Key Points
Checkpoint blockade via anti–PD-1 mAbs was associated with a high overall response rate in relapsed Hodgkin lymphoma allo-HCT patients.
Checkpoint blockade via anti–PD-1 mAbs after allo-HCT can be complicated by rapid onset of severe and treatment-refractory GVHD.
Abstract
Given the limited treatment options for relapsed lymphoma post–allogeneic hematopoietic cell transplantation (post–allo-HCT) and the success of programmed death 1 (PD-1) blockade in classical Hodgkin lymphoma (cHL) patients, anti–PD-1 monoclonal antibodies (mAbs) are increasingly being used off-label after allo-HCT. To characterize the safety and efficacy of PD-1 blockade in this setting, we conducted a multicenter retrospective analysis of 31 lymphoma patients receiving anti–PD-1 mAbs for relapse post–allo-HCT. Twenty-nine (94%) patients had cHL and 27 had ≥1 salvage therapy post–allo-HCT and prior to anti–PD-1 treatment. Median follow-up was 428 days (range, 133-833) after the first dose of anti–PD-1. Overall response rate was 77% (15 complete responses and 8 partial responses) in 30 evaluable patients. At last follow-up, 11 of 31 patients progressed and 21 of 31 (68%) remain alive, with 8 (26%) deaths related to new-onset graft-versus-host disease (GVHD) after anti–PD-1. Seventeen (55%) patients developed treatment-emergent GVHD after initiation of anti–PD-1 (6 acute, 4 overlap, and 7 chronic), with onset after a median of 1, 2, and 2 doses, respectively. GVHD severity was grade III-IV acute or severe chronic in 9 patients. Only 2 of these 17 patients achieved complete response to GVHD treatment, and 14 of 17 required ≥2 systemic therapies. In conclusion, PD-1 blockade in relapsed cHL allo-HCT patients appears to be highly efficacious but frequently complicated by rapid onset of severe and treatment-refractory GVHD. PD-1 blockade post–allo-HCT should be studied further but cannot be recommended for routine use outside of a clinical trial.
Introduction
Allogeneic hematopoietic cell transplantation (allo-HCT) can be a curative therapy for a subset of advanced lymphoma patients, including those with relapsed classical Hodgkin lymphoma.1-3 Although allo-HCT may elicit immunological graft-versus-tumor (GVT) effects, these immune responses can be misdirected toward normal host organs, resulting in acute and chronic graft-versus-host disease (GVHD). The pathophysiology of acute GVHD (aGVHD) and chronic GVHD (cGVHD) involves the activation and proliferation of donor T cells.4,5
The programmed death 1 (PD-1) pathway serves as a checkpoint to limit T-cell–mediated immune responses. Blocking the PD-1 receptor on T cells results in T-cell activation and proliferation and can induce a potent immunotherapeutic antitumor effect.6-8 The therapeutic efficacy of monoclonal antibodies (mAbs) targeting the PD-1 receptor in classical Hodgkin lymphoma (cHL) has been demonstrated in recent publications.9-11 Anti–PD-1 mAbs are also being investigated across other lymphoma subtypes and malignancies.12 Preclinical studies show that PD-1 blockade can augment the GVT effect when given posttransplant.13-15 Given the limited treatment options for lymphoma patients relapsing post–allo-HCT and the promising clinical and preclinical studies with PD-1 blockade, many clinicians are considering off-label use in this setting. However, PD-1 blockade in a murine allo-HCT aGVHD model was shown to exacerbate GVHD-related mortality.16,17
There have been a few cases of severe and even fatal transplant-related complications, including GVHD, when anti–PD-1 mAbs were given for disease control prior to allo-HCT,18 which has led to a package insert warning.19 Less is known about the safety and efficacy of anti–PD-1 mAbs when administered after allo-HCT. To date, most case reports20-28 and 2 case series29,30 suggest it can be given safely and is effective. However, due to concerns about small numbers of patients and the possibility of reporting bias, we conducted a large multicenter retrospective study to better characterize the risks and benefits of PD-1 blockade after allo-HCT. Our main objectives were to (1) collect data on the efficacy of PD-1 blockade after allo-HCT and (2) evaluate the risk of GVHD after PD-1 blockade in patients who have undergone allo-HCT and identify any associated risk factors.
Methods
We contacted 10 US transplant programs with the highest volumes of lymphoma patients undergoing allo-HCT (during 2014-2015), as provided by the Center for International Blood and Marrow Transplant Research, to query individual transplant centers’ use of PD-1 mAbs in lymphoma patients after an allo-HCT. Additional sites were contacted based on recommendation from these initial 10 sites. In total, 23 US transplant centers were surveyed, and 15 of the 23 centers reported treating ≥1 patient with anti–PD-1 mAbs after allo-HCT for relapsed lymphoma. All sites obtained institutional review board approval for the retrospective chart review. None of the patients received anti–PD-1 mAbs prior to allo-HCT. Medical records of all patients were reviewed by participating investigators. Baseline (pretransplant) and treatment variables were collected, along with last follow-up and cause of death. Response assessments to anti–PD-1 mAbs were determined by the treating provider according to revised Lugano criteria (though not centrally).31 Treatment-emergent (acute or chronic) GVHD was defined as GVHD that developed after starting anti–PD-1 and meeting one of the following criteria: (1) new-onset GVHD in a patient without prior history of GVHD or (2) recurrence of GVHD in a patient with prior history of GVHD and requiring a new GVHD-directed treatment. aGVHD and cGVHD stage/grade and responses were scored according to Consensus and National Institutes of Health (NIH) criteria, respectively.32,33 All GVHD scoring and response data were performed independently by the patients’ treating physicians. Specimens from biopsies to confirm GVHD were reviewed by pathologists at participating institutions. Deaths from GVHD were defined according to recent guidelines published by the Center for International Blood and Marrow Transplant Research.34
Descriptive statistics were used to summarize clinical outcomes. Continuous variables were compared using Wilcoxon rank sum test. Categorical variables were compared using χ2 test (or Fisher’s exact test in instances where observed counts were <5 for any of the categorizations). Individuals who did not develop GVHD after anti–PD-1 treatment were compared with individuals who developed GVHD after anti–PD-1 treatment. Cumulative incidence curves were created, and median and mean survival estimates were calculated using Kaplan-Meier product-limit estimates for both progression-free and overall survival.
Results
Patient characteristics prior to treatment with anti–PD-1
We identified 31 lymphoma patients (29 cHL, 1 transformed follicular lymphoma, and 1 with follicular lymphoma and cHL) treated with an anti–PD-1 mAb (28 nivolumab and 3 pembrolizumab) for relapsed disease after allo-HCT. Patients were generally treated according to the prescribing information on the package label (3 mg/kg nivolumab every 2 weeks and 200 mg pembrolizumab every 3 weeks) with adjustments after initial dose(s) for adverse events per provider discretion. Table 1 highlights patient characteristics prior to treatment with anti–PD-1. Median age at time of transplantation was 37 years (range, 20-68). Eighteen patients (58%) were male and 25 (81%) were white. Thirty of 31 patients (97%) received a reduced-intensity conditioning regimen. Donor source varied with 16 (52%) matched sibling, 10 (32%) matched unrelated donors, 1 (3%) mismatch unrelated donor, and 4 (13%) haploidentical related donors. Graft sources included peripheral blood (n = 29) or bone marrow (n = 2). All patients received calcineurin inhibitor (CNI)–based GVHD prophylaxis. Five patients additionally received posttransplant cyclophosphamide (PTCy) (4 haploidentical and 1 matched unrelated donor). Twenty-seven patients (87%) received at least 1 salvage therapy (median, 2; range, 0-6) post–allo-HCT relapse and before initiating anti–PD-1 (see supplemental Table 1, available on the Blood Web site). These salvage therapies included brentuximab vedotin (n = 16, 52%), lenalidomide (n = 3, 10%), donor lymphocyte infusion (DLI) (n = 5, 16%), and ipilimumab (n = 3, 10%). Chimerism analysis prior to anti–PD-1 treatment was complete donor cell chimeric (ie, ≥95% T-cell donor cells) in 27 of 29 (93%) patients. Two patients were mixed donor cell chimeric, and in 2 patients, chimerism information was not available. Patients started anti–PD-1 treatment a median of 2.2 years after transplantation (range, 4.8 months to 9 years). Nineteen of 31 patients (61%) had experienced GVHD prior to administration of anti–PD-1, but in the majority, it was not active at the time of anti–PD-1 initiation. Eight patients (26%) were still receiving immunosuppressive therapy at the time of anti–PD-1 administration.
Characteristics of 31 patients treated with anti–PD-1 mAbs for relapsed disease after allo-HCT
| . | All patients (N = 31) . | No GVHD after PD-1 (n = 14) . | Developed GVHD after PD-1 (n = 17) . | P . |
|---|---|---|---|---|
| Age (y) at time of allo-HCT, median (range) | 37 (20-68) | 36 (20-56) | 37 (21-68) | .57* |
| Male | 18 (58) | 8 (57) | 10 (59) | 1.0† |
| White | 25 (81) | 10 (71) | 15 (88) | .37† |
| Disease type | .49‡ | |||
| Hodgkin’s lymphoma | 29 (94) | 14 (100) | 15 (88) | |
| Other lymphoma | 2 (6) | 0 | 2 (12) | |
| Donor type | .02‡ | |||
| Matched sibling | 16 (52) | 4 (28) | 12 (71) | |
| Matched unrelated | 10 (32) | 5 (36) | 5 (29) | |
| Mismatch unrelated | 1 (3) | 1 (7) | 0 | |
| Haploidentical related | 4 (13) | 4 (29) | 0 | |
| Peripheral blood graft source | 29 (94) | 12 (86) | 17 (100) | .20‡ |
| Reduced intensity conditioning | 30 (97) | 14 (100) | 17 (94) | 1.0‡ |
| Prior history of GVHD before to PD-1 | 19 (61) | 7 (50) | 12 (71) | .29† |
| aGVHD prior to PD-1 | 6 (19) | 3 (21) | 3 (17) | .79‡ |
| cGVHD prior to PD-1 | 15 (48) | 6 (43) | 9 (53) | .58‡ |
| Receiving systemic IST at time of PD-1 | 8 (26) | 5 (36) | 3 (18) | .41‡ |
| Complete donor cell chimeric (ie, ≥95% T-cell donor cells) | 27 (93)§ | 12 (86) | 15 (88) | 1.0‡ |
| Days from allo-HCT to administration of anti–PD-1, median (range) | 790 (146-3289) | 920 (146-3289) | 740 (196-1902) | .45* |
| PD-1 treatment | 1.0‡ | |||
| Nivolumab | 28 (90) | 13 (93) | 15 (88) | |
| Pembrolizumab | 3 (10) | 1 (7) | 2 (12) | |
| Disease responded (CR 1 PR) to anti–PD-1 treatment | 23 (77)|| | 11 (79) | 12 (71) | .70‡ |
| Complete responders | 15 (50)|| | 6 (43) | 9 (53) | .58† |
| . | All patients (N = 31) . | No GVHD after PD-1 (n = 14) . | Developed GVHD after PD-1 (n = 17) . | P . |
|---|---|---|---|---|
| Age (y) at time of allo-HCT, median (range) | 37 (20-68) | 36 (20-56) | 37 (21-68) | .57* |
| Male | 18 (58) | 8 (57) | 10 (59) | 1.0† |
| White | 25 (81) | 10 (71) | 15 (88) | .37† |
| Disease type | .49‡ | |||
| Hodgkin’s lymphoma | 29 (94) | 14 (100) | 15 (88) | |
| Other lymphoma | 2 (6) | 0 | 2 (12) | |
| Donor type | .02‡ | |||
| Matched sibling | 16 (52) | 4 (28) | 12 (71) | |
| Matched unrelated | 10 (32) | 5 (36) | 5 (29) | |
| Mismatch unrelated | 1 (3) | 1 (7) | 0 | |
| Haploidentical related | 4 (13) | 4 (29) | 0 | |
| Peripheral blood graft source | 29 (94) | 12 (86) | 17 (100) | .20‡ |
| Reduced intensity conditioning | 30 (97) | 14 (100) | 17 (94) | 1.0‡ |
| Prior history of GVHD before to PD-1 | 19 (61) | 7 (50) | 12 (71) | .29† |
| aGVHD prior to PD-1 | 6 (19) | 3 (21) | 3 (17) | .79‡ |
| cGVHD prior to PD-1 | 15 (48) | 6 (43) | 9 (53) | .58‡ |
| Receiving systemic IST at time of PD-1 | 8 (26) | 5 (36) | 3 (18) | .41‡ |
| Complete donor cell chimeric (ie, ≥95% T-cell donor cells) | 27 (93)§ | 12 (86) | 15 (88) | 1.0‡ |
| Days from allo-HCT to administration of anti–PD-1, median (range) | 790 (146-3289) | 920 (146-3289) | 740 (196-1902) | .45* |
| PD-1 treatment | 1.0‡ | |||
| Nivolumab | 28 (90) | 13 (93) | 15 (88) | |
| Pembrolizumab | 3 (10) | 1 (7) | 2 (12) | |
| Disease responded (CR 1 PR) to anti–PD-1 treatment | 23 (77)|| | 11 (79) | 12 (71) | .70‡ |
| Complete responders | 15 (50)|| | 6 (43) | 9 (53) | .58† |
Values represent n (%) of patients unless otherwise indicated. PD-1 indicates nivolumab or pembrolizumab.
IST, immunosuppressive therapy.
Wilcoxon rank sum test.
χ2 test.
Fisher’s exact test.
Two unknown; of the 2 patients with mixed chimera prior to anti–PD-1 treatment, 1 patient developed aGVHD.
One unknown.
Disease responses after anti–PD-1 treatment
Median follow-up for survivors was 428 days (range, 133-833) after the first dose of anti–PD-1, with 21 of 31 (68%) patients alive at the last follow-up. Patients received a median of 2 doses of anti–PD-1 (range, 1-31). Overall response rate (ORR = complete response [CR] + partial response [PR]) to anti–PD-1 was 77% (95% confidence interval, 58% to 90%) in the 30 response assessable patients (15 CRs, 8 PRs, 3 stable disease [SD], and 4 progressive disease). The 1 patient without cHL attained SD. Thus, among the 30 patients with cHL, the ORR was 79%. At last follow-up, 11 of 31 patients progressed, including 2 cHL patients who achieved a CR after anti–PD-1. Median progression-free survival was 591 days (95% confidence interval, 400-644). Median overall survival could not be calculated, because 21 of the 31 patients were still alive at study conclusion (mean, 400 days) (supplemental Figure 1) In the 9 patients who had chimerism testing after anti–PD-1 treatment, all remained fully chimeric. The 2 patients with mixed chimerism prior to anti–PD-1 treatment were not tested again afterward.
Risk of treatment-emergent GVHD after anti–PD-1 treatment
Seventeen patients (55%) developed GVHD after the initiation of anti-PD-1 treatment (6 aGVHD, 4 overlap, and 7 cGVHD; supplemental Tables 2 and 3). Median number of doses prior to onset of aGVHD, overlap, and cGVHD was 1 (range, 1-2), 2 (range, 1-2), and 2 (range, 1-25), respectively. Only 1 patient with treatment-emergent GVHD received >2 doses of anti–PD-1 prior to GVHD onset (patient 12 received 25 doses). Median days to aGVHD, overlap, and cGVHD onset after administration of anti–PD-1 was 16 (range, 8-28), 21 (range, 12-28), and 14 (range, 1-350), respectively.
We compared baseline clinical characteristics to determine associated risk factors for developing treatment-emergent GVHD after anti–PD-1 treatment (Table 1). GVHD after anti–PD-1 treatment was observed most frequently in matched sibling donor transplants. None of the haploidentical related transplants (n = 4) developed GVHD after anti–PD-1 treatment; all 4 received PTCy in addition to CNI as GVHD prevention. The one unrelated donor recipient who received PTCy developed severe cGVHD 14 days after anti–PD-1. Receipt of immune modulating therapies post–allo-HCT and prior to anti–PD-1, including lenalidomide, DLI, and ipilimumab, did not appear to be associated with increased risk of GVHD post anti–PD-1, although the numbers were small (supplemental Table 1). Two of 3 patients who received ipilimumab had GVHD (both chronic) in CR prior to anti-PD-1. One developed GVHD after anti–PD-1 (patient 12). Of the 5 patients who received a DLI, 3 had GVHD (2 acute, 1 chronic) in CR prior to anti–PD-1, and these 3 patients developed GVHD after PD-1 blockade (patients 4, 10, and 12). Eight of 31 (26%) patients were receiving systemic immunosuppression at the time of anti–PD-1 administration; 3 of these 8 patients developed GVHD after anti–PD-1 treatment (P = .41). Disease response was also not associated with increased risk of GVHD (P = .7).
Prior to administration of anti–PD-1, 19 of 31 patients (61%) had experienced GVHD (4 aGVHD, 2 aGVHD followed by cGVHD, and 13 cGVHD). The majority of the 19 with a history of GVHD were in CR and off immunosuppression at time of anti–PD-1 administration (13 CR, 4 PR, and 2 SD). Twelve of the 19 patients (63%) with a history of GVHD prior to anti–PD-1 treatment developed treatment-emergent GVHD after receiving anti–PD-1 (P = .29). All 4 patients with aGVHD prior to anti–PD-1 treatment were in CR upon initiation of PD-1 blockade. Among these 4 patients, 3 (75%) developed GVHD after anti–PD-1 treatment (1 aGVHD and 2 overlap). Neither of the 2 patients with a history of both aGVHD and cGVHD (both in CR) developed treatment-emergent GVHD after anti–PD-1 treatment. For the 13 with a history of only cGVHD, 9 developed treatment-emergent GVHD after anti–PD-1 treatment (2 aGVHD, 2 overlap, and 5 cGVHD). Six had active cGVHD (PR + SD) at time of anti–PD-1 administration; 3 of these 6 patients (50%) developed treatment-emergent worsening of GVHD after anti–PD-1 treatment (1 aGVHD, 1 overlap, and 1 cGVHD; patients 2, 6, and 11). Notably, there were 5 patients without a history of GVHD who developed treatment-emergent GVHD after anti–PD-1 treatment (patients 3, 7, 9, 13, and 15).
Stage/grade and site of aGVHD and cGVHD after anti–PD-1 administration
We recorded time of onset, site involved, and stage of aGVHD and cGVHD developing after anti–PD-1 treatment (Figure 1; Tables 2 and 3). Ten of 31 (32%) patients developed treatment-emergent aGVHD (including 4 with overlap), which was severe in 6 (19%) (5 grade IV and 1 grade III). Eight of these 10 patients had aGVHD confirmed by biopsy after anti–PD-1 administration. Three of 4 cases with stage IV liver aGVHD were biopsy proven. The most common sites of aGVHD were liver and skin.
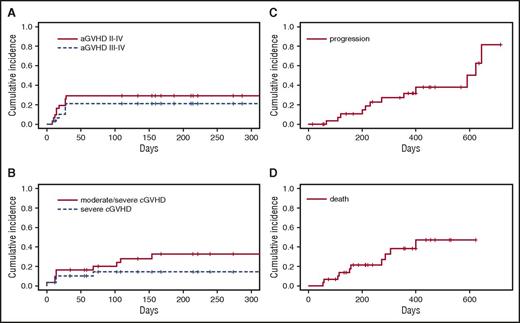
Cumulative incidence of clinically significant events after anti–PD-1 administration. (A) Time from anti–PD-1 administration to development of treatment-emergent grade II-IV and grade III-IV aGVHD. (B) Time from anti–PD-1 administration to treatment-emergent moderate/severe and severe cGVHD. (C) Time from anti–PD-1 administration to lymphoma progression. (D) Time from anti–PD-1 administration to death. aGVHD and cGVHD stage/grade and responses were scored according to Consensus and NIH criteria, respectively.
Cumulative incidence of clinically significant events after anti–PD-1 administration. (A) Time from anti–PD-1 administration to development of treatment-emergent grade II-IV and grade III-IV aGVHD. (B) Time from anti–PD-1 administration to treatment-emergent moderate/severe and severe cGVHD. (C) Time from anti–PD-1 administration to lymphoma progression. (D) Time from anti–PD-1 administration to death. aGVHD and cGVHD stage/grade and responses were scored according to Consensus and NIH criteria, respectively.
Frequency of treatment-emergent aGVHD by site of disease after anti–PD-1 administration
| . | aGVHD stage . | |||
|---|---|---|---|---|
| Stage 1 (n = 4) . | Stage 2 (n = 1) . | Stage 3 (n = 8) . | Stage 4 (n = 6) . | |
| Skin | 1 | 1 | 5 | 1 |
| GI | 0 | 0 | 1 | 1 |
| Liver | 3 | 0 | 2 | 4 |
| . | aGVHD stage . | |||
|---|---|---|---|---|
| Stage 1 (n = 4) . | Stage 2 (n = 1) . | Stage 3 (n = 8) . | Stage 4 (n = 6) . | |
| Skin | 1 | 1 | 5 | 1 |
| GI | 0 | 0 | 1 | 1 |
| Liver | 3 | 0 | 2 | 4 |
aGVHD stage/grade and responses were scored according to Consensus criteria. Each episode of GVHD was recorded as either acute or chronic, although some patients experienced aGVHD and cGVHD, which were counted as mutually exclusive events.
GI, gastrointestinal.
Frequency of treatment-emergent cGVHD by site of disease after anti–PD-1 administration
| . | cGVHD score . | ||
|---|---|---|---|
| 1 (mild); n = 11 . | 2 (moderate); n = 10 . | 3 (severe); n = 5 . | |
| Skin | 4 | 3 | 0 |
| GI | 1 | 3 | 0 |
| Liver | 2 | 1 | 2 |
| Ocular | 2 | 1 | 1 |
| Oral | 2 | 1 | 2 |
| Joints/fascia | 0 | 1 | 0 |
| . | cGVHD score . | ||
|---|---|---|---|
| 1 (mild); n = 11 . | 2 (moderate); n = 10 . | 3 (severe); n = 5 . | |
| Skin | 4 | 3 | 0 |
| GI | 1 | 3 | 0 |
| Liver | 2 | 1 | 2 |
| Ocular | 2 | 1 | 1 |
| Oral | 2 | 1 | 2 |
| Joints/fascia | 0 | 1 | 0 |
cGVHD stage/grade and responses were scored according to NIH criteria. Each episode of GVHD was recorded as either acute or chronic, although some patients experienced aGVHD and cGVHD, which were counted as mutually exclusive events.
Eleven of 31 (35%) patients developed treatment-emergent cGVHD after anti–PD-1 administration (including 4 with overlap), which was severe in 4 (13%) (4 severe, 4 moderate, and 3 mild). Of the 11 patients developing cGVHD after anti–PD-1 administration, only 2 developed cGVHD after anti–PD-1 treatment without any history of GVHD (at 102 and 108 days after initiating anti–PD-1 treatment). The most common sites of cGVHD were liver and skin.
Response to treatment of GVHD occurring after anti–PD-1 administration
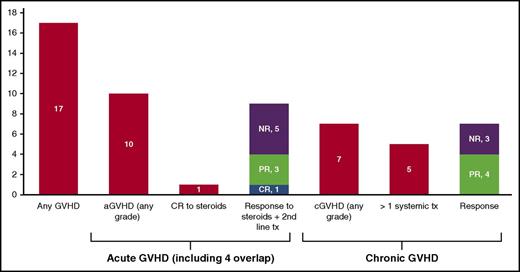
Of the 10 patients who developed aGVHD (including 4 with overlap), 9 required a second systemic therapy after inadequate response to steroids alone. Ultimately, 5 of 10 (50%) responded to treatment (2 CRs and 3 PRs) (Figure 2). The median number of systemic therapies for aGVHD was 3 (range, 1-4). Systemic treatments for aGVHD included steroids (n = 10, 100%), CNI (n = 6, 60%), mycophenolate mofetil (n = 4, 40%), extracorporeal photopheresis (n = 2, 20%), anti–thymocyte globulin (N = 2, 20%), rituximab (n = 2, 20%), and basiliximab (n = 1, 10%). Only 1 patient responded to steroids alone, achieving a CR. The other patient who achieved a CR developed stage IV hepatic aGVHD (peak bilirubin, 20.4 mg/dL) and responded to anti–thymocyte globulin treatment after being refractory to steroids and mycophenolate mofetil. The 3 patients with partial responses all required second line systemic therapy in addition to steroids but are alive at a median follow-up of 255 days (range, 129-263) from onset of aGVHD. Four of 5 nonresponders died (patients 4, 6, 7, and 10). The primary cause of death in all 4 patients was GVHD; the secondary cause of death in 2 patients was progressive disease. An additional patient died of multiorgan failure after 6 doses of anti–PD-1. This patient had a history of cGVHD prior to anti–PD-1 and was in partial remission on immunosuppression at time of anti–PD-1 administration. After anti–PD-1 treatment, her course was complicated by adrenal crisis, bilateral pleural effusions, and severe gastrointestinal bleed leading to death. There was no treatment-emergent cGVHD after anti–PD-1 administration, and at autopsy, these events could not be attributed to GVHD or disease.
Frequency of treatment-emergent aGVHD and cGVHD and responses to treatment of GVHD. aGVHD and cGVHD stage/grade and responses were scored according to Consensus and NIH criteria, respectively. CR, complete response; NR, no response; PR, partial response; tx, treatment.
Frequency of treatment-emergent aGVHD and cGVHD and responses to treatment of GVHD. aGVHD and cGVHD stage/grade and responses were scored according to Consensus and NIH criteria, respectively. CR, complete response; NR, no response; PR, partial response; tx, treatment.
Of the 7 patients who developed cGVHD, 4 of 7 (57%) responded to treatment (4 PRs). (Figure 2) The median number of systemic therapies for cGVHD was 2 (range, 0-4). Treatments for cGVHD included systemic steroids (n = 5, 57%), CNI (n = 3, 43%), extracorporeal photopheresis (n = 3, 43%), topical or local therapy (n = 3, 43%), mycophenolate mofetil (n = 2, 29%), rituximab (n = 1, 14%), and sirolimus (n = 1, 14%). Five patients required systemic steroids in addition to a second-line systemic cGVHD therapy. One responder required topical treatment only. Four of 7 patients with treatment-emergent cGVHD died (patients 13, 14, 16, and 17). The primary cause of death in all 4 patients was GVHD; the secondary cause of death in 1 patient was progressive disease.
Discussion
Allo-HCT is an important treatment strategy for lymphoma patients,35-38 including elderly patients.39,40 As treatment-related mortality from allo-HCT continues to decline with lower-intensity preparative regimens and better supportive care,41 treatment of relapsed disease is increasingly common. Approximately 30% of allografted lymphoma patients relapse after allo-HCT,35-37,42,43 and this rate is likely higher among patients with cHL.44 Improved post–allo-HCT treatment strategies are needed. In our multicenter retrospective study, we observed an ORR of 77% (CR 50%) with 11 of 31 relapses at last follow-up in a heavily pretreated cohort (mostly cHL). In a smaller cohort of patients who relapsed post–allo-HCT, Herbaux and colleagues reported an ORR of 95% (CR 42%) in 20 cHL patients treated with nivolumab.45 In relapsed/refractory cHL phase 1/2 trials using pembrolizumab and nivolumab, the ORR ranged from 65% to 87% (CR 16% to 28%) with median progression-free survival of ∼1 year.9-11 Several murine models show that PD-1 blockade restores and enhances GVT postallograft without increased GVHD mortality.13-15 Norde et al show that checkpoint blockade post–allo-HCT reverses T-cell exhaustion and immune escape, thereby theoretically improving GVT.46 Checkpoint blockade as a method to improve GVT and decrease relapse after allo-HCT could be a rational approach for lymphoma and perhaps other hematological malignancies if it could be managed safely.
In our study, while disease response rates were high, the frequency of GVHD after anti–PD-1 treatment was also high. We observed that 17 of 31 patients (55%) developed either aGVHD or cGVHD after PD-1 blockade, and in 16 of these 17 patients, this occurred after 1 or 2 doses. Responses to treatment of GVHD were poor, with 14 of 17 patients requiring ≥2 systemic therapies, and 8 deaths were related to treatment-emergent GVHD (4 aGVHD and 4 cGVHD). Prior case reports and a case series with varying allograft donor sources, histories of GVHD, immunosuppression at time of anti–PD-1 administration, and dosing schedules (including starting at lower doses of anti–PD-1) report no cases of treatment-refractory GVHD when treated with anti–PD-1 mAbs after allograft.20-24,27-29,45 In a French cohort, treatment-emergent aGVHD occurred in 6 patients (30%) after anti–PD-1 treatment, including 2 deaths attributed to GVHD, although GVHD only occurred in patients with a history of GVHD.30 In our study, the majority of treatment-emergent GVHD occurred in patients with a prior history of GVHD; however, we observed treatment-emergent GVHD in 5 patients without a prior history of GVHD. History of GVHD and immunosuppression at time of anti–PD-1 administration were not associated with treatment-emergent GVHD after anti–PD-1 treatment (Table 1). In some murine models, checkpoint blockade administered before and within days after transplant was shown to accelerate GVHD lethality.16,47 In a cohort of 39 patients receiving anti–PD-1 prior to allo-HCT, there was a high incidence of GVHD, including 3 deaths from early aGVHD.18 In the French series, time between allo-HCT and anti–PD-1 treatment was shorter in patients who developed treatment-emergent GVHD.30 In our study, we did not observe a significant difference in GVHD frequency relative to the timing of anti–PD-1 treatment following allo-HCT, although most of our patients received anti–PD-1 ≥2 years after allo-HCT. It remains possible that anti–PD-1 administration during the earlier phase of transplantation would trigger severe GVHD secondary to decreased PD-1/PD-L1 ligation early post–allo-HCT.48 Donor source, type of GVHD prophylaxis, history of GVHD, dose and timing of anti–PD-1 therapy, and immunosuppression at time of anti–PD-1 administration should be considered as variables potentially influencing the development of GVHD in clinical trials evaluating treatment with checkpoint blockade after allo-HCT.
Of the 17 (55%) patients who developed GVHD after anti–PD-1 administration, 10 (32%) were classified as developing aGVHD (including 4 overlap), and 7 (23%) were classified as having cGVHD. The majority experienced cutaneous and hepatic GVHD, and 8 of 17 patients (47%) experienced stage ≥3 or severe cutaneous or liver GVHD (ie, rash ≥50% body surface area and bilirubin ≥6). Typically, hepatic GVHD is a rare occurrence in allo-HCT, yet we observed 5 deaths associated with hepatic GVHD. Outside the context of allo-HCT, grade 3-5 (Common Terminology Criteria for Adverse Events) hepatic (bilirubin >3× upper limit of normal) and dermatologic (rash ≥50% body surface area) toxicities from anti–PD-1 mAbs are rare.49 In phase 1 studies with nivolumab and pembrolizumab for treatment of relapsed and refractory cHL, grade 3 hepatic and dermatologic toxicities were observed in <5% of patients, and there were no drug-related grade 4-5 events.9,11 One case of fatal hepatic toxicity has been reported after anti–PD-1 mAb treatment in a lung cancer patient.50,51 While most of our patients had biopsy findings of GVHD, the clinical and histologic differences between GVHD and anti–PD-1 toxicity are not clearly defined. Nevertheless, early onset of severe and fatal events occurred more frequently than expected when anti–PD-1 mAbs were administered post–allo-HCT. Our retrospective study is also the first to show treatment-emergent cGVHD after anti–PD-1. We hypothesize that there are context-dependent functions of the PD-1/PD-L1 axis in allo-HCT, including timing of pathway activation and organ-specific immunogenicity, donor vs host expression, and peripheral tolerance, which affect risk of aGVHD and cGVHD.13,16,17 Future studies should aim to improve our understanding of the pathobiology of these events.
Multiple prior studies have investigated immune-modulating strategies to augment GVT. In previous phase 1/1b trials, 57 patients with relapsed disease (26 lymphoma) post–allo-HCT were treated with an alternate checkpoint inhibitor, ipilimumab (anti–CTLA4 mAb), with several objective responses in the lymphoma cohort.52,53 In these trials, there were 4 cases of GVHD requiring systemic treatment; all had resolution of GVHD with steroids and discontinuation of ipilimumab.52,53 This suggests there may be important differences in both safety and efficacy comparing CTLA4 to PD-1 blockade in the post–allo-HCT setting. Additional immune modulating strategies in lymphoma patients after allo-HCT include lenalidomide and DLI. Lenalidomide has antilymphoma activity,54-56 but it has also been shown to herald new-onset GVHD, although incidence and outcome is not well reported.57-59 After DLI, ∼60% of patients develop grade ≥2 GVHD, and half of these patients die of GVHD-related complications.60-62 In light of the multiple ongoing investigations using cellular/immunotherapy, comparative studies on the risks and benefits of allo-HCT and immune-modulating strategies post–allo-HCT, including checkpoint blockade, are needed to rank safety and efficacy in lymphoma patients.
Any clinical research based on retrospective review of medical records is flawed by a degree of bias, including selection bias that may have resulted in an inaccurate estimate of the incidence of GVHD after anti–PD-1. We acknowledge the potential histologic overlap between anti–PD-1 toxicity and GVHD, which may have led to an overestimation of hepatitis and dermatologic toxicity as GVHD. In our study, the majority of patients had biopsy findings consistent with GVHD, although misclassification is still possible. In regards to our assessment of safety after anti–PD-1, we only recorded the incidence of GVHD. However, it is well characterized that anti–PD-1 mAb treatment can result in immune-related toxicities, including pneumonitis, colitis, hepatitis, and other immunologic phenomenon.63 In our study, these events were not recorded, but they should be investigated in future studies. Irrespective of these limitations, multiple patients in our study experienced a clinical presentation consistent with severe rapid-onset and treatment-refractory GVHD frequently involving the liver, which is typically a rare event, and providers should be cognizant of this potentially fatal and morbid complication. Administration of anti–PD-1 after allo-HCT should be done with extreme caution and preferably within the context of a clinical trial. The reports using ipilimumab52,53 and lower doses of anti–PD-1 mAbs27,52 suggest there may be a dose-dependent or context-dependent risk of developing GVHD after checkpoint blockade. Given that preclinical studies suggest checkpoint blockade increases GVT without worsening GVHD, in combination with our high responses in this heavily pretreated cohort, a phase 1 study to determine the optimal phase 2 dosing after allo-HCT is warranted.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are deeply indebted to their patients, who risked their lives on decisions made under heavy uncertainty; some with excellent and some with devastating outcomes, but all with great courage.
This work was supported in part by National Cancer Institute of the National Institutes of Health grant L30 CA189070.
Authorship
Contribution: B.M.H. designed the research, collected, analyzed, and interpreted data, and wrote the manuscript; D.A. performed statistical analysis and edited the manuscript; M.H. designed the research, collected data, and edited the manuscript; P.A., M.E.F., R.M., M.K., A.S.K., A.S., A.M., S.G., T.S.F., P.H., R.L., L.A., M.J., A.B., S.B., V.B., D.S., S.M., R.N., Y.-B.C., and B.R.B. collected data and edited the manuscript; J.G. designed the research, collected data, and edited the manuscript; and S.M.D. designed the research, collected, analyzed, and interpreted data, and edited the manuscript.
Conflict-of-interest disclosure: M.H. received research funding from Merck. P.A. received research funding and consultancy fees from Bristol-Myers Squibb and Merck. M.K. received consultancy fees from Seattle Genetics. A.S. received research funding from American Porphyria Foundation and Astellas and an honorarium from Alexion and Spectrum. S.G. received consultancy fees from Onyx and Seattle Genetics, and research funding from Janssen. P.H. received research funding from Merck, and an honorarium from Bristol-Myers Squibb. V.B. received consultancy fees from Seattle Genetics. The remaining authors declare no competing financial interests.
Correspondence: Bradley M. Haverkos, Division of Hematology, University of Colorado School of Medicine, Denver, CO 80045; e-mail: bradley.haverkos@ucdenver.edu; and Steven M. Devine, Division of Hematology, The Ohio State University, Columbus, OH 43210; e-mail: steven.devine@osumc.edu.