Key Points
Patients registered with low VWF have significant bleeding phenotypes that cannot be explained by concomitant bleeding disorders.
Low VWF levels in the range of 30 to 50 IU/dL are predominantly due to reduced VWF synthesis/secretion rather than enhanced clearance.
Abstract
Critical clinical questions remain unanswered regarding diagnosis and management of patients with low von Willebrand factor (VWF) levels (30-50 IU/dL). To address these questions, the Low VWF Ireland Cohort (LoVIC) study investigated 126 patients registered with low VWF levels. Despite marginally reduced plasma VWF levels, International Society of Thrombosis and Haemostasis Bleeding Assessment Tool (ISTH BAT) confirmed significant bleeding phenotypes in the majority of LoVIC patients. Importantly, bleeding tendency did not correlate with plasma VWF levels within the 30 to 50 IU/dL range. Furthermore, bleeding phenotypes could not be explained by concurrent hemostatic defects. Plasma factor VIII to VWF antigen (VWF:Ag) ratios were significantly increased in LoVIC patients compared with controls (P < .0001). In contrast, VWF propeptide to VWF:Ag ratios >3 were observed in only 6% of the LoVIC cohort. Furthermore, platelet-VWF collagen binding activity levels were both significantly reduced compared with controls (P < .05). In response to 1-desamino-8-D-arginine vasopressin (DDAVP), peak VWF:Ag levels exceeded 100 IU/dL in 88% of patients and was sustained >100 IU/dL after 4 hours in 72% of subjects. In conclusion, our novel data suggest that low VWF levels can be associated with significant bleeding and are predominantly due to reductions in VWF synthesis and/or constitutive secretion. Although enhanced VWF clearance may contribute to the pathophysiology in some individuals, the absolute reduction in VWF plasma half-life is usually mild and not sufficient to significantly impact upon the duration of DDAVP-induced VWF response. This trial was registered at www.clinicaltrials.gov as #NCT03167320.
Introduction
von Willebrand disease (VWD) represents the most common inherited bleeding disorder and is caused by either deficiency or dysfunction of von Willebrand factor (VWF).1 Reduced plasma VWF levels have been reported across many ethnic groups with an estimated population prevalence in the order of 1%.2,3 Moreover, significant bleeding symptoms due to reduced VWF levels have been reported in ∼1 in 1000 of the normal population.4 According to International Society of Thrombosis and Haemostasis (ISTH) classification criteria published in 2006, VWD is classified into 3 major categories.5 Type 1 VWD is responsible for a vast majority of cases (∼75%) and is characterized by a partial quantitative deficiency of functionally normal VWF. Significant phenotypic heterogeneity exists among patients with type 1 VWD, which is perhaps unsurprising given that plasma VWF antigen (VWF:Ag) levels in these patients range between 1 and 50 IU/dL (normal range 50-200 IU/dL). Type 2 VWD accounts for 25% of cases and results from the expression of a functionally abnormal VWF molecule. Finally, in rare patients with type 3 VWD (1 per million population), there is virtually complete absence of VWF.
The genetic mutations and biological mechanisms that lead to type 2 and type 3 VWD have been extensively characterized.1,6-9 In contrast, however, the molecular mechanisms involved in the pathogenesis of the more common type 1 VWD remain poorly understood. However, this area has advanced in recent years following a number of large type 1 VWD cohort studies.10-15 These studies reported VWF gene mutations in ∼65% of patients with type 1 VWD.10-13 Furthermore, VWF coding mutations were significantly more common in patients with plasma VWF levels <30 IU/dL.10-12 In contrast, VWF mutations were less common in patients with more intermediate plasma VWF levels (30-50 IU/dL).10,11,16 Interestingly, linkage studies have shown that reduced plasma VWF levels in this latter cohort result from unknown mechanisms independent of the VWF gene.17,18 On the basis of these cumulative data, the US National Heart, Lung, and Blood Institute (NHLBI)19 and the UK Haemophilia Centre Doctors Organization20 have both recommended modification of the previous ISTH VWD classification criteria. In particular, the NHLBI and UK Haemophilia Centre Doctors Organization proposed that patients with partial quantitative VWF deficiencies and bleeding phenotypes should be considered in 2 distinct subsets.19,20 Patients with more marked reductions in plasma VWF levels (<30 IU/dL) are likely to have VWF gene mutations, exhibit autosomal dominant inheritance, and should be labeled “Type 1 VWD.” In contrast, patients with intermediate plasma VWF:Ag levels (30-50 IU/dL) should be considered in a distinct category labeled “Low VWF levels.” Because the pathophysiology and heritability of Low VWF levels remain largely undefined, diagnosis and management of these patients continue to pose significant clinical challenges.21-23 Moreover, the “Low VWF levels” category has significant public health ramifications given that an estimated ∼0.4% of the general population will be affected. Consequently, for example, this diagnosis will apply to an estimated 1.3 million people in the United States alone.
As highlighted in the NHLBI consensus guidelines,19 a number of critical clinical questions remain unanswered regarding the diagnosis and management of patients with low VWF levels. First, more clinical information is required to properly elucidate the relationship that exists between low VWF levels and clinical bleeding phenotype. Second, evidence-based and standardized diagnostic criteria are needed for establishing the diagnosis of low VWF. Finally, the molecular mechanism(s) responsible for the moderate reduction in plasma VWF levels in these patients needs to be defined. In order to address these questions, we established the Low VWF Ireland Cohort (LoVIC) study. In contrast to the previously published type 1 VWD cohort studies, this prospective longitudinal cohort study focused exclusively on investigating patients with low VWF levels.
Methods
Subjects
In Ireland, all patients with inherited bleeding disorders are registered on the Irish National Bleeding Disorder Registry and have annual clinical reviews performed in a single National Coagulation Centre (NCC). A total of 283 Irish adults (>18 years old) are registered with a diagnosis of low VWF levels (defined as significant personal bleeding history and lowest plasma VWF levels ≥30 IU/dL but <50 IU/dL measured on 2 separate occasions at least 3 months apart). Patients with low VWF were recruited into the LoVIC study from those attending the NCC between October 2014 and June 2016. Pregnant patients were excluded from the trial. Blood samples from age- and sex-matched healthy volunteer Irish adult controls were also collected where indicated. The LoVIC study was approved by the St James’ Hospital Research Ethics Committee, and written informed consent was obtained from all participants.
Phenotypic evaluation
A bleeding questionnaire was administered to each patient at time of enrollment by a consultant hematologist specializing in thrombosis and hemostasis. Only bleeding symptoms and procedures prior to original diagnosis were included. For each low VWF patient, this questionnaire was then used to calculate scores for both the Condensed Molecular and Clinical Markers for the Diagnosis and Management of Type 1 VWD (Condensed MCMDM-1 VWD)24,25 and the ISTH Bleeding Assessment Tool (BAT).26 In keeping with previous reports, ISTH BAT was considered abnormal if bleeding scores were >3 for male or >5 for female patients.16,26 Again, as previously reported, Condensed MCMDM-1 VWD scores ≥4 were considered abnormal for both sexes.24,25
Coagulation laboratory studies
All hemostasis testing was performed in a single National Hemostatic Reference Laboratory in St James’ Hospital, Dublin. Plasma VWF:Ag levels were measured using a latex particle enhanced immunoturbidimetric assay (HemosIL VWF antigen assay; Instrumentation Laboratories, Milan, Italy) on an automated coagulometer (ACL Top 700; Instrumentation Laboratories). VWF collagen binding activity (VWF:CB) was performed using a commercial enzyme-linked immunosorbent assay according to the procedure recommended by the manufacturer (Technoclone Techonozym, Vienna, Austria). Plasma VWF ristocetin cofactor activity (VWF:RCo) levels were determined by standard platelet agglutination on a Sysmex CS2100i Analyzer (Siemens Healthcare, Marburg, Germany). Platelet aggregation studies were performed using standard light transmission aggregometry. Platelet nucleotides were assessed using luminescent methods with an ATP Kit SL (Biothema, Handen, Sweden). Plasma clotting factor activity levels (FII, FV, FVII, FVIII, FIX, FX, FXI, FXII) were determined by clotting-based assays using appropriate factor-deficient plasmas. VWF multimer analyses were performed by sodium dodecyl sulfate agarose gel electrophoresis in the laboratory of Ulrich Budde (Hamburg, Germany) as previously described.27
VWF:pp and platelet VWF assays
Plasma VWF propeptide (VWF:pp) levels were determined by enzyme-linked immunosorbent assay using monoclonal antibodies CLB-Pro 35 and CLB-Pro 14.3-HRP (Sanquin, The Netherlands) as previously described.28 Platelet VWF:Ag and VWF:CB levels were measured essentially as previously described.29 In brief, a platelet pellet was generated from platelet-rich plasma by centrifugation at 1000g for 10 minutes and then resuspended in in Tris HCl buffer containing Protease Inhibitor Cocktail I (Calbiochem, Merck, UK). Finally, platelets were lysed using Triton X-100 (0.5%) and repeated snap freeze–thaw cycles in liquid nitrogen.
VWF sequencing
VWF was sequenced using a custom genetic array. All DNA samples were diluted to the same initial concentration (25 ng/μL) and were amplified using custom primers designed to avoid the VWF pseudogene. The library preparation was performed using TruSeq Custom Amplicon v1.5 Library Prep kit (Illumina, San Diego, CA) according to the manufacturer’s instructions. Briefly, the primers were hybridized by cooling for 80 minutes from 95°C to 40°C. After the extension-ligation and polymerase chain reaction amplification were carried out, the pool of amplicons were labeled with a barcode (index adapters) incorporated into each set of polymerase chain reactions. Bead-based normalization of the quantity of DNA in each library was performed using AMPure XP (Beckman Coulter, Inc, Brea, CA). Pooled libraries were sequenced on a MiSeq sequencer (Illumina) by the Genomics and Microarray Core of University of Colorado Denver using the 300-pb paired-end sequencing. The average reads per bases quality for all samples is at least 69% ≥ Q30.
Bioinformatics analysis
Reads that passed the quality filter step (fastq file) were mapped to the reference human genome sequence (hg19) with GSNAP (Genomic Short-read Nucleotide Alignment Program). The snpEff (variant annotation) was used to classify variants (single-nucleotide variants [SNVs]) and to cross-reference all variants across different genetic variation databases. Using the dbNSFP (database for nonsynonymous SNPs’ functional predictions), we determined the impact of any changes seen using different algorithms. ExAC and 1000 genomes database were used to determine the frequency of SNVs. All changes were compared with existing VWF variation databases: the Human Gene Mutation Database (www.hgmd.cf.ac.uk) and the European Association for Haemophilia and Allied Disorders Coagulation Factor Variants Database (www.eahad-db.org), as well as the published literature.
Statistical methods
Normally distributed laboratory data were expressed using means, and continuous variables, such as bleeding scores, were expressed as medians. Descriptive statistics are presented as frequencies and percentages (n, %). The Mann-Whitney U test was used to test the differences in bleeding scores between groups. The change in VWF levels over time within the same patient group was assessed with a paired Student t test. A value of P < .05 was considered statistically significant. Statistical analyses were performed using Prism 6 for Mac OSX, Version 6.0g (GraphPad, La Jolla, CA).
Results
Low VWF Ireland cohort
One hundred twenty-six adult Irish patients from 118 kindreds with a registered diagnosis of low VWF were recruited into the LoVIC study. The majority of patients were women (112/126; 89%), and median age at enrollment was 38.8 years (range 18-72 years). These values were very similar to those for the entire Irish national low VWF database (n = 283; women 81%; median age 39 years). Within the LoVIC cohort, 69 subjects had lowest recorded plasma VWF:Ag, VWF:RCo, and VWF:CB levels all within the 30- to 50-IU/dL range. In a further 32 subjects, lowest levels of both VWF:RCo and VWF:CB were between 30 and 50 IU/dL, but plasma VWF:Ag levels were consistently >50 IU/dL. Finally, isolated reductions (in the range 30-50 IU/dL) in VWF:RCo only (n = 22), or VWF:CB only (n = 3), were also observed. Six (5%) patients were tested on 2 separate occasions; 49 (39%) patients were tested on 3 or 4 separate occasions, and 71 (56%) patients underwent testing on ≥5 separate visits. The median range of time spanning testing was 0.5 years for patients tested on 2 occasions; 2.8 years for patients tested on 3 or 4 separate occasions, and 6.7 years for patients tested on ≥5 separate visits. For patients tested on 3 to 4 occasions, 85% of plasma VWF levels were in the low VWF range. For those patients tested on ≥5 separate visits (median 7 samples), plasma VWF levels consistent with a diagnosis of low VWF levels were observed on 76% of occasions. Interestingly, blood group O phenotype was significantly more common in the LoVIC cohort (112/126 individuals; 88.9%) compared with the normal Irish population (55%).
Patients with low VWF have significant bleeding
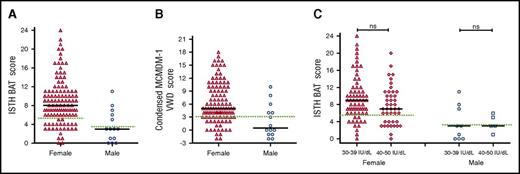
Although a number of different BATs have been used to objectively assess bleeding in patients with type 1 VWD,14,24,26,30-33 their utility has not previously been specifically studied in a cohort of patients with low VWF. We found that the majority of patients with low VWF had significant bleeding histories, as determined using either the ISTH BAT (Figure 1A) or the Condensed MCMDM-1 VWD score (Figure 1B), respectively. The bleeding histories appeared disproportionate compared with the relatively modest reduction in plasma VWF levels. For example, among female patients with low VWF, 86/112 (77%) had positive ISTH BAT scores (≥6) and 37% had scores ≥10. Previous studies have demonstrated that in patients with type 1 VWD, bleeding scores inversely correlate with plasma VWF levels.15,24,31,34 We observed no significant difference in ISTH BAT or Condensed MCMDM-1 VWD scores for LoVIC patients with lowest VWF levels in the 30 to 39 IU/dL range, compared with those with lowest VWF levels in the 40 to 50 IU/dL range (median ISTH BAT scores 9 vs 7 for female patients; P = .09; 3 vs 3 male patients; P = .7) (Figure 1C; supplemental Figure 1, available on the Blood Web site). Cumulatively, these findings demonstrate that BATs may be useful in the diagnosis of low VWF levels and confirm the majority of patients registered with low VWF have significant bleeding histories. Importantly, however, this bleeding does not correlate with plasma VWF levels within the 30 to 50 IU/dL range.
Patients with low VWF have significant bleeding phenotypes. (A) ISTH BAT scores by sex, females, n = 112 (triangles, median = 8), and males, n = 14 (circles, median = 3). (B) Condensed MCMDM-1 VWD bleeding scores by sex. Black lines indicate median scores in females, n = 112 (triangles, median 5), and males, n = 14 (circles, median 0.5). (C) ISTH BAT scores studied in LoVIC subgroups with lowest plasma VWF levels in the range 30 to 39 IU/dL (females, triangles; males, circles) compared with patients with lowest VWF levels in the range 40 to 49 IU/dL (females, diamonds; males, squares). No significant difference was seen for either females (median 9 vs 7, P = .09, ns = not significant) or males (median 3 vs 3, P = .7). The upper limit of bleeding scores considered normal for each score is indicated by the green dotted line; median is shown by the black line.
Patients with low VWF have significant bleeding phenotypes. (A) ISTH BAT scores by sex, females, n = 112 (triangles, median = 8), and males, n = 14 (circles, median = 3). (B) Condensed MCMDM-1 VWD bleeding scores by sex. Black lines indicate median scores in females, n = 112 (triangles, median 5), and males, n = 14 (circles, median 0.5). (C) ISTH BAT scores studied in LoVIC subgroups with lowest plasma VWF levels in the range 30 to 39 IU/dL (females, triangles; males, circles) compared with patients with lowest VWF levels in the range 40 to 49 IU/dL (females, diamonds; males, squares). No significant difference was seen for either females (median 9 vs 7, P = .09, ns = not significant) or males (median 3 vs 3, P = .7). The upper limit of bleeding scores considered normal for each score is indicated by the green dotted line; median is shown by the black line.
Bleeding in low VWF is not explained by concomitant bleeding disorders
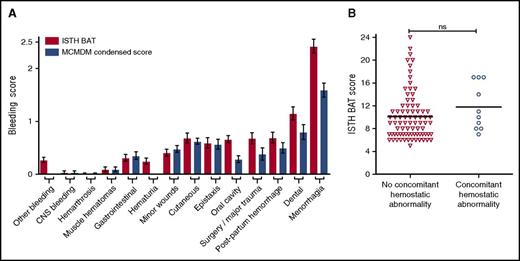
To determine the type of bleeding complications experienced by LoVIC patients, individual domain scores within both BATs were examined (Figure 2A). Highest bleeding scores were observed in the menorrhagia and dental domains, respectively. Ninety-seven (86.6%) of the women enrolled in the cohort described symptoms of menorrhagia (changing pads/tampons more frequently than every 2 hours; clots and flooding; bleeding longer than 7 days), with 82 (73.4%) female patients describing 2 or more of these symptoms. Of the patients with menorrhagia, 76 (78%) had symptoms since menarche, and 70 (72%) had attended for medical review. Of these women who attended complaining of menorrhagia, 65 (67%) were treated with hormonal therapy (oral contraceptive pill or intrauterine device) and 35 (36%) required iron. Twenty-three (24%) LoVIC patients with significant menorrhagia underwent dilation and curettage, and a hysterectomy was ultimately performed for menorrhagia in 8/97 (8%).
Bleeding in low VWF is not explained by concomitant bleeding disorders. (A) Comparison of ISTH BAT scores (red bars) and condensed MCMDM-1 VWD scores (blue bars) for all patients (n = 126) according to individual bleeding subdomains. Data are represented as mean ± standard error of the mean of bleeding scores. (B) ISTH BAT scores were not significantly different for LoVIC patients with concomitant coagulation disorders (circles, n = 10) compared with those without (triangles, n = 81) (median scores 10.5 vs 9.0; P = .15). CNS, central nervous system.
Bleeding in low VWF is not explained by concomitant bleeding disorders. (A) Comparison of ISTH BAT scores (red bars) and condensed MCMDM-1 VWD scores (blue bars) for all patients (n = 126) according to individual bleeding subdomains. Data are represented as mean ± standard error of the mean of bleeding scores. (B) ISTH BAT scores were not significantly different for LoVIC patients with concomitant coagulation disorders (circles, n = 10) compared with those without (triangles, n = 81) (median scores 10.5 vs 9.0; P = .15). CNS, central nervous system.
To investigate the pathophysiology underlying this bleeding phenotype, further hemostatic studies were performed in the 91 LoVIC patients with positive bleeding scores. Additional mild coagulation defects were identified in only 10 subjects (Table 1). Furthermore, there was no significant difference in bleeding scores for patients with low VWF and concomitant hemostatic abnormalities (n = 10) compared with low VWF patients (n = 81) who had no other abnormalities (median ISTH BAT scores 10.5 vs 9; P = .15) (Figure 2B). Recent studies have reported that a decrease in high-molecular-weight multimers may be present in some patients with normal VWF:RCo and VWF:CB, and even normal VWF:RCo/VWF:Ag ratios.35,36 However, plasma VWF multimer analysis demonstrated abnormal multimer patterns in only 4 LoVIC patients (3.2%) (supplemental Figure 2). Altogether, these data demonstrate that, in most patients, the bleeding phenotype cannot be explained by the presence of detectable concomitant bleeding disorders.
Concomitant hemostatic abnormalities identified
| Concomitant hemostatic defect . | n . | Sex . | ISTH BAT score . | Factor level (IU/dL) . | Normal reference range (IU/dL) . |
|---|---|---|---|---|---|
| Factor deficiency | |||||
| Factor V | 1 | M* | 11 | 30 | 70-137 |
| Factor VII | 2 | F, F | 10, 17 | 31, 39 | 70-170 |
| Factor VIII (confirmed carrier) | 2 | F, F* | 17, 14 | 28, 39 | 60-136 |
| Factor XI | 2 | M, F | 5, 8 | 50, 58 | 72-152 |
| Factor XIII | 1 | F | 17 | 48 | 73-160 |
| Platelet functional defect | |||||
| No response to adrenaline | 1 | M* | 11 | ||
| Reduced response to ADP and adrenaline | 1 | F | 7 | ||
| Reduced response to AA and adrenaline | 1 | F | 8 | ||
| One-phase ADP | 1 | F* | 14 |
| Concomitant hemostatic defect . | n . | Sex . | ISTH BAT score . | Factor level (IU/dL) . | Normal reference range (IU/dL) . |
|---|---|---|---|---|---|
| Factor deficiency | |||||
| Factor V | 1 | M* | 11 | 30 | 70-137 |
| Factor VII | 2 | F, F | 10, 17 | 31, 39 | 70-170 |
| Factor VIII (confirmed carrier) | 2 | F, F* | 17, 14 | 28, 39 | 60-136 |
| Factor XI | 2 | M, F | 5, 8 | 50, 58 | 72-152 |
| Factor XIII | 1 | F | 17 | 48 | 73-160 |
| Platelet functional defect | |||||
| No response to adrenaline | 1 | M* | 11 | ||
| Reduced response to ADP and adrenaline | 1 | F | 7 | ||
| Reduced response to AA and adrenaline | 1 | F | 8 | ||
| One-phase ADP | 1 | F* | 14 |
Patients with low VWF levels and significant bleeding histories (n = 91) were screened for additional coagulation disorders (see “Methods” for details). Concomitant hemostatic abnormalities were identified in only 10 individuals (in 2 subjects, combined platelet function defects and coagulopathies were found).
Combined mild factor deficiency and platelet function defect.
AA, arachdonic acid; ADP, adenosine 5′-diphosphate.
Plasma VWF levels normalize with age in some patients with low VWF
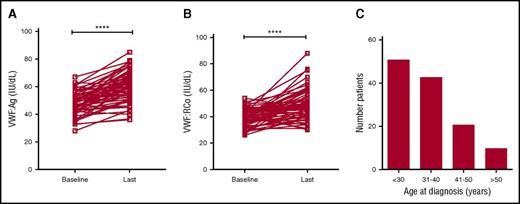
Recent studies have shown that plasma VWF levels increase with age in some patients with type 1 VWD.37,38 To investigate the effects of age on plasma VWF levels in patients with low VWF, the clinical records for all subjects enrolled in the LoVIC trial were reviewed. Sixty-four patients were identified who had been followed in the NCC for >5 years. The mean initial age of this subgroup of patients was 36.4 years (range 15-66 years), and mean duration of follow-up was 8.5 years (range 5-17 years). At time of initial diagnosis, mean VWF:Ag and VWF:RCo levels in this cohort were 47.8 and 39.4 IU/dL, respectively (Figure 3A-B). However, by the time of their most recent follow-up, mean VWF:Ag (60.6 IU/dL; P < .0001) and VWF:RCo (49.0 IU/dL; P < .0001) had both significantly increased (Figure 3A-B). Overall, mean increases of 1.9 IU/dL per year for VWF:Ag and 1.5 IU/dL per year for VWF:RCo were observed. Importantly, in 29 patients with low VWF levels, both VWF:Ag and VWF:RCo levels corrected to within the normal range during the duration of follow-up. Given this effect of age on plasma VWF levels in both type 1 VWD and low VWF, the clinical records for all LoVIC patients were reviewed to define age at original diagnosis. Interestingly, first diagnosis of low VWF was made over a wide range of ages (Figure 3C). For example, although 51 patients (40%) were diagnosed before 30 years of age, a further 10 patients (8%) were not diagnosed until they were older than 50 years. In keeping with previous studies in patients with type 1 VWD,31 a trend for increasing ISTH BAT score according to age at initial low VWF diagnosis was also observed (data not shown). Collectively, these data demonstrate that plasma VWF antigen and activity levels increase with aging in patients with low VWF levels, and in some patients, will correct to within the normal range. Nevertheless, it is also clear that low VWF may be diagnosed for the first time in older patients, many of whom will have previously had unrecognized significant bleeding histories.
Plasma VWF levels normalize with age in some patients with low VWF. (A) In a subgroup of 64 LoVIC patients followed in the NCC for >5 years, plasma VWF:Ag levels were significantly higher at last follow-up compared with baseline levels (mean VWF:Ag at baseline = 47.8 IU/dL vs 60.6 IU/dL at last follow-up; ****P < .0001). (B) Similarly, plasma VWF:RCo levels were also significantly higher at last follow-up compared with baseline levels (mean VWF:RCo at baseline = 39.4 IU/dL vs 49.0 IU/dL at last follow-up; ****P < .0001). (C) Age at original registration of patients with a diagnosis with low VWF levels (n = 126).
Plasma VWF levels normalize with age in some patients with low VWF. (A) In a subgroup of 64 LoVIC patients followed in the NCC for >5 years, plasma VWF:Ag levels were significantly higher at last follow-up compared with baseline levels (mean VWF:Ag at baseline = 47.8 IU/dL vs 60.6 IU/dL at last follow-up; ****P < .0001). (B) Similarly, plasma VWF:RCo levels were also significantly higher at last follow-up compared with baseline levels (mean VWF:RCo at baseline = 39.4 IU/dL vs 49.0 IU/dL at last follow-up; ****P < .0001). (C) Age at original registration of patients with a diagnosis with low VWF levels (n = 126).
Pathophysiology underlying low VWF
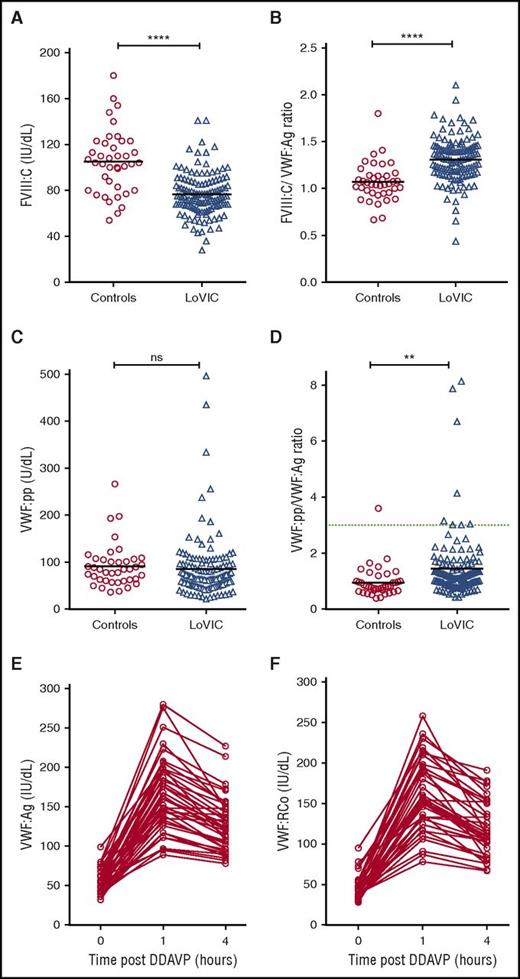
To investigate the pathophysiology underlying low VWF levels, plasma FVIII:C and VWF:pp levels were defined for each LoVIC patient. Although plasma FVIII:C levels were reduced in LoVIC patients compared with normal controls (mean 76 IU/dL vs 105 IU/dL; P < .0001; Figure 4A), nevertheless 104/126 (82.5%) patients had FVIII:C levels within the local normal range (60-136 IU/dL). Elevated FVIII:C/VWF:Ag ratios have been used to define patients with type 1 VWD caused by decreased VWF synthesis and/or secretion.39 Interestingly, plasma FVIII:C/VWF:Ag ratios were also significantly increased in LoVIC patients compared with normal controls (mean 1.3 vs 1.07; P < .0001) (Figure 4B). Similarly, previous studies have demonstrated that an increased VWF:pp/VWF:Ag ratio >3 can be used to define patients with type 1 VWD due to enhanced VWF clearance (type 1C VWD).16,39-41 In a minority of patients with low VWF, increased plasma VWF:pp/VWF:Ag ratios were also observed (Figure 4C-D). For example, the VWF:pp/VWF:Ag ratio was >2 in only 18 (14%) subjects, and >3 in only 8 (6.3%) of the total LoVIC cohort (Figure 4D). However, in contrast to patients with type 1C VWD,39-41 the increase in the VWF:pp/VWF:Ag ratio observed in patients with low VWF in comparison with controls was more subtle (mean 1.44 vs 0.95, P < .01). Taken together, the FVIII:C/VWF:Ag and VWF:pp/VWF:Ag ratio data demonstrate that the reduced plasma VWF levels in patients with low VWF are predominantly attributable to decreased VWF synthesis and/or secretion rather than enhanced VWF clearance.
Reduced plasma VWF levels in patients with low VWF are due to both decreased VWF synthesis/secretion and subtly enhanced VWF clearance. (A) Plasma FVIII:C levels were significantly reduced in patients with low VWF (n = 126, triangles) compared with normal controls (n = 40, circles) (mean FVIII:C levels 76 IU/dL vs 105 IU/dL; ****P < .0001). (B) FVIII:C/VWF:Ag ratios were significantly increased in LoVIC patients compared with controls (mean ratio 1.3 vs 1.07; ****P < .0001). (C) Plasma VWF:pp levels in LoVIC patients were not significantly different from those in normal controls (mean VWF:pp levels 91.6 U/dL vs 85.4 U/dL; P = .58). (D) VWF:pp/VWF:Ag ratios were significantly increased in LoVIC patients compared with controls (mean ratio 1.42 vs 0.95; **P < .01). Elevated VWF:pp/VWF:Ag ratios >3 (green dotted line) were identified in only 8 patients. Mean values for each group are indicated by a black line. In a subgroup of 40 LoVIC patients, plasma VWF:Ag (E) and VWF:RCo (F) levels were measured at baseline and subsequently repeated at 1 hour and 4 hours following intravenous DDAVP administration.
Reduced plasma VWF levels in patients with low VWF are due to both decreased VWF synthesis/secretion and subtly enhanced VWF clearance. (A) Plasma FVIII:C levels were significantly reduced in patients with low VWF (n = 126, triangles) compared with normal controls (n = 40, circles) (mean FVIII:C levels 76 IU/dL vs 105 IU/dL; ****P < .0001). (B) FVIII:C/VWF:Ag ratios were significantly increased in LoVIC patients compared with controls (mean ratio 1.3 vs 1.07; ****P < .0001). (C) Plasma VWF:pp levels in LoVIC patients were not significantly different from those in normal controls (mean VWF:pp levels 91.6 U/dL vs 85.4 U/dL; P = .58). (D) VWF:pp/VWF:Ag ratios were significantly increased in LoVIC patients compared with controls (mean ratio 1.42 vs 0.95; **P < .01). Elevated VWF:pp/VWF:Ag ratios >3 (green dotted line) were identified in only 8 patients. Mean values for each group are indicated by a black line. In a subgroup of 40 LoVIC patients, plasma VWF:Ag (E) and VWF:RCo (F) levels were measured at baseline and subsequently repeated at 1 hour and 4 hours following intravenous DDAVP administration.
To further investigate the molecular mechanisms underlying low VWF levels, we examined plasma VWF:Ag and VWF:RCo responses following DDAVP administration in a subset of 40 LoVIC patients. In all patients studied, plasma VWF:Ag and VWF:RCo levels increased >50 IU/dL at 1 hour following DDAVP (Figure 4E-F). Moreover, in 35/40 (87.5%) of these patients, the post-DDAVP peak levels exceeded 100 IU/dL. Importantly, the response to DDAVP was also sustained. Both VWF:Ag and VWF:RCo remained >50 IU/dL after 4 hours in all subjects, and in 29/40 (72.5%), plasma VWF levels were still >100 IU/dL. These DDAVP responses demonstrate that Weibel Palade body stores of VWF are maintained in patients with low VWF, and that the regulated pathway of VWF secretion is intact. Furthermore, the sustained plasma VWF response observed following DDAVP supports the hypothesis that in most patients enhanced VWF clearance does not play a major role in the pathogenesis of low VWF levels.
We next examined the genotype of subjects with low VWF levels. We detected VWF gene sequence variations in 60.3% of the LoVIC cohort (Table 2). Importantly, previously described damaging VWF variants, or sequence variations predicted to be damaging, were observed in only 39.7% of patients with low VWF levels. Three of the 4 patients with abnormal multimers were from the same large kindred and shared the previously described p.Arg2464Cys substitution.42 Finally, 2 patients with a type 2N mutation (p.Arg854Gln) were identified.
Only 39.7% of patients with low VWF were found to have a damaging VWF gene variant
| HGVSc . | SNV . | MAF . | Mutation . | Number of patients . | Classification . |
|---|---|---|---|---|---|
| c.3692A>G c.3686T>G | rs61749368 rs61749367 | 0.0022/224 0.0024/235 | p.Asn1231Thr p.Val1229Gly | 9 | Type 1 VWD |
| c.4196G>A | rs1800382 | 0.0087/1048 | p.Arg1399His | 6 | Collagen IV and VI binding defects |
| c.4751A>G | rs1800386 | 0.0029/356 | p.Tyr1584Cys | 11 | Type 1 VWD |
| c.3797C>T | rs61749370 | 0.0007/89 | p.Pro1266Leu | 3 | Type 1 VWD |
| c.5557C>T | rs61750612 | 0.00002/2 | p.Arg1853* | 2 | Type 1 VWD |
| c.7390C>T | rs61751286 | 0.00004/5 | p.Arg2464Cys | 4 | Type 1 VWD abnormal multimers pattern |
| c.7520_7521delGG | p.Arg2507fs | 3 | Type 1 VWD | ||
| c.6187C>T | rs61750615 | 0.0127/1545 | p.Pro2063Ser | 1 | Type 1 VWD |
| c.5791C>T | p.Gln1931* | 1 | Type 1 VWD | ||
| c.2561G>A | rs41276738 | 0.0031/374 | p.Arg854Gln | 2 | Type 2N VWD |
| c.6853_6856delAGCG | p.Ser2285fs | 1 | Type 1 VWD | ||
| c.7770+1G>A | rs200770256 | splice donor variant | 1 | Damaging in all algorithms | |
| c.2515delG | p.Gly839fs | 1 | Type 1 VWD | ||
| c.8084C>G | rs76459136 | 0.0002/24 | p.Pro2695Arg | 2 | Mainly damaging in algorithms |
| c.4696C>T | rs61750112 | p.Arg1566* | 1 | Type 1 VWD | |
| c.1548T>A | p.Tyr516* | 1 | Type 1 VWD | ||
| c.7627C>T | p.Gln2543* | 2 | Type 1 VWD | ||
| c.4944delT | p.Pro1648fs | 1 | type 1 VWD | ||
| c.6553C>T | rs569962285 | 0.00003/3 | p.Arg2185Trp | 2 | Damaging in all algorithms |
| HGVSc . | SNV . | MAF . | Mutation . | Number of patients . | Classification . |
|---|---|---|---|---|---|
| c.3692A>G c.3686T>G | rs61749368 rs61749367 | 0.0022/224 0.0024/235 | p.Asn1231Thr p.Val1229Gly | 9 | Type 1 VWD |
| c.4196G>A | rs1800382 | 0.0087/1048 | p.Arg1399His | 6 | Collagen IV and VI binding defects |
| c.4751A>G | rs1800386 | 0.0029/356 | p.Tyr1584Cys | 11 | Type 1 VWD |
| c.3797C>T | rs61749370 | 0.0007/89 | p.Pro1266Leu | 3 | Type 1 VWD |
| c.5557C>T | rs61750612 | 0.00002/2 | p.Arg1853* | 2 | Type 1 VWD |
| c.7390C>T | rs61751286 | 0.00004/5 | p.Arg2464Cys | 4 | Type 1 VWD abnormal multimers pattern |
| c.7520_7521delGG | p.Arg2507fs | 3 | Type 1 VWD | ||
| c.6187C>T | rs61750615 | 0.0127/1545 | p.Pro2063Ser | 1 | Type 1 VWD |
| c.5791C>T | p.Gln1931* | 1 | Type 1 VWD | ||
| c.2561G>A | rs41276738 | 0.0031/374 | p.Arg854Gln | 2 | Type 2N VWD |
| c.6853_6856delAGCG | p.Ser2285fs | 1 | Type 1 VWD | ||
| c.7770+1G>A | rs200770256 | splice donor variant | 1 | Damaging in all algorithms | |
| c.2515delG | p.Gly839fs | 1 | Type 1 VWD | ||
| c.8084C>G | rs76459136 | 0.0002/24 | p.Pro2695Arg | 2 | Mainly damaging in algorithms |
| c.4696C>T | rs61750112 | p.Arg1566* | 1 | Type 1 VWD | |
| c.1548T>A | p.Tyr516* | 1 | Type 1 VWD | ||
| c.7627C>T | p.Gln2543* | 2 | Type 1 VWD | ||
| c.4944delT | p.Pro1648fs | 1 | type 1 VWD | ||
| c.6553C>T | rs569962285 | 0.00003/3 | p.Arg2185Trp | 2 | Damaging in all algorithms |
Variants were identified in low VWF cohort by custom genetic array (see “Methods”) that causes unequivocally VWD or are predictively damaging in VWF by bioinformatics algorithms. p.Asn1231Thr and p.Val1229Gly possibly occur on the same haplotype. Minor allele frequencies (MAF) were obtained from ExAC browser (http://exac.broadinstitute.org).
HGVSc, Human Genome Variation Society notation in the complementary DNA.
Termination or stop codon.
Platelet VWF levels are reduced in patients with low VWF
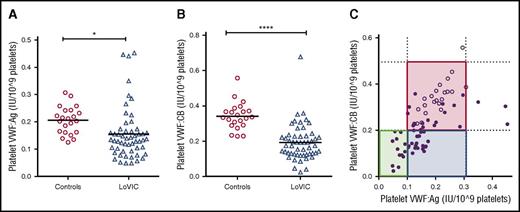
Platelet (plt)-VWF accounts for 10% to 20% of the total VWF:Ag present in normal platelet-rich plasma and is enriched in hemostatically active high-molecular-weight multimers.43,44 To determine whether quantitative and/or qualitative abnormalities in plt-VWF may influence bleeding phenotype in patients with low VWF, we studied plt-VWF:Ag and VWF:CB in 49 consecutive patients enrolled in the LoVIC study. In keeping with the hypothesis that reduced VWF synthesis plays a key role in the pathogenesis underlying low VWF levels, plt-VWF:Ag and plt-VWF:CB levels were both significantly reduced in LoVIC patients compared with healthy controls (mean plt-VWF:Ag 0.16 IU/109 platelet vs 0.21 IU/109 platelet, P < .05; mean plt-VWF:CB 0.19 IU/109 platelet vs 0.34 IU/109 platelet; P < .0001) (Figure 5A-B). Mannucci et al previously described 3 distinct subgroups (“platelet-low,” “platelet-normal,” and “platelet-discordant,” respectively) based upon plt-VWF:Ag and plt-VWF activity levels in a study of 17 patients with type 1 VWD.44,45 Interestingly, we identified these same 3 subgroups in patients with low VWF (Figure 5C). However, although previous studies suggested that platelet VWF levels may influence bleeding risk in patients with type 1 VWD (such that bleeding is attenuated in “platelet-normal” patients),45-47 we observed no significant increase in bleeding for low VWF patients with reduced compared with normal plt-VWF:Ag or plt-VWF:CB levels (supplemental Figure 3A-B). Thus, although plt-VWF levels may be informative in terms of elucidating the biology underlying low VWF, it is not clear that they significantly impact upon bleeding phenotype.
Platelet VWF levels are significantly reduced in patients with low VWF. Following platelet lysis, platelet VWF:Ag (A) and platelet VWF:CB levels (B) were determined in 54 patients with low VWF levels. (A) Platelet VWF:Ag was significantly reduced in LoVIC patients (n = 54, triangles) compared with controls (n = 22, circles) (mean values 0.16 vs 0.21 IU/109 platelets; *P < .05). (B) Platelet VWF:CB levels were also significantly reduced in LoVIC patients (triangles) compared with controls (circles) (mean values 0.19 vs 0.34 IU/109 platelets; P < .0001). (C) On the basis of platelet VWF:Ag and platelet VWF:CB levels, patients with low VWF levels were subdivided into 3 distinct groups of “platelet-low” (green box), “platelet-normal” (red box), and “platelet-discordant” (blue box). LoVIC patients are indicated as filled circles and healthy controls as open circles.
Platelet VWF levels are significantly reduced in patients with low VWF. Following platelet lysis, platelet VWF:Ag (A) and platelet VWF:CB levels (B) were determined in 54 patients with low VWF levels. (A) Platelet VWF:Ag was significantly reduced in LoVIC patients (n = 54, triangles) compared with controls (n = 22, circles) (mean values 0.16 vs 0.21 IU/109 platelets; *P < .05). (B) Platelet VWF:CB levels were also significantly reduced in LoVIC patients (triangles) compared with controls (circles) (mean values 0.19 vs 0.34 IU/109 platelets; P < .0001). (C) On the basis of platelet VWF:Ag and platelet VWF:CB levels, patients with low VWF levels were subdivided into 3 distinct groups of “platelet-low” (green box), “platelet-normal” (red box), and “platelet-discordant” (blue box). LoVIC patients are indicated as filled circles and healthy controls as open circles.
Discussion
Data from large recent cohort studies have significantly advanced our understanding of the pathophysiology underpinning type 1 VWD. In particular, these findings have refined diagnosis and management for patients with type 1 VWD (plasma VWF:Ag levels <30 IU/dL). These patients typically have dominant-negative VWF gene missense mutations and present with a significant bleeding phenotype that has high heritability. In contrast, however, diagnosis and treatment of patients with modestly reduced plasma VWF levels in the range of 30 to 50 IU/dL continue to pose significant clinical challenges.21,23 Although recent expert consensus guidelines have recommended amendment of the ISTH VWD guidelines to differentiate between type 1 VWD and low VWF,19,20,48 individual guidelines have proposed different plasma VWF thresholds for assigning a diagnosis of low VWF (either 30-4048 or 30-50 IU/dL,19,20 respectively). Furthermore, because the molecular mechanisms underlying low VWF have not been defined, genetic counseling and defining optimal treatment strategies remain problematic for this large group of patients. The LoVIC study is the first cohort study to focus specifically on patients with low VWF. Our findings demonstrate that significant bleeding complications are present in the majority of patients with low VWF. Moreover, we further show that this bleeding can be objectively assessed using either the ISTH BAT or the Condensed MCMDM-1 VWD score. On the basis of these data, we propose that diagnosis of low VWF should therefore involve (1) a confirmed bleeding phenotype objectively verified by the ISTH BAT and (2) reduced plasma VWF levels (VWF:Ag and/or VWF:RCo and/or VWF:CB) consistently in the range of 30 to 50 IU/dL.
The observation that patients registered with low VWF levels have significant bleeding phenotypes has important clinical implications. In particular, it is interesting that the highest scores were observed in the menorrhagia domain using both BATs. For example, almost two-thirds of all female patients in the cohort had required hormonal treatment of menorrhagia. Further studies will be required to determine the mechanisms underlying the etiology of this significant menorrhagia in women with low VWF. Importantly, however, we also found that, even if gynecological bleeding (menorrhagia and postpartum hemorrhage combined) scores were removed, bleeding scores still remained significantly elevated in female patients with low VWF levels. Because the normal plasma VWF range is 50 to 200 IU/dL, by definition 2.5% of the general population will have plasma VWF levels <50 IU/dL. Thus, in Ireland alone, an estimated 115 000 individuals will have VWF levels <50 IU/dL. In contrast, however, a total of only 476 Irish patients (283 adults and 193 children) are currently registered with low VWF levels. Given these findings, there are clearly additional adults and children with low VWF levels present in the Irish population who have not yet come to medical attention, including some individuals who may not have a significant bleeding phenotype despite their reduced VWF levels. Collectively, these data suggest that, although low VWF levels in the 30 to 50 IU/dL range contribute to bleeding risk, nevertheless moderately reduced VWF levels alone are not sufficient to cause a bleeding phenotype. Rather, our data support the hypothesis that additional factors beyond plasma VWF levels contribute to bleeding phenotype in the LoVIC cohort.
Although reduced FVIII:C levels contribute to bleeding risk in some patients with VWD, plasma FVIII:C levels were normal in 82% of our low VWF cohort. In addition, VWF multimer patterns were unremarkable in the vast majority of LoVIC patients with bleeding phenotypes. Of note, we observed only 4 patients with abnormal multimer patterns, all of whom shared the same mutation (p.Arg2464Cys).42 Finally, previous studies have reported that other hemostatic abnormalities may be present in patients with moderate reductions in VWF levels that present with significant bleeding.49-52 In contrast, we identified concomitant coagulation abnormalities in only 11% of LoVIC patients with a bleeding phenotype. Moreover, these additional coagulation defects were mild, and importantly, there was no significant difference in bleeding scores for low VWF patients with or without concomitant hemostatic abnormalities. Altogether, these data highlight that the pathophysiology underlying the significant bleeding phenotype in most patients with low VWF levels remains poorly understood. However, given the mucocutaneous nature of the bleeding complications observed, it seems likely that these patients may have additional disorders (eg, involving primary hemostasis, thrombin generation, or fibrinolysis) such that their bleeding is due only in part to a moderate reduction in VWF levels.49 Additional studies will be needed to elucidate the molecular mechanisms responsible for the bleeding diathesis in patients with low VWF and the importance of ABO blood group in this context. Nevertheless, it seems likely that these mechanisms may overlap with those involved in patients registered with “Bleeding Disorders of Unknown Cause.” In this context, it is interesting that plasma VWF antigen and activity levels increased significantly with age in patients with low VWF, and in some patients, corrected to within the normal range. Given that patients with low VWF likely have additional undefined factors contributing to the etiology of their bleeding phenotype, further studies will be required to determine whether this normalization in plasma VWF levels will necessarily result in correction of bleeding phenotype in all patients.
Recent studies have clearly demonstrated that enhanced VWF clearance plays an important role in the pathogenesis of both type 1 and type 2 VWD.39-41 In contrast, we found that increased plasma VWF:pp/VWF:Ag ratios were present in only a minority of patients with low VWF (6% patients with ratio >3). However, the FVIII:C/VWF:Ag ratio was significantly increased in LoVIC patients. Furthermore, platelet VWF:Ag was also significantly reduced in the low VWF cohort compared with normal individuals. Collectively, these novel findings suggest that low VWF levels are due in part to reductions in VWF synthesis and/or constitutive secretion. Although enhanced VWF clearance may contribute to the pathophysiology in some individuals with low VWF, the absolute reduction in VWF plasma half-life is usually mild and not sufficient to significantly impact upon the duration of DDAVP-induced VWF response. Our findings therefore confirm that DDAVP can be used successfully to correct both VWF antigen and activity levels in patients with low VWF. Furthermore, these data suggest that little is to be gained from performing DDAVP trials at diagnosis in these patients. Rather, VWF responses could simply be confirmed following first use of DDAVP treatment.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by an Irish Health Research Board Health Research Award (HRA-POR-2014-529) (J.S.O’D.) and a Science Foundation Ireland Principal Investigator Award (11/PI/1066) (J.S.O’D.).
Authorship
Contribution: M.L., S.A., S.S., N.D., K.L.J., J.M.O’S., M.R., M.M.D., M.N., and U.B. performed experiments; M.L., S.A., N.M.O’C., K.R., B.W., M.B., K.L.J., R.J.S.P., U.B., P.J., J.D.P., and J.S.O’D. designed the research and analyzed the data; and all authors were involved in writing and reviewing the paper.
Conflict-of-interest disclosure: M.L. has received research funding from Baxalta and has served on advisory boards for Baxalta. P.J. receives research funding from CSL Behring, Octapharma, and Shire and has served on advisory boards for CSL Behring and Shire. J.S.O’D. has served on the speaker’s bureau for Baxter, Bayer, Novo Nordisk, Boehringer Ingelheim, Leo Pharma, and Octapharma and has also served on the advisory boards of Baxter, Bayer, Octapharma CSL Behring, Daiichi Sankyo, Boehringer Ingelheim, and Pfizer. J.S.O’D. has received research grant funding awards from Baxter, Bayer, Pfizer, and Novo Nordisk. The remaining authors declare no competing financial interests.
Correspondence: James S. O’Donnell, Irish Centre for Vascular Biology, Royal College of Surgeons in Ireland, Ardilaun House, 111 St. Stephen's Green, Dublin 2, Ireland; e-mail jamesodonnell@rcsi.ie.
References
Author notes
M.L. and S.A. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal