2Con MCL cells. (C) Representative data showing an intracellular pulse staining of BrdU in BACH2KO or BACH2Con MCL cells (left). The population of BACH2KO cells in S phase was normalized to the control cells. Data are shown as the mean ± SD from 2 independent experiments (right). (D) mRNA levels of cell-cycle–related factors in BACH2KO or BACH2Con MCL cells. Each value from quantitative reverse-transcription PCR (qRT-PCR) was normalized to GAPDH and is presented as the mean ± SD. from 3 independent experiments. NS, not significant; *P < .05; **P < .01 (vs control cells; Student t test).
To elucidate the mechanisms involving enhanced cell growth in BACH2KO cells, cell survival and cell proliferation were analyzed. FACS analyses based on Annexin V/7-AAD staining revealed a survival advantage of the BACH2KO cells, especially in the late phase of the time-course studies. At day 6, BACH2KO cells were alive more than the control cells (supplemental Figure 1E). To directly assess MCL cell proliferation upon BACH2 silencing, we tracked cell division by staining cells with PKH26, a fluorescent dye that decreases fluorescence intensity after each division.17 Similar to what was observed in cell growth, BACH2KO cells showed a rapid decline in PKH26 labeling compared with control cells (supplemental Figure 1F). Correspondingly, cell cycle distribution showed 10% to 20% more BACH2KO cells in S phase and G2/M phase compared with control cells (Figure 1B). Intracellular pulse staining for BrdU incorporation also revealed higher amounts of BACH2KO cells in S phase (Figure 1C). We further measured cell cycle–related factors and found increased levels of several cyclin genes involved in G1/S progression and G2/M phase as well as increased expression of cell cycle regulators; the expression of tumor suppressors, such as TP53 and CDKN1B, was also decreased (Figure 1D). Based on these data, BACH2 downregulation contributes to both enhanced MCL cell survival and increased cell proliferation, thereby leading to dominant growth of BACH2KO cells.
BACH2 deletion promotes colony formation and cell adhesion
Since BACH2 is a downstream effector of PAX5 during normal B-cell development,10,18-21 we investigated whether silencing BACH2 increases colony formation and cell adhesion in a similar manner as PAX5.21 BACH2 deletion led to enhanced colony-forming abilities compared with control cells (Figure 2A; supplemental Figure 2A), suggesting that BACH2 negatively contributes to the overall oncogenic mechanism such as proliferation in MCL.
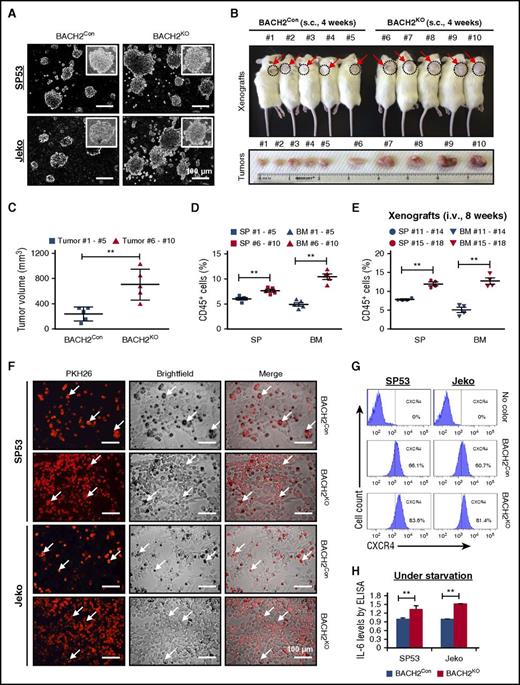
BACH2 deletion contributes to increased colony-formation abilities and increases cell adhesion. (A) Representative colonies of BACH2KO and BACH2Con MCL cells in MethoCult medium were photographed under a microscope. Scale bar, 100 μm. (B) BACH2KO or BACH2Con SP53 MCL cells (3.5 × 106) were subcutaneously (s.c.) injected into NOD/SCID mice (n = 10). Mice 1 to 5 were injected with BACH2Con cells, and mice 6 to 10 were injected with BACH2KO cells. Xenografts were sacrificed 4 weeks postinjection. Dotted circles represent the area of subcutaneous tumors (top). Tumors were isolated and photographed against an inch ruler (bottom). (C) The size of the tumors in each group was measured. The results are shown as the mean ± SD. **P < .01 (vs control xenografts; Student t test). (D) Human leukocyte cells were isolated from the spleen (SP) and bone marrow (BM) using anti–human CD45-MicroBeads, and the number of CD45+ cells was counted using trypan blue staining. The percentage of CD45+ cells in each organ was calculated, and data are presented as the mean ± SD. **P < .01 (vs control xenografts; Student t test). (E) BACH2KO SP53 cells or control cells (3 × 106) were IV injected (i.v.) into NOD/SCID mice (n = 8). Mice 11 to 14 were injected with BACH2Con cells, and mice 15 to 18 were injected with BACH2KO cells. Xenografts were sacrificed 8 weeks postinjection. SP and BM were collected and stained for human leukocyte cells using anti–human CD45 antibody. Samples were then analyzed for CD45+ cells using FACS analysis. The percentage of CD45+ cells in each organ was calculated, and data are presented as the mean ± SD. **P < .01 (vs control xenografts; Student t test). (F) Representative microscopic images of adherent MCL cells in a coculture setting. Either BACH2KO or BACH2Con MCL cells were stained with PKH26 prior to seeding onto a preestablished monolayer of HS5 BMSCs. Scale bar, 100 μm. The arrows point to the representative MCL cells adhered to bone marrow stromal cells. (G) CXCR4 levels in BACH2KO or BACH2Con cells were analyzed by flow cytometry, the percentage of CXCR4+ cells in the population is indicated. (H) BACH2KO or BACH2Con MCL cells were serum starved for 48 hours. IL-6 levels were measured using enzyme-linked immunosorbent assay. The colorimetric values in the BACH2KO cells were normalized to the control values. The results are shown as the mean ± SD from 3 independent experiments. **P < .01 (vs control cells; Student t test).
BACH2 deletion contributes to increased colony-formation abilities and increases cell adhesion. (A) Representative colonies of BACH2KO and BACH2Con MCL cells in MethoCult medium were photographed under a microscope. Scale bar, 100 μm. (B) BACH2KO or BACH2Con SP53 MCL cells (3.5 × 106) were subcutaneously (s.c.) injected into NOD/SCID mice (n = 10). Mice 1 to 5 were injected with BACH2Con cells, and mice 6 to 10 were injected with BACH2KO cells. Xenografts were sacrificed 4 weeks postinjection. Dotted circles represent the area of subcutaneous tumors (top). Tumors were isolated and photographed against an inch ruler (bottom). (C) The size of the tumors in each group was measured. The results are shown as the mean ± SD. **P < .01 (vs control xenografts; Student t test). (D) Human leukocyte cells were isolated from the spleen (SP) and bone marrow (BM) using anti–human CD45-MicroBeads, and the number of CD45+ cells was counted using trypan blue staining. The percentage of CD45+ cells in each organ was calculated, and data are presented as the mean ± SD. **P < .01 (vs control xenografts; Student t test). (E) BACH2KO SP53 cells or control cells (3 × 106) were IV injected (i.v.) into NOD/SCID mice (n = 8). Mice 11 to 14 were injected with BACH2Con cells, and mice 15 to 18 were injected with BACH2KO cells. Xenografts were sacrificed 8 weeks postinjection. SP and BM were collected and stained for human leukocyte cells using anti–human CD45 antibody. Samples were then analyzed for CD45+ cells using FACS analysis. The percentage of CD45+ cells in each organ was calculated, and data are presented as the mean ± SD. **P < .01 (vs control xenografts; Student t test). (F) Representative microscopic images of adherent MCL cells in a coculture setting. Either BACH2KO or BACH2Con MCL cells were stained with PKH26 prior to seeding onto a preestablished monolayer of HS5 BMSCs. Scale bar, 100 μm. The arrows point to the representative MCL cells adhered to bone marrow stromal cells. (G) CXCR4 levels in BACH2KO or BACH2Con cells were analyzed by flow cytometry, the percentage of CXCR4+ cells in the population is indicated. (H) BACH2KO or BACH2Con MCL cells were serum starved for 48 hours. IL-6 levels were measured using enzyme-linked immunosorbent assay. The colorimetric values in the BACH2KO cells were normalized to the control values. The results are shown as the mean ± SD from 3 independent experiments. **P < .01 (vs control cells; Student t test).
To verify the in vitro results, we subcutaneously transplanted BACH2KO SP53 cells or control cells into NOD/SCID mice. BACH2KO MCL xenograft mice developed larger tumors compared with the control xenografts (Figure 2B), and those differences in the tumor volumes were statistically significant (Figure 2C). Further analyses of these xenografts showed increased CD45+ human cells in the SP and BM upon BACH2 silencing (Figure 2D). IV transplanted mice displayed even higher tumor engraftment in the SP and BM of BACH2KO xenograft mice (Figure 2E; supplemental Figure 2B). Since MCL cells have increased tumor dispersal to the BM upon BACH2 silencing, we analyzed MCL cell adhesion with human bone marrow stroma cells (BMSCs). PKH26-labeled BACH2KO MCL cells showed markedly increased cell adhesion to the HS5 BMSCs (Figure 2F; supplemental Figure 2C).
MCL cell homing to the BM requires the expression and activation of chemokine receptors and the release of cytokines. For example, CXCR4, an important chemokine receptor for chemokine stromal-cell–derived factor 1, plays essential roles in hematopoietic stem cell homing to the BM.22 Interleukin-6 (IL-6) is another key cytokine involved in the growth and progression of hematological malignancies. IL-6 is secreted by BMSCs (eg, HS5 cells) and has been considered as a microenvironment-dependent survival agent for cancer cells.21,23-27 Therefore, we examined CXCR4 and autocrine IL-6 levels upon BACH2 silencing. CXCR4 levels were significantly higher in BACH2KO MCL cells than in control cells (Figure 2G). We then serum-starved cells, which can induce IL-6 secretion in MCL cells.24 Compared with control cells, BACH2KO cells presented more IL-6 secretion (Figure 2H), and messenger RNA (mRNA) levels of IL-6R (IL-6 receptor) and IL-6ST (IL-6 signal transducer) were also increased (supplemental Figure 2D). Collectively, our data support the notion that BACH2 downregulation in MCL leads to enhanced tumor formation and dispersal by upregulating CXCR4 and IL-6 production.
Decreased BACH2 levels are associated with a poor prognosis in MCL patients and contribute to MCL dispersal and progression
To determine the clinical relevance of our findings, we examined the relative BACH2 levels in cells isolated from MCL patients with different stages of disease. We conducted comprehensive BACH2 analyses using only CD19+ B cells isolated from primary MCL samples rather than using whole tissue extracts that contain different immune cells and stromal cells (supplemental Table 1).
Compared with normal PB and normal BM, CD19+ B cells from MCL patient samples showed reduced BACH2 levels (Figure 3A), supporting its tumor suppressor–like role in MCL. Interestingly, BACH2 levels in CD19+ cells from BM were significantly decreased compared with PB samples (Figure 3B). These data suggest that BACH2 downregulation in CD19+ B cells may facilitate MCL dispersal to the BM. Given that advanced MCL patients often demonstrate BM involvement, deregulated BACH2 levels may be a focal signaling node for MCL dispersal.
Decreased BACH2 levels are associated with an unfavorable prognosis in MCL patients and promote tumor dispersal. (A) CD19+ B cells isolated from either apheresis blood samples (MCL patients, n = 27; healthy donors, n = 9) (left) or BM samples (MCL patients, n = 8; healthy donors, n = 8) (right) were used to measure the BACH2 mRNA levels using qRT-PCR. Each condition was run in triplicate with the values normalized to GAPDH. (B) BACH2 mRNA levels were downregulated in MCL cells isolated from BM (n = 8) compared with MCL isolated from blood samples (n = 27). (C) CD19+ cells isolated from MCL blastoid patients (n = 10) contained lower levels of BACH2 than CD19+ cells from MCL nonblastoid patients (n = 17). (D) MKI67 mRNA levels were measured in CD19+ B cells isolated from MCL blastoid subtypes (n = 5) or non-blastoid subtypes (n = 5). (E) CD19+ cells isolated from MCL patients with gastrointestinal (GI) dispersal (n = 5) contained lower levels of BACH2 compared with those from MCL patients without GI dispersal (n = 22). (F) The overall survival of MCL patients was significantly lower in BACH2low MCL patients than in BACH2high MCL patients (P = .027, Mantel-Cox curve analysis). BACH2high and BACH2low refer to the upper and lower 50% of BACH2 levels in MCL patients, respectively. (G) The overall survival of MCL patients was analyzed in Oncomine database using the upper and lower 25% of BACH2 levels in MCL patients with a cutoff of 10 years (P = .042, Mantel-Cox curve analysis). (H) Heatmap of BACH2 mRNA levels in subcutaneous tumor cells, SP, or BM from xenografts bearing MCL cells (n = 5, #1-#5). The fold change in expression compared with #1 xenograft tumor cells is indicated by the color intensity, with green representing reduced expression and red representing elevated expression. The results are shown as the mean ± SD. *P < .05; **P < .01 (Student t test).
Decreased BACH2 levels are associated with an unfavorable prognosis in MCL patients and promote tumor dispersal. (A) CD19+ B cells isolated from either apheresis blood samples (MCL patients, n = 27; healthy donors, n = 9) (left) or BM samples (MCL patients, n = 8; healthy donors, n = 8) (right) were used to measure the BACH2 mRNA levels using qRT-PCR. Each condition was run in triplicate with the values normalized to GAPDH. (B) BACH2 mRNA levels were downregulated in MCL cells isolated from BM (n = 8) compared with MCL isolated from blood samples (n = 27). (C) CD19+ cells isolated from MCL blastoid patients (n = 10) contained lower levels of BACH2 than CD19+ cells from MCL nonblastoid patients (n = 17). (D) MKI67 mRNA levels were measured in CD19+ B cells isolated from MCL blastoid subtypes (n = 5) or non-blastoid subtypes (n = 5). (E) CD19+ cells isolated from MCL patients with gastrointestinal (GI) dispersal (n = 5) contained lower levels of BACH2 compared with those from MCL patients without GI dispersal (n = 22). (F) The overall survival of MCL patients was significantly lower in BACH2low MCL patients than in BACH2high MCL patients (P = .027, Mantel-Cox curve analysis). BACH2high and BACH2low refer to the upper and lower 50% of BACH2 levels in MCL patients, respectively. (G) The overall survival of MCL patients was analyzed in Oncomine database using the upper and lower 25% of BACH2 levels in MCL patients with a cutoff of 10 years (P = .042, Mantel-Cox curve analysis). (H) Heatmap of BACH2 mRNA levels in subcutaneous tumor cells, SP, or BM from xenografts bearing MCL cells (n = 5, #1-#5). The fold change in expression compared with #1 xenograft tumor cells is indicated by the color intensity, with green representing reduced expression and red representing elevated expression. The results are shown as the mean ± SD. *P < .05; **P < .01 (Student t test).
The blastoid variant of MCL is a highly aggressive rare form of MCL that has a worse prognosis compared with classical MCL.28,29 Further analysis revealed that blastoid subtypes contained significantly lower BACH2 levels and higher Ki-67 levels than nonblastoid types (Figure 3C-D; supplemental Figure 3). We next analyzed the BACH2 levels of MCL patients who have a history of gastrointestinal dispersal, which is very common in MCL.30 BACH2 levels were remarkably decreased in CD19+ tumor cells isolated from the patients who presented with gastrointestinal infiltration compared with patients who did not (Figure 3E). The overall survival of patients with BACH2low was also significantly lower than that of patients with BACH2high (Figure 3F), and a similar result was observed when the publically available MCL database was used (Figure 3G).31 We further compared the BACH2 mRNA levels in CD45+ human cells isolated from different organs of the xenograft mice, including the SP and BM. Consistent with the observations in the MCL patient samples (Figure 3B), the BACH2 levels were significantly lower in the BM compared with subcutaneous tumors or SP (Figure 3H).
HIF-1α expression is inversely correlated with BACH2 expression during hypoxia
BM and secondary lymphoid organs such as SP provide well-described chronic hypoxic microenvironments for developing lymphocytes32 ; HIF-1α, a key regulator of cell adaptation to hypoxia, contributes to cancer cell survival, progression, and chemoresistance in human cancers.33,34 Since BACH2KO cells increased their dispersal to SP and BM (Figure 2D-E) and BACH2 levels were significantly lower in the BM of both MCL patients and xenografts (Figure 3B,H), we then hypothesized that decreased BACH2 levels in hypoxic microenvironments could be due to HIF-1α activity. HIF-1α levels were increased in CD45+ human cells isolated from xenograft organs, while BACH2 levels were correspondingly decreased (Figure 4A). Further calculation of the coefficient of determination (R2) based on the relative values revealed an inverse correlation between HIF-1α expression and BACH2 expression (Figure 4B), suggesting that HIF-1α is likely a potential regulator of BACH2.
HIF-1α negatively correlates with BACH2 expression under hypoxia. (A) Immunoblots of CD45+ human cells for HIF-1α and BACH2 in subcutaneous tumors, SP, or BM from xenograft mice bearing MCL cells (n = 5, #1-#5). GAPDH was used as a loading control. The normalized values for HIF-1α and BACH2 in each lane are indicated. (B) Linear regression analysis of the relative BACH2 and HIF-1α expression levels based on densitometry analyses of immunoblots shown in panel A. R2 values of each xenograft are indicated. (C) MCL cells were cultured under hypoxia (at 1% O2) at the indicated times. HIF-1α and BACH2 levels were measured by immunoblotting with GAPDH as a loading control. The normalized values for HIF-1α and BACH2 in each lane are indicated. (D) Immunoblots of MCL cells for HIF-1α and BACH2 in the presence or absence of CoCl2 treatment (200 μmol/L) for 24 h. The BACH2/GAPDH ratios are indicated under each lane. (E) CRISPR-Cas9–mediated HIF-1αKO MCL cells were generated with mock-transfected cells (HIF-1αCon) as a control. HIF-1αKO or HIF-1αCon cells were incubated in the presence or absence of CoCl2 for 24 hours. HIF-1α and BACH2 levels were detected by immunoblotting. (F) HIF-1αKO MCL cells were cultured under hypoxia for 4 hours with HIF-1αCon cells as a control. HIF-1α and BACH2 levels were detected by immunoblotting.
HIF-1α negatively correlates with BACH2 expression under hypoxia. (A) Immunoblots of CD45+ human cells for HIF-1α and BACH2 in subcutaneous tumors, SP, or BM from xenograft mice bearing MCL cells (n = 5, #1-#5). GAPDH was used as a loading control. The normalized values for HIF-1α and BACH2 in each lane are indicated. (B) Linear regression analysis of the relative BACH2 and HIF-1α expression levels based on densitometry analyses of immunoblots shown in panel A. R2 values of each xenograft are indicated. (C) MCL cells were cultured under hypoxia (at 1% O2) at the indicated times. HIF-1α and BACH2 levels were measured by immunoblotting with GAPDH as a loading control. The normalized values for HIF-1α and BACH2 in each lane are indicated. (D) Immunoblots of MCL cells for HIF-1α and BACH2 in the presence or absence of CoCl2 treatment (200 μmol/L) for 24 h. The BACH2/GAPDH ratios are indicated under each lane. (E) CRISPR-Cas9–mediated HIF-1αKO MCL cells were generated with mock-transfected cells (HIF-1αCon) as a control. HIF-1αKO or HIF-1αCon cells were incubated in the presence or absence of CoCl2 for 24 hours. HIF-1α and BACH2 levels were detected by immunoblotting. (F) HIF-1αKO MCL cells were cultured under hypoxia for 4 hours with HIF-1αCon cells as a control. HIF-1α and BACH2 levels were detected by immunoblotting.
To gain more insight into the relationship between HIF-1α and BACH2, we cultured MCL cells under hypoxic conditions. Lower BACH2 levels resulted in extended MCL survival, because a significant increase in cell growth was observed in BACH2KO MCL cells at 1% O2 compared with controls (supplemental Figure 4A). Under hypoxia, HIF-1α levels rapidly increased and peaked at 4 hours, and levels gradually declined to baseline within 24 hours (Figure 4C). Meanwhile, BACH2 protein levels were reciprocally decreased from their basal levels (at 0 hours) under hypoxia (Figure 4C; supplemental Figure 4B). Treatment of MCL cells with CoCl2, a chemical inducer of HIF-1α,35 also showed similar patterns, including a decrease in BACH2 levels (Figure 4D).
To further validate BACH2 regulation by HIF-1α, we silenced HIF-1α (HIF-1αKO) in MCL cells using a lentivirus-mediated CRISPR-Cas9 system. HIF-1α deletion resulted in the loss of BACH2 repression in MCL cells treated with CoCl2 (Figure 4E), and similar observations were made in HIF-1αKO cells under hypoxia (Figure 4F). Our findings provide convincing evidence that HIF-1α expression negatively correlates with BACH2 expression in MCL and that HIF-1α is a potential regulator of BACH2.
BACH2 is a direct transcriptional target repressed by HIF-1α
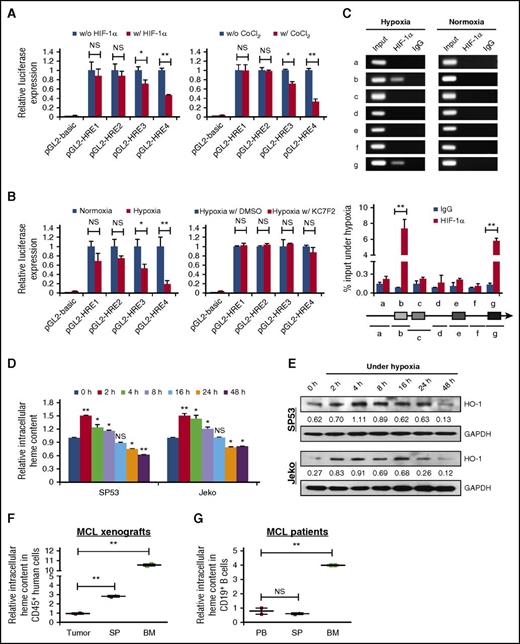
To further delineate HIF-1α–mediated BACH2 repression, we analyzed whether BACH2 is a direct transcriptional target of HIF-1α. A promoter analysis of the BACH2 gene identified 4 putative hypoxia-responsive element (HRE) binding sites (5′-ACGTG-3′) within the BACH2 proximal promoter and 5′ untranslated region (−1000/−996, −938/−934, −370/−366, and +161/+165) (supplemental Figure 5A). Serial deletions of the promoter region were constructed with difficulty in cloning because of an extremely high GC content in some regions (supplemental Figure 5B). Under normal conditions, the luciferase activity of pGL2-HRE3 and pGL2-HRE4 was significantly decreased in cells when cotransfected with HIF-1α expression plasmids compared with the controls. The similar results were obtained in cells subjected to CoCl2 treatment (Figure 5A). Culturing the cells under hypoxia significantly reduced the luciferase activity to a greater extent; in contrast, pretreatment with KC7F2, a selective inhibitor of HIF-1α synthesis,36,37 reversed luciferase activity following hypoxia (Figure 5B; supplemental Figure 5C). ChIP analyses in SP53 cells following hypoxia confirmed two regions (DNA fragments b and g) of the BACH2 promoter containing HIF-1α–binding sites (Figure 5C; supplemental Figure 5D). These findings strongly support that HIF-1α suppresses BACH2 expression during hypoxia by direct binding to HRE sites on the BACH2 promoter.
HIF-1α and heme are responsible for BACH2 repression under hypoxia. (A) 293T cells were transfected with truncated promoter plasmids with or without HIF-1α expression plasmids (left) or in the presence or absence of CoCl2 (24 hours prior to harvest) (right). An empty pGL2-basic plasmid was used as a negative control. The Renilla luciferase reporter pRL-SV40 was used as an internal control for normalization. Luciferase activity was measured 48 hours after transfection. The results are presented as the relative luciferase activity compared with the cells without HIF-1α expression or without CoCl2 treatment. (B) 293T cells were transfected with truncated promoter plasmids. During a 48-hour transfection, cells were cultured at 1% O2 for 4 hours with or without HIF-1α inhibitor KC7F2 (40 μmol/L), and luciferase activity was measured. Relative luciferase activity compared with that of cells cultured in normal conditions or with dimethyl sulfoxide (DMSO) (control) are indicated. (C) DNA fragments (a-g) amplified by PCR following ChIP assays are indicated (top). SP53 cells were cultured at 1% O2 for 4 hours. Chromatin was immunoprecipitated with antibodies against HIF-1α or control immunoglobulin G (IgG). Total chromatin before immunoprecipitation (input) was used as positive control for PCR. Samples processed under normoxia were used as negative controls. Further quantitative analyses of the ChIP assays in SP53 cells following hypoxia were analyzed using qRT-PCR and are presented as the mean ± SD values from 2 independent experiments (bottom). (D) Intracellular heme content was measured in MCL cells cultured under hypoxia at the indicated time. Each condition was run in duplicate with the values normalized to the controls (at 0 hours). (E) HO-1 protein levels were detected by immunoblotting using MCL cells cultured under hypoxia at the indicated time, with GAPDH as a loading control. HO-1/GAPDH values in each lane are indicated. (F) Intracellular heme levels of CD45+ human cells in subcutaneous tumors, SP, or BM from xenograft mice bearing MCL cells (n = 2). Values normalized to subcutaneous tumors are indicated. (G) CD19+ B cells isolated from PB, SP, or BM samples (n = 6) were used to measure the cellular heme levels. The values normalized to PB are indicated. NS, not significant; *P < .05; **P < .01 (vs control cells; Student t test).
HIF-1α and heme are responsible for BACH2 repression under hypoxia. (A) 293T cells were transfected with truncated promoter plasmids with or without HIF-1α expression plasmids (left) or in the presence or absence of CoCl2 (24 hours prior to harvest) (right). An empty pGL2-basic plasmid was used as a negative control. The Renilla luciferase reporter pRL-SV40 was used as an internal control for normalization. Luciferase activity was measured 48 hours after transfection. The results are presented as the relative luciferase activity compared with the cells without HIF-1α expression or without CoCl2 treatment. (B) 293T cells were transfected with truncated promoter plasmids. During a 48-hour transfection, cells were cultured at 1% O2 for 4 hours with or without HIF-1α inhibitor KC7F2 (40 μmol/L), and luciferase activity was measured. Relative luciferase activity compared with that of cells cultured in normal conditions or with dimethyl sulfoxide (DMSO) (control) are indicated. (C) DNA fragments (a-g) amplified by PCR following ChIP assays are indicated (top). SP53 cells were cultured at 1% O2 for 4 hours. Chromatin was immunoprecipitated with antibodies against HIF-1α or control immunoglobulin G (IgG). Total chromatin before immunoprecipitation (input) was used as positive control for PCR. Samples processed under normoxia were used as negative controls. Further quantitative analyses of the ChIP assays in SP53 cells following hypoxia were analyzed using qRT-PCR and are presented as the mean ± SD values from 2 independent experiments (bottom). (D) Intracellular heme content was measured in MCL cells cultured under hypoxia at the indicated time. Each condition was run in duplicate with the values normalized to the controls (at 0 hours). (E) HO-1 protein levels were detected by immunoblotting using MCL cells cultured under hypoxia at the indicated time, with GAPDH as a loading control. HO-1/GAPDH values in each lane are indicated. (F) Intracellular heme levels of CD45+ human cells in subcutaneous tumors, SP, or BM from xenograft mice bearing MCL cells (n = 2). Values normalized to subcutaneous tumors are indicated. (G) CD19+ B cells isolated from PB, SP, or BM samples (n = 6) were used to measure the cellular heme levels. The values normalized to PB are indicated. NS, not significant; *P < .05; **P < .01 (vs control cells; Student t test).
Heme and HIF-1α collectively repress BACH2 expression
Despite the transcriptional repression by HIF-1α, the degree of BACH2 repression did not coincide with the peak HIF-1α expression. BACH2 expression was dramatically decreased at 2 hours, while HIF-1α expression peaked at 4 hours (Figure 4C). This suggests that there could be a secondary mechanism involved in BACH2 downregulation. Heme, an iron-protoporphyrin involved in multiple biological processes,38,39 was reported to induce Bach2 degradation in murine B cells.40 Heme biosynthesis is regulated by environmental oxygen41 ; for example, exposure of cancer cells to hypoxia leads to an increase in cellular heme content.42 Therefore, we examined whether decreased BACH2 expression requires additional regulation by heme content during hypoxia.
As expected, the intracellular heme content was significantly increased during the first 8 hours of hypoxic exposure compared with controls, indicating that low oxygen resulted in a transient elevation of heme levels in MCL. Importantly, heme content peaked at 2 hours after hypoxia induction, which coincides with the nadir of BACH2 levels (Figure 5D). Accordingly, the mRNA and protein levels of heme oxygenase-1 (HO-1), an enzyme highly induced by heme,39,40 were also elevated (Figure 5E; supplemental Figure 5E).
In MCL xenografts, CD45+ human cells isolated from the SP and BM exhibited a gradual increase of heme content compared with subcutaneous tumors (Figure 5F). Consistent results were observed in primary BM cells from MCL patients, although no difference was observed between PB and SP samples (Figure 5G). Our data demonstrated that HIF-1α–driven transcriptional repression and heme-induced protein degradation are collectively responsible for the repression of BACH2 under hypoxia.
BACH2 accelerates HIF-1α degradation via downregulation of PHD3 under normoxia
Under normoxic conditions, PHD enzymes hydroxylate key proline residues within the oxygen-dependent degradation domain of HIF-1α subunits; this hydroxylation event leads to rapid von Hippel Lindau–dependent ubiquitination and proteasomal degradation.43,44 The activity of PHD depends on the tissue oxygen tension; under hypoxic conditions, PHD activity is inhibited, which results in HIF-1α stabilization.44 Interestingly, Phd3 is one of the target genes directly repressed by Bach2.16 Therefore, we further investigated whether BACH2 can modulate HIF-1α degradation via PHD3 under normoxia.
In BACH2KO MCL cells, mRNA and protein levels of PHD3 were upregulated (Figures 6A). In addition, we observed an increased turnover of HIF-1α in BACH2KO SP53 cells following CoCl2 exposure in the presence of cycloheximide (Figure 6B; supplemental Figure 6A). Conversely, ectopic expression of BACH2 in 293T cells led to a decreased level of PHD3 (Figure 6C; supplemental Figure 6B-C) and resulted in delayed HIF-1α degradation with an increased half-life (Figure 6D; supplemental Figure 6A). Compared with control cells, the degradation of HIF-1α can be rescued by the proteasome inhibitor MG132 (Figure 6B-C), indicating that BACH2 protects HIF-1α from proteasome-dependent degradation. To determine whether the altered HIF-1α stability is due to BACH2 downstream target PHD3, we further silenced PHD3 in SP53 cells; loss of PHD3 expression significantly increased HIF-1α stability (Figure 6E), and similar observations were in 293T cells (Figure 6F). These data suggested that PHD3 is partially responsible for HIF-1α degradation, since ablations of PHD3 expression only delayed instead of prevented HIF-1α degradation. This may be due to the upregulation of PHD2 levels to compensate for the loss of PHD3 (supplemental Figure 6D).45,46
BACH2 modulates HIF-1α degradation by suppressing PHD3 under normoxia. (A) mRNA (top) and protein (bottom) levels of PHD3 were measured using qRT-PCR and immunoblots in BACH2KO or BACH2Con MCL cells. Each qRT-PCR value was normalized to GAPDH and represents the mean ± SD. GAPDH was used as a loading control for immunoblots. (B) Schematic of the experimental design (top). BACH2Con or BACH2KO SP53 MCL cells were treated with CoCl2 (200 μmol/L) for 16 hours followed by removal of CoCl2-containing medium and addition of cycloheximide (CHX; 50 μg/mL) or MG132 (5 μmol/L) at the indicated time. HIF-1α levels were determined by immunoblotting with GAPDH as a loading control (bottom). (C) PHD3 levels were measured in 293T cells transient transfected with BACH2 expression plasmids (pEGFP-BACH2) for 48 hours. The pEGFP-N1 empty vector was used as a control. GAPDH was used as a loading control. (D) 293T cells were transiently transfected with HIF-1α or BACH2 expression plasmids for 48 hours followed by addition of CHX (50 μg/mL) or MG132 (5 μmol/L) at the indicated time. The pEGFP-N1 empty vector was used as a control. HIF-1α stability was measured by immunoblotting with GAPDH as a loading control. (E) The knockout efficiency of CRISPR-Cas9–mediated deletion of PHD3 in SP53 MCL cells (left).The control cells were mock-transfected cells (PHD3Con). PHD3KO or PHD3Con SP53 cells were treated with CoCl2 (200 μmol/L) for 16 hours followed by removal of CoCl2 and addition of CHX (50 μg/mL) at the indicated time. HIF-1α levels were determined by immunoblotting with GAPDH as a loading control. (F) The knockout efficiency of CRISPR-Cas9–mediated deletion of PHD3 in 293T cells (left). Manipulated 293T cells were transiently transfected with HIF-1α expression plasmids for 48 hours. Cells treated with CHX were harvested at the indicated times. HIF-1α levels were measured by immunoblotting with GAPDH as a loading control.
BACH2 modulates HIF-1α degradation by suppressing PHD3 under normoxia. (A) mRNA (top) and protein (bottom) levels of PHD3 were measured using qRT-PCR and immunoblots in BACH2KO or BACH2Con MCL cells. Each qRT-PCR value was normalized to GAPDH and represents the mean ± SD. GAPDH was used as a loading control for immunoblots. (B) Schematic of the experimental design (top). BACH2Con or BACH2KO SP53 MCL cells were treated with CoCl2 (200 μmol/L) for 16 hours followed by removal of CoCl2-containing medium and addition of cycloheximide (CHX; 50 μg/mL) or MG132 (5 μmol/L) at the indicated time. HIF-1α levels were determined by immunoblotting with GAPDH as a loading control (bottom). (C) PHD3 levels were measured in 293T cells transient transfected with BACH2 expression plasmids (pEGFP-BACH2) for 48 hours. The pEGFP-N1 empty vector was used as a control. GAPDH was used as a loading control. (D) 293T cells were transiently transfected with HIF-1α or BACH2 expression plasmids for 48 hours followed by addition of CHX (50 μg/mL) or MG132 (5 μmol/L) at the indicated time. The pEGFP-N1 empty vector was used as a control. HIF-1α stability was measured by immunoblotting with GAPDH as a loading control. (E) The knockout efficiency of CRISPR-Cas9–mediated deletion of PHD3 in SP53 MCL cells (left).The control cells were mock-transfected cells (PHD3Con). PHD3KO or PHD3Con SP53 cells were treated with CoCl2 (200 μmol/L) for 16 hours followed by removal of CoCl2 and addition of CHX (50 μg/mL) at the indicated time. HIF-1α levels were determined by immunoblotting with GAPDH as a loading control. (F) The knockout efficiency of CRISPR-Cas9–mediated deletion of PHD3 in 293T cells (left). Manipulated 293T cells were transiently transfected with HIF-1α expression plasmids for 48 hours. Cells treated with CHX were harvested at the indicated times. HIF-1α levels were measured by immunoblotting with GAPDH as a loading control.
BACH2 blockade confers drug resistant properties to MCL
We next questioned the implication of decreased BACH2 expression in MCL therapy and sought to determine whether reduced BACH2 levels contribute to MCL drug resistance. Bortezomib-resistant cells in MCL differentiate to plasma-like cells21,47 ; these cells display elevated expression of CD138 and CD38 and upregulation of the transcription factors IRF4 and PRDM1, all of which mediate plasma cell differentiation.21,47 BACH2 represses Prdm1 expression via Maf recognition elements during B-cell development.3 BACH2 also delays the terminal differentiation of B cells by inhibiting Prdm1 and provides adequate time for class switch recombination.1 Upon plasma cell differentiation, either decreased BACH2 expression or cytoplasmic translocation is necessary for proper differentiation to plasma cells.48 Therefore, we investigated the chemotherapy resistance of BACH2KO cells, especially to bortezomib. BACH2 deletion in MCL led to a significant increase in bortezomib resistance, while re-expression of BACH2 sensitized MCL cells to bortezomib (Figure 7A). BACH2KO cells also presented increased mRNA levels of PRDM1 and IRF4 (supplemental Figure 7A) as well as elevated IRF4 protein levels (Figure 7B), all of which are the signature of MCL cells that have undergone plasmacytic differentiation.47 The plasma cell markers CD138/CD38 (supplemental Figure 7B), especially the CD138 (Figure 7C) were also dramatically increased in BACH2KO cells, indicating that reduced BACH2 confers bortezomib resistance properties to MCL by promoting plasmacytic differentiation.
BACH2 blockade confers drug-resistant properties to MCL by promoting plasmacytic differentiation. (A) BACH2KO or BACH2KO-OE MCL cells were treated with bortezomib for 24 hours. Cell viability was determined using 3-(4,5-dimethylthiazol-2-yl)-2,5-dimethyltetrazolium bromide; 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide assays. Controls were mock-transfected cells. Data are presented as the mean ± S.D. from 3 independent experiments. (B) Immunoblots for IRF4 in BACH2KO or BACH2Con cells with GAPDH as a loading control. (C) CD138 levels in BACH2KO or BACH2Con MCL cells were measured using FACS analysis (left), and the percentage of CD138+ cells in each group is indicated in the diagram (data are from 2 independent experiments) (right). **P < .01 (vs control cells; Student t test). (D) Under hypoxic stress, stabilized HIF-1α protein and increased heme synthesis are responsible for the decreased BACH2 levels at both the transcriptional and protein levels, thereby promoting MCL survival and progression in hypoxic microenvironments. BACH2 in turn modulates HIF-1α stability by downregulating PHD3 under normoxic conditions, indicating a fine-tuned interconnected loop between BACH2 and HIF-1α in MCL.
BACH2 blockade confers drug-resistant properties to MCL by promoting plasmacytic differentiation. (A) BACH2KO or BACH2KO-OE MCL cells were treated with bortezomib for 24 hours. Cell viability was determined using 3-(4,5-dimethylthiazol-2-yl)-2,5-dimethyltetrazolium bromide; 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide assays. Controls were mock-transfected cells. Data are presented as the mean ± S.D. from 3 independent experiments. (B) Immunoblots for IRF4 in BACH2KO or BACH2Con cells with GAPDH as a loading control. (C) CD138 levels in BACH2KO or BACH2Con MCL cells were measured using FACS analysis (left), and the percentage of CD138+ cells in each group is indicated in the diagram (data are from 2 independent experiments) (right). **P < .01 (vs control cells; Student t test). (D) Under hypoxic stress, stabilized HIF-1α protein and increased heme synthesis are responsible for the decreased BACH2 levels at both the transcriptional and protein levels, thereby promoting MCL survival and progression in hypoxic microenvironments. BACH2 in turn modulates HIF-1α stability by downregulating PHD3 under normoxic conditions, indicating a fine-tuned interconnected loop between BACH2 and HIF-1α in MCL.
In addition to bortezomib, we further tested other anticancer agents. Similar to what was observed, BACH2 deletion displayed lower sensitivity to these chemotherapy drugs, which was reversed by BACH2 re-expression (supplemental Figure 7C). These findings suggest that silencing BACH2 likely increases the threshold for drug-induced apoptosis by activating cyclin-dependent kinases49 or by modifying the cytotoxic effects of drugs,7,48 which deserves further investigation.
Discussion
In the present study, we demonstrated that downregulation of BACH2 increases MCL-cell proliferation and enhanced tumor dispersal in vitro and in vivo, suggesting a tumor suppressor–like role of BACH2 in MCL. Multiple lines of evidence demonstrate that the BACH2 locus is a hotspot for viral integrations and transpositions that lead to disruption of wild-type BACH2 expression.50-53 Based on the data available through the cBioPortal for Cancer Genomics, >10% of DLBCL patients have BACH2 deletion and ∼3% of DLBCL patients have a BACH2 mutation (data not shown), implying that loss of BACH2 function is most likely a pathogenic factor in lymphoma. In addition to genetic variations of BACH2, epigenetic modifications have been reported as another possible mechanism for BACH2 downregulation. For example, histone modifications within the BACH2 promoter were observed in BCR-ABL–positive chronic myeloid leukemia cells.20 However, the exact mechanism involved in reducing BACH2 expression in MCL remains unclear.
In this study, we discovered a significant reduction of BACH2 in the BM from MCL patients. In xenograft models, human B cells from the SP and BM also contained lower BACH2 levels than cells from subcutaneous tumors. These findings highlight the crucial roles of BACH2 in the pathogenesis and progression of MCL in specific microenvironments. Therefore, our focus is to figure out by which mechanisms BACH2 is downregulated under hypoxic stress in MCL.
The BM microenvironment, especially the osteoblastic niche, is characterized by a very low oxygen tension, which is thought to help maintain the undifferentiated states of hematopoietic stem/progenitor cells.54 However, other cells, including infiltrating cancer cells in hypoxic BM, are threatened when cells are deprived of oxygen. Numerous studies have shown that oxygen deficiency leads to an accumulation of heme content,41,42,55,56 thereby accelerating rather than diminishing the production of mitochondrial ROS.57-60 These events trigger diverse functional responses to ensure oxygen consumption and metabolism during hypoxia.59 In accordance with these findings, we found a transient increase of intracellular heme levels in MCL cells during the first few hours of incubation at 1% O2. Compared with xenograft tumors and PB samples from MCL patients, MCL cells within the BM also showed elevated heme levels. Since excessive heme is highly toxic to nearly all cells,61,62 the human body is equipped with various defense mechanisms. HO-1 is one of the key mechanisms that lead to increased heme breakdown. Indeed, we found a consistent and corresponding increase in HO-1 levels in MCL cells under hypoxia, indicating a fine-tuned feedback loop in the heme/HO-1 metabolic pathway to properly balance intracellular heme levels within a certain range.
Intriguingly, BACH2 is involved in this network to facilitate MCL survival under stress. In mice, heme inhibits the DNA-binding activity of Bach2 and induces Bach2 degradation in B cells.40 HO-1, an important enzyme involved in heme degradation, is a direct downstream target of Bach2.16,40 These findings were further confirmed in our results, implying a subtle feedback loop among the heme–BACH2–HO-1 axis in MCL cells under hypoxia. Apart from heme-induced degradation of BACH2, HIF-1α–dependent transcriptional repression is another important factor involved in BACH2 downregulation under hypoxia. HIF-1α stabilization is a crucial event that activates multiple genes and cellular processes in response to low oxygen levels.63-65 In contrast, HIF-1α is also featured as a transcriptional repressor under hypoxia in several tumors.66-71 For example, HIF-1α induces the downregulation of the transcription factor CCAAT/enhancer-binding protein-α67 or the tumor suppressor RECK68 by binding to its promoter under hypoxia. In our study, we showed for the first time that HIF-1α negatively drives BACH2 transcription in MCL cells in hypoxic conditions via direct binding. Therefore, MCL cells within the BM may favor cancer survival by lowering the expression of the tumor suppressor BACH2 via transcriptional repression of HIF-1α and heme-induced posttranscriptional effects if oxygen availability becomes limited (Figure 7D). Nonetheless, the exact mechanisms of how reduced BACH2 alters the microenvironment to promote MCL survival and which pathways are likely involved require further study.
In addition to BACH2 regulation by HIF-1α under hypoxia, BACH2 can itself delay HIF-1α degradation by suppressing PHD3 in normoxia, suggesting a reciprocal interaction between BACH2 and HIF-1α under different conditions (Figure 7D). We previously reported that bortezomib-induced ROS can drive the nuclear translocation of BACH2 in MCL cells, where it acts as a transcriptional repressor of Maf recognition element/AU-rich element sequences, thereby leading to cell apoptosis by suppressing antioxidative and antiapoptotic genes.48 Interestingly, HIF-1α is constitutively expressed in tumor tissues from DLBCL patients undergoing chemotherapies.72 Antioxidant treatments lead to HIF-1α inhibition in mice bearing a MYC-inducible lymphoma.73 These findings, to some extent, suggest that BACH2 is more likely involved in the regulatory network of ROS and HIF-1α in normoxic conditions. Our results undoubtedly add a new layer to the understanding of this complicated regulatory loop.
In conclusion, our study identifies crucial biological roles of BACH2 in lymphoma progression and dispersal. Aberrant BACH2 levels may serve as a new predictor for advanced MCL patients. Importantly, our discovery of the correlation between HIF-1α and BACH2 provides the first proof of MCL survival and development in chronic hypoxic microenvironments. Further exploration of the mechanism responsible for the reduced BACH2 levels in MCL patients will allow for tailored antilymphoma treatments and more specific targeted therapies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Sanat Dave and the Anderson Hematopathology Tissue Bank for providing clinical samples for this study. This work was supported by grants from the American Cancer Society (Scholar Award RSG-11-157) and the National Institutes of Health, National Cancer Institute (grant R01CA181319) (N.M.).
Authorship
Contribution: H.Z. performed the experiments, analyzed the data, and wrote and revised the paper; Z.C. assisted the experiments; R.N.M. and L.J.M. provided clinical samples and assisted the clinical analyses; and N.M. supervised the experiments and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Nami McCarty, University of Texas-Health Science Center at Houston, 1825 Pressler St, IMM-630H, Houston, TX 77030; e-mail: nami.mccarty@uth.tmc.edu.





![Figure 7. BACH2 blockade confers drug-resistant properties to MCL by promoting plasmacytic differentiation. (A) BACH2KO or BACH2KO-OE MCL cells were treated with bortezomib for 24 hours. Cell viability was determined using 3-(4,5-dimethylthiazol-2-yl)-2,5-dimethyltetrazolium bromide; 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide assays. Controls were mock-transfected cells. Data are presented as the mean ± S.D. from 3 independent experiments. (B) Immunoblots for IRF4 in BACH2KO or BACH2Con cells with GAPDH as a loading control. (C) CD138 levels in BACH2KO or BACH2Con MCL cells were measured using FACS analysis (left), and the percentage of CD138+ cells in each group is indicated in the diagram (data are from 2 independent experiments) (right). **P < .01 (vs control cells; Student t test). (D) Under hypoxic stress, stabilized HIF-1α protein and increased heme synthesis are responsible for the decreased BACH2 levels at both the transcriptional and protein levels, thereby promoting MCL survival and progression in hypoxic microenvironments. BACH2 in turn modulates HIF-1α stability by downregulating PHD3 under normoxic conditions, indicating a fine-tuned interconnected loop between BACH2 and HIF-1α in MCL.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/130/6/10.1182_blood-2017-02-767293/4/m_blood767293f7.jpeg?Expires=1767755472&Signature=GK0B6btlPKkfkZdAVut6ufo~7gnBd4hYubAX9xZG8Mf97Yfj1vKFTgxQW7pH2sqUPdj6ie0Cvv7wVogyIqP5TK1Gb5L1a17fKfNAMu~NpuCoinLvYMqSO5Qt7BtywBmGdEZD1qqKGpvRWLsq8sNN1hPDXwm2k~wN~WC8sjtg-EDfAbBGmjLny5kN6tew7zV22qTPKRk6e-wxBW-xd2kJQQCcJINXs~jk4Y4E8L7FXPbk1yU1xSk9GgPP~nZkLqClSeKjLmEPtxu7YSUx4Hc2LWs97UHet9Ae8XcURBr2IiFVDk2PrlYvQR1HwLHUuqda9xnZyvzzWEFnbBqblEUP2w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal