Key Points
The complexity and dynamics of mutations significantly impact on response, progression, and prognosis in midostaurin-treated advSM patients.
Abstract
In advanced systemic mastocytosis (advSM), disease evolution is often triggered by KIT mutations (D816V in >80% of cases) and by additional mutations (eg, in SRSF2, ASXL1, and/or RUNX1 [S/A/Rpos in >60% of cases]). In a recently reported phase 2 study, midostaurin, a multikinase/KIT inhibitor, demonstrated an overall response rate (ORR) of 60% in advSM but biomarkers predictive of response are lacking. We evaluated the impact of molecular markers at baseline and during follow-up in 38 midostaurin-treated advSM patients. The median overall survival (OS) was 30 months (95% confidence interval, 6-54) from start of midostaurin. ORR and OS were significantly different between S/A/Rneg (n = 12) and S/A/Rpos (n = 23) patients (ORR: 75% vs 39%, P = .04; OS: P = .01, HR 4.5 [1.3-16.2]). Depending on the relative reduction of the KIT D816V expressed allele burden (EAB) at month 6, patients were classified as KIT responders (≥25%, n = 17) or KIT nonresponders (<25%, n = 11). In univariate analyses at month 6, reduction of KIT D816V EAB ≥25%, tryptase ≥50%, and alkaline phosphatase ≥50% were significantly associated with improved OS. In multivariate analysis, only KIT D816V EAB reduction ≥25% remained an independent on-treatment marker for improved OS (P = .004, HR 6.8 [1.8-25.3]). Serial next-generation sequencing analysis of 28 genes in 16 patients revealed acquisition of additional mutations or increasing variant allele frequency in K/NRAS, RUNX1, IDH2, or NPM1 associated with progression in 7 patients. In midostaurin-treated advSM patients, the complexity and dynamics of mutational profiles significantly affect response, progression, and prognosis.
Introduction
Systemic mastocytosis (SM) is a rare myeloid neoplasm characterized by clonal expansion of mast cells (MCs) in various organ systems. Advanced SM (advSM), which comprises aggressive SM (ASM), SM with an associated hematologic neoplasm (SM-AHN), and mast cell leukemia (MCL), has a poor prognosis.1-3 In patients with SM-AHN, the SM component can resemble indolent SM, ASM, or MCL.2-4
SM is characterized by somatically acquired, activating mutations in the gene encoding the receptor tyrosine kinase KIT, most commonly KIT D816V, which is seen >80% of all SM patients. Recent data, however, have highlighted that the molecular pathogenesis of advSM is complex with 1 or more additional mutations (eg, in ASXL1, CBL, JAK2, RUNX1, SRSF2, or TET2) present in >60% of advSM patients.5-7 These additional mutations are usually acquired prior to KIT D816V, thereby indicating a multimutated stem cell disease.8 The KIT D816V expressed allele burden (EAB) and the presence and number of additional molecular aberrations, notably in SRSF2, ASXL, or RUNX1 (S/A/Rpos), have a strong adverse impact on disease phenotype and prognosis.6,9,10 A new risk classification was recently proposed including clinical and molecular parameters (eg, splenomegaly, elevated alkaline phosphatase [AP], and S/A/Rpos).11
A recently reported phase 2 study (www.clinicaltrials.gov as #NCT00782067) exploring the efficacy and safety of midostaurin, a multikinase/KIT inhibitor, in patients with advSM demonstrated an overall response rate (ORR) of 60%. Notably, achievement of responses was significantly associated with an improved outcome.12 However, the impact of the clonal architecture on responsiveness to midostaurin and the mechanisms of progression are unknown. In the current study, we therefore sought to evaluate the impact of the presence and dynamics of molecular markers at baseline and during follow-up on response, progression, and prognosis in midostaurin-treated advSM patients.
Materials and methods
Patients, diagnosis, and response criteria
The diagnosis of SM was established according to the World Health Organization classification.2,3,13,14 All bone marrow (BM) biopsies were evaluated by reference pathologists of the European Competence Network on Mastocytosis (H.-P.H. and K.S.). The diagnosis of ASM was based on the presence of 1 or more C-findings. Only measurable C-findings were eligible for this study: transfusion-independent and dependent cytopenia(s); liver function abnormalities (increased alanine aminotransferase, aspartate aminotransferase, and/or total bilirubin); hypoalbuminemia; and medically documented weight loss ≥10% in the 6 months prior to the study. Response was evaluated according to Valent criteria.15 The study design adhered to the tenets of the Declaration of Helsinki and was approved by the institutional review board of the Medical Faculty of Mannheim, Heidelberg University, as part of the ‘German Registry on Disorders of Eosinophils and Mast Cells.’ All patients gave written informed consent.
Quantitative assessment of KIT D816V
Quantitative assessments of KIT D816V EAB were performed using allele-specific quantitative real-time reverse-transcriptase polymerase chain reaction analysis on RNA/complementary DNA as previously described. It was also shown that quantitative measurements on RNA were equivalent to DNA.9 Assessments were performed at baseline and at least every 3 months during and after stopping treatment. Baseline was defined as last measurement prior to first dose of treatment.
Targeted next-generation sequencing (NGS) analysis
Next-Generation Deep Amplicon Sequencing by 454 FLX amplicon chemistry (Roche, Penzberg, Germany) was performed at baseline in all patients to investigate 18 candidate genes. The customized sequencing panel targeted the hotspot or complete coding regions of the following 18 genes: ASXL1, CBL, ETV6, EZH2, IDH1, IDH2, JAK2, KRAS, NPM1, NRAS, RUNX1, SETBP1, SF3B1, SRSF2, TET2, TP53, U2AF1, and ZRSR2.5 Sequential NGS, with consistent detection sensitivity of variant allele frequency (VAF) down to 3%, was performed using the 18 gene panel given previously or a wider 28 gene panel (as previously described)16 in 16 patients with signs of progressive disease.17-20 The sequential NGS approach is based on library preparation by the Access Array Technology (Fluidigm, San Francisco, CA) and sequencing on the MiSeq Instrument (Illumina, San Diego, CA). Gene mutations were annotated using the reference sequence of the Ensembl Transcript ID (Ensembl release 85: July 2016).
Statistical analyses
Statistical analyses considered clinical, laboratory, and molecular parameters obtained at the time of diagnosis, start of treatment, and at multiple time points during treatment. Overall survival (OS) was defined as time from start of treatment to date of death or last contact. Differences in the distribution of continuous variables between categories were analyzed by the Mann-Whitney U test (for comparison of 2 groups). For categorical variables, Fisher’s exact test was used. For KIT D816V EAB reduction, receiver operating characteristic curve with a time-dependent survival probability were used to identify optimal cutoff points. OS probabilities were estimated using the Kaplan-Meier method and compared by the log-rank test for univariate analysis. For the estimation of hazard ratios (HRs) and multivariate analysis, the Cox proportional hazard regression model was used. P values <.05 (2-sided) were considered significant. There was no adjustment for multiple testing as all analyses were explorative. SPSS version 22.0 (IBM Corporation, Armonk, NY) and SAS version 9.2 (SAS Institute, Cary, NC) were used for statistical analysis.
Results
Clinical characteristics at baseline
Between 2009 and 2015, 38 advSM patients (median age 67 years, range 48-76, 69% male) were treated with midostaurin at our institution (international phase 2 study on the efficacy and safety of midostaurin in advSM [www.clinicialtrials.gov, #NCT00782067], n = 20; compassionate use program, funded by Novartis Pharma, n = 18). There were no differences in eligibility criteria and treatment design between the phase 2 study and the compassionate use program. All patients started at 100 mg twice daily. Fifteen of 35 (43%) patients had at least 1 prior therapy (median 1, range 1-4).
Diagnoses at baseline were as follows: SM-AHN, n = 22; ASM, n = 5; MCL, n = 4; MCL-AHN, n = 7.1,3,13,14 The AHN comprised chronic myelomonocytic leukemia (n = 15), myelodysplastic syndrome/myeloproliferative neoplasm unclassified (MDS/MPN-U, n = 11), and chronic eosinophilic leukemia (n = 3). KIT mutation status was as follows: KIT D816V (n = 35, 92%), KIT D816Y (n = 1), KIT D816 negative (n = 2). Three of 38 (8%) patients stopped midostaurin within the first 4 months because of intolerance (nausea, vomiting) and were excluded from further analyses. Clinical parameters at diagnosis of the 35 remaining patients are shown in Table 1.
Clinical, laboratory, and treatment characteristics and outcome of 35 patients with advSM, stratified by absence (S/A/Rneg) or presence (S/A/Rpos) of mutations in the SRSF2/ASXL1/RUNX1 (S/A/R) gene panel
| Variables . | All patients . | S/A/Rneg . | S/A/Rpos . | P* . |
|---|---|---|---|---|
| No. of patients | 35 | 12 | 23 | |
| Age in y, median (range) | 67 (48-76) | 64 (48-76) | 68 (55-75) | NS |
| Males, n (%) | 24 (69) | 5 (42) | 19 (83) | .02 |
| Diagnosis | ||||
| ASM, n (%) | 4 (6) | 4 (33) | 0 | NS |
| MCL, n (%) | 4 (11) | 3 (25) | 1 (4) | NS |
| SM-AHN | 27 (83) | 5 (42) | 22 (96) | .01 |
| SM, n (%) | 20 (57) | 4 (80) | 16 (73) | NS |
| MCL, n (%) | 7 (26) | 1 (20) | 6 (27) | NS |
| Disease-related parameters | ||||
| Hemoglobin (G/dL) median (range) | 10 (6-15) | 11 (7-15) | 9 (6-14) | .04 |
| <10 g/dL, n (%) | 21 (60) | 4 (33) | 17 (74) | .03 |
| Platelets (×109/L) median (range) | 97 (7-426) | 113 (30-344) | 94 (7-426) | NS |
| <100 × 109/L, n (%) | 20 (57) | 6 (50) | 14 (61) | NS |
| Hemoglobin <10 g/dL and/or platelets <100 × 109/L, n (%) | 30 (86) | 8 (67) | 22 (96) | .04 |
| MC infiltration in BM (%) median (range) | 50 (15-95) | 40 (15-90) | 50 (20-95) | NS |
| Serum tryptase (µg/D) median (range) | 246 (33-1690) | 214 (65-1690) | 296 (33-1200) | NS |
| >200 µg/L, n (%) | 21 (60) | 7 (58) | 14 (61) | NS |
| KIT D816V+ EAB in PB (%) median (range) | 40 (4-64) | 35 (4-63) | 40 (7-64) | NS |
| >30%, n (%) | 24 (69) | 8 (67) | 16 (70) | NS |
| Albumin (g/L) median (range) | 34 (23-45) | 34 (23-43) | 34 (26-45) | NS |
| <35 g/L, n (%) | 21 (60) | 7 (58) | 14 (61) | NS |
| AP (U/L) median (range) | 287 (61-1067) | 179 (61-479) | 355 (134-1067) | .004 |
| >150 U/L, n (%) | 29 (83) | 8 (67) | 22 (96) | .04 |
| Spleen volume (mL) median (range), n = 28 | 918 (333-2058) | 800 (333-1796) | 919 (338-2058) | NS |
| Marked splenomegaly (≥1200 mL) | 10 (36) | 2 (22) | 8 (42) | NS |
| Treatment-related parameters | ||||
| Time on midostaurin therapy (mo) median (range) | 13 (1-88) | 13 (5-88) | 11 (1-57) | NS |
| ≥6 mo on midostaurin, n (%) | 29 (83) | 11 (92) | 18 (78) | NS |
| Number of patients on midostaurin at time of reporting | 11 (31) | 7 (58) | 4 (17) | .02 |
| Outcome | ||||
| Median OS, mo (95% CI) | 30 (6-54) | NR | 27 (16-38) | .01† |
| Death, n (%) | 20 (57) | 3 (25) | 17 (74) |
| Variables . | All patients . | S/A/Rneg . | S/A/Rpos . | P* . |
|---|---|---|---|---|
| No. of patients | 35 | 12 | 23 | |
| Age in y, median (range) | 67 (48-76) | 64 (48-76) | 68 (55-75) | NS |
| Males, n (%) | 24 (69) | 5 (42) | 19 (83) | .02 |
| Diagnosis | ||||
| ASM, n (%) | 4 (6) | 4 (33) | 0 | NS |
| MCL, n (%) | 4 (11) | 3 (25) | 1 (4) | NS |
| SM-AHN | 27 (83) | 5 (42) | 22 (96) | .01 |
| SM, n (%) | 20 (57) | 4 (80) | 16 (73) | NS |
| MCL, n (%) | 7 (26) | 1 (20) | 6 (27) | NS |
| Disease-related parameters | ||||
| Hemoglobin (G/dL) median (range) | 10 (6-15) | 11 (7-15) | 9 (6-14) | .04 |
| <10 g/dL, n (%) | 21 (60) | 4 (33) | 17 (74) | .03 |
| Platelets (×109/L) median (range) | 97 (7-426) | 113 (30-344) | 94 (7-426) | NS |
| <100 × 109/L, n (%) | 20 (57) | 6 (50) | 14 (61) | NS |
| Hemoglobin <10 g/dL and/or platelets <100 × 109/L, n (%) | 30 (86) | 8 (67) | 22 (96) | .04 |
| MC infiltration in BM (%) median (range) | 50 (15-95) | 40 (15-90) | 50 (20-95) | NS |
| Serum tryptase (µg/D) median (range) | 246 (33-1690) | 214 (65-1690) | 296 (33-1200) | NS |
| >200 µg/L, n (%) | 21 (60) | 7 (58) | 14 (61) | NS |
| KIT D816V+ EAB in PB (%) median (range) | 40 (4-64) | 35 (4-63) | 40 (7-64) | NS |
| >30%, n (%) | 24 (69) | 8 (67) | 16 (70) | NS |
| Albumin (g/L) median (range) | 34 (23-45) | 34 (23-43) | 34 (26-45) | NS |
| <35 g/L, n (%) | 21 (60) | 7 (58) | 14 (61) | NS |
| AP (U/L) median (range) | 287 (61-1067) | 179 (61-479) | 355 (134-1067) | .004 |
| >150 U/L, n (%) | 29 (83) | 8 (67) | 22 (96) | .04 |
| Spleen volume (mL) median (range), n = 28 | 918 (333-2058) | 800 (333-1796) | 919 (338-2058) | NS |
| Marked splenomegaly (≥1200 mL) | 10 (36) | 2 (22) | 8 (42) | NS |
| Treatment-related parameters | ||||
| Time on midostaurin therapy (mo) median (range) | 13 (1-88) | 13 (5-88) | 11 (1-57) | NS |
| ≥6 mo on midostaurin, n (%) | 29 (83) | 11 (92) | 18 (78) | NS |
| Number of patients on midostaurin at time of reporting | 11 (31) | 7 (58) | 4 (17) | .02 |
| Outcome | ||||
| Median OS, mo (95% CI) | 30 (6-54) | NR | 27 (16-38) | .01† |
| Death, n (%) | 20 (57) | 3 (25) | 17 (74) |
CI, confidence interval; NR, not reached; NS, not significant; PB, peripheral blood.
The P values refer to the Mann-Whitney U test, Fisher’s exact test, or log-rank tests comparing S/A/Rneg and S/A/Rpos patients.
Log-rank test comparing S/A/Rneg and S/A/Rpos patients.
Response rates, progression, and survival on midostaurin
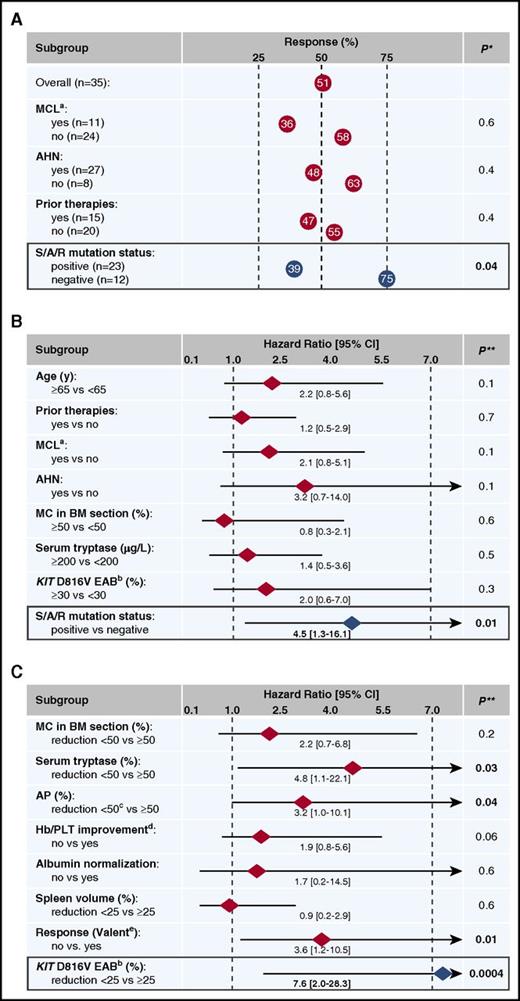
The median time from diagnosis of advSM to start of midostaurin was 12 months (range 1-60) and the median treatment time was 13 months (range 1-88). Responses according to Valent criteria at month 6 (n = 29) or at the end of treatment because of progression/death prior to month 6 (n = 6) were as follows: ORR, 51% (18/35 patients) with major response, n = 12 (34%), and partial response (PR), n = 6 (17%); stable disease, n = 8 (23%), and progressive disease, n = 9 (26%). ORRs were not statistically different between patients with or without MCL, AHN, or prior therapies (Figure 1A).
Response and OS depending on various baseline and on-treatment parameters. Response rates (A) and OS (B) in 35 midostaurin-treated advSM patients according to various baseline parameters. Only the mutational status in the SRSF2/ASXL1/RUNX1 (S/A/R) gene panel was a significant parameter for prediction of response and OS. (C) On-treatment response parameters in 28 patients at month 6: significant reductions of serum tryptase, AP, KIT D816V EAB, and any response according to Valent criteria were identified as favorable prognostic parameters concerning OS. In multivariate analysis, only a KIT D816V EAB reduction <25% remained an independent risk factor for poor OS. Hb, hemoglobin; PLT, platelets. *, P values refer to Fisher’s exact tests; **, P values refer to the log-rank tests. a, MCL patients with and without AHN; b, KIT D816V in PB; c, AP reduction ≥50% or normalization; d, Cheson criteria for transfusions; e, response criteria according to Valent.15
Response and OS depending on various baseline and on-treatment parameters. Response rates (A) and OS (B) in 35 midostaurin-treated advSM patients according to various baseline parameters. Only the mutational status in the SRSF2/ASXL1/RUNX1 (S/A/R) gene panel was a significant parameter for prediction of response and OS. (C) On-treatment response parameters in 28 patients at month 6: significant reductions of serum tryptase, AP, KIT D816V EAB, and any response according to Valent criteria were identified as favorable prognostic parameters concerning OS. In multivariate analysis, only a KIT D816V EAB reduction <25% remained an independent risk factor for poor OS. Hb, hemoglobin; PLT, platelets. *, P values refer to Fisher’s exact tests; **, P values refer to the log-rank tests. a, MCL patients with and without AHN; b, KIT D816V in PB; c, AP reduction ≥50% or normalization; d, Cheson criteria for transfusions; e, response criteria according to Valent.15
During follow-up, secondary MCL (sMCL), sMCL with secondary AML (sMCL-sAML), and SM-sAML were diagnosed in 4, 1, and 1 patient(s), respectively. In 5/6 (83%) cases, the disease evolved from SM-AHN within a median time of 14 months (range 6-58) from start of midostaurin. The median OS was 30 months (95% CI, 6-54) from start of midostaurin and 45 months (95% CI, 34-56) from diagnosis of advSM. Twenty of 35 (57%) patients died, 6 of them within the first 6 months because of early progressive disease.
Impact of mutations in the S/A/R gene panel on response, progression, and survival
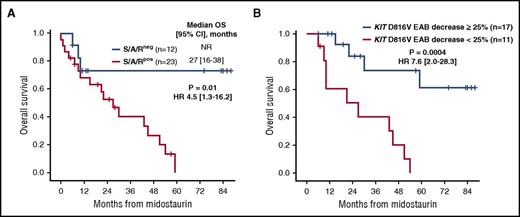
At baseline, significant differences between S/A/Rpos (n = 23) and S/A/Rneg (n = 12) patients were observed for hemoglobin <10 g/dL and elevated AP >150 U/L (Table 1). Five of 6 (83%) patients who stopped treatment prior to month 6 because of progression/death were S/A/Rpos and the remaining S/A/Rneg patient had MCL. Number of patients on midostaurin at the time of last contact (17% vs 58%, P = .02), ORR (39% vs 75%, P = .04; Figure 1A) and OS (median not reached vs 27 months, 95% CI [16-38], P = .01; HR 4.0, 95% CI [1.3-16.2]; Figure 1B) were all significantly in favor of S/A/Rneg patients. Long-term survival >5 years (n = 5) was only observed in S/A/Rneg patients. Of note, 5/6 (83%) patients with progression to sMCL or sAML were S/A/Rpos.
Within an unselected historical control group (‘German Registry on Disorders of Eosinophils and Mast Cells’) of advSM patients without midostaurin treatment (n = 50), the molecular profile was available in 30 patients; 17/30 (57%) patients (SM-AHN, n = 15; MCL-AHN, n = 1; ASM, n = 1) were S/A/Rpos. The median OS calculated from diagnosis of advSM in those 17 patients without midostaurin treatment was 14 months compared with 40 months in 23 S/A/Rpos patients (SM-AHN, n = 16; MCL-AHN, n = 6; MCL, n = 1) with midostaurin treatment (P = .004, HR 3.0 [1.4-6.5]). Progression to sAML was not different between the 2 cohorts (4/23 [17%] vs 3/17 [18%]).
KIT D816V allele burden changes during treatment with midostaurin
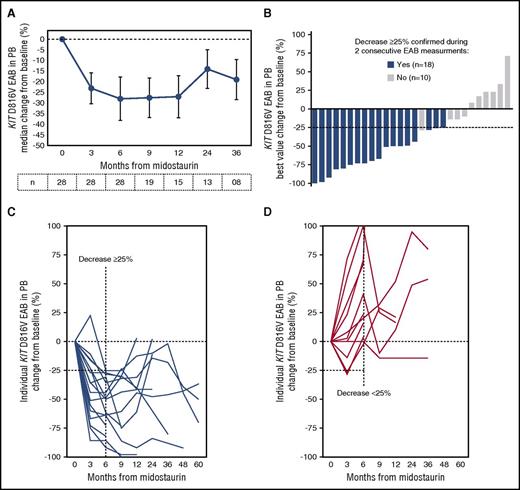
For the 28 KIT D816V positive patients with at least 6 months on midostaurin, the KIT D816V EAB in PB was assessed at baseline and at least every 3 months (Figure 2A). The best median response in all patients was −29% (range −100 to 71). Nineteen (68%) and 13 patients (46%) had a relative KIT D816V EAB reduction of ≥25% or ≥50%, respectively, which were independent of baseline EAB (Figure 2B).
KIT D816V EAB changes on midostaurin. (A) Mean percent change (±SE) of the KIT D816V EAB in PB from baseline in 28 advSM patients treated with midostaurin. (B) Waterfall plot of the best percentage change of KIT D816V EAB in individual patients at any time. EAB changes over time in individual patients with ≥25% (C) or <25% (D) reduction of KIT D816V EAB at month 6. SE, standard error.
KIT D816V EAB changes on midostaurin. (A) Mean percent change (±SE) of the KIT D816V EAB in PB from baseline in 28 advSM patients treated with midostaurin. (B) Waterfall plot of the best percentage change of KIT D816V EAB in individual patients at any time. EAB changes over time in individual patients with ≥25% (C) or <25% (D) reduction of KIT D816V EAB at month 6. SE, standard error.
As a result of receiver operating characteristic analysis, patients were classified according to the KIT D816V EAB reduction at month 6 as KIT-responders (≥25% reduction, n = 17) or KIT-nonresponders (<25% reduction, n = 11) (Figures 2C-D). KIT-responders were found to have significantly higher ORR (13/17 vs 2/11 patients, P = .006), a median longer time on midostaurin (25 months vs 9 months, P = .01) and improved OS (median not reached vs 27 months, 95% CI [7-51], P = .0004) (Table 2; Figures 1C and 3B). All 11 KIT-nonresponders were found to have multimutated advSM (S/A/Rpos, n = 9, 82%; TET2/EZH2, n = 1; TET2, n = 1). The mutation profile of the KIT EAB responders (n = 17) were as follows: S/A/Rpos (n = 9, 53%), TET2/CBL/JAK2 (n = 1), TET2/JAK2 (n = 1), TET2 (n = 1), no mutations (n = 4). Of note, all patients (n = 4/17, 24%) who lost their molecular response (KIT D816V EAB reduction <25%) sustainably (during follow up) were also S/A/Rpos.
Clinical, laboratory, molecular, and treatment characteristics and outcome of 28 patients with advSM, stratified by KIT D816V EAB reduction at month 6 (≥25% vs <25%)
| Variables . | All patients . | Decrease of KIT D816V . | P* . | |
|---|---|---|---|---|
| EAB ≥25% . | EAB <25% . | |||
| No. of patients (n) | 28 | 17 | 11 | |
| Age in y, median (range) | 65 (48-76) | 68 (48-76) | 63 (55-75) | NS |
| Males, n (%) | 19 (68) | 10 (59) | 9 (82) | NS |
| Diagnosis | ||||
| ASM, n (%) | 3 (11) | 3 (18) | 0 | |
| MCL, n (%) | 2 (7) | 2 (12) | 0 | |
| SM-AHN | 23 (82) | 12 (71) | 11 (100) | NS |
| SM, n (%) | 19 (83) | 10 (83) | 9 (82) | |
| MCL, n (%) | 4 (17) | 2 (17) | 2 (18) | |
| Any response according Valent criteria | 15 (54) | 13 (76) | 2 (18) | .006 |
| Molecular profile | ||||
| S/A/Rpos, n (%) | 18 (65) | 9 (53) | 9 (82) | NS |
| Other mutation, n (%) | 6 (21) | 4 (24) | 2 (18) | |
| No mutation, n (%) | 4 (14) | 4 (24) | 0 | |
| Loss of KIT response†, n (%) | 4 (24) | — | ||
| S/A/Rpos, n (%) | 4 (24) | — | ||
| Median time on midostaurin, mo (range) | 19 (6-88) | 25 (6-88) | 9 (7-35) | .01 |
| Number of patients on midostaurin at time of reporting, n (%) | 10 (36) | 10 (59) | 0 | .002 |
| Outcome | ||||
| Median OS, mo (95% CI) | 45 (17-73) | NR | 27 (7-51) | .0004‡ |
| Death, n (%) | 14 (50) | 4 (24) | 10 (91) | |
| Variables . | All patients . | Decrease of KIT D816V . | P* . | |
|---|---|---|---|---|
| EAB ≥25% . | EAB <25% . | |||
| No. of patients (n) | 28 | 17 | 11 | |
| Age in y, median (range) | 65 (48-76) | 68 (48-76) | 63 (55-75) | NS |
| Males, n (%) | 19 (68) | 10 (59) | 9 (82) | NS |
| Diagnosis | ||||
| ASM, n (%) | 3 (11) | 3 (18) | 0 | |
| MCL, n (%) | 2 (7) | 2 (12) | 0 | |
| SM-AHN | 23 (82) | 12 (71) | 11 (100) | NS |
| SM, n (%) | 19 (83) | 10 (83) | 9 (82) | |
| MCL, n (%) | 4 (17) | 2 (17) | 2 (18) | |
| Any response according Valent criteria | 15 (54) | 13 (76) | 2 (18) | .006 |
| Molecular profile | ||||
| S/A/Rpos, n (%) | 18 (65) | 9 (53) | 9 (82) | NS |
| Other mutation, n (%) | 6 (21) | 4 (24) | 2 (18) | |
| No mutation, n (%) | 4 (14) | 4 (24) | 0 | |
| Loss of KIT response†, n (%) | 4 (24) | — | ||
| S/A/Rpos, n (%) | 4 (24) | — | ||
| Median time on midostaurin, mo (range) | 19 (6-88) | 25 (6-88) | 9 (7-35) | .01 |
| Number of patients on midostaurin at time of reporting, n (%) | 10 (36) | 10 (59) | 0 | .002 |
| Outcome | ||||
| Median OS, mo (95% CI) | 45 (17-73) | NR | 27 (7-51) | .0004‡ |
| Death, n (%) | 14 (50) | 4 (24) | 10 (91) | |
The P values refer to the Mann-Whitney U test, Fisher’s exact test, or log-rank tests comparing decrease of KIT D816V EAB ≥25% vs <25%.
Loss of KIT D816V response during follow-up (after month 6).
Log-rank test comparing decrease of KIT D816V EAB ≥25% vs <25%.
OS depending on the presence and dynamic of molecular parameters on midostaurin. (A) Kaplan-Meier estimates of OS depending on mutational status in the SRSF2/ASXL1/RUNX1 (S/A/R) gene panel (S/A/Rpos vs S/A/Rneg) at baseline. (B) OS depending on the expressed KIT D816V allele burden (EAB) reduction at month 6 in PB (≥25% vs <25%). HR, hazard ratio 95% confidence interval; NR, not reached.
OS depending on the presence and dynamic of molecular parameters on midostaurin. (A) Kaplan-Meier estimates of OS depending on mutational status in the SRSF2/ASXL1/RUNX1 (S/A/R) gene panel (S/A/Rpos vs S/A/Rneg) at baseline. (B) OS depending on the expressed KIT D816V allele burden (EAB) reduction at month 6 in PB (≥25% vs <25%). HR, hazard ratio 95% confidence interval; NR, not reached.
Univariate and multivariate analyses of on-treatment variables
Univariate analyses of key clinical (measureable) parameters (MC infiltration in BM, serum tryptase level, AP, cytopenia, albumin) and molecular markers (KIT D816V EAB) and their response at month 6, identified reduction of serum tryptase (P = .03, HR 4.8 [1.1-22.1]), reduction of AP (P = .04, HR 3.2 [1.0-10.1]), reduction of KIT D816V EAB (P = .0004, HR 7.6 [2.0-28.3]), and any response (major response and PR) according to Valent criteria (P = .01, HR 3.6 [1.2-10.5]) as favorable prognostic variables regarding OS. In multivariate analysis, only KIT D816V EAB reduction <25% remained an independent risk factor for poor OS (P = .004, HR 6.8 [1.8-25.3]; Figures 1C and 3B).
Serial NGS analyses during treatment with midostaurin
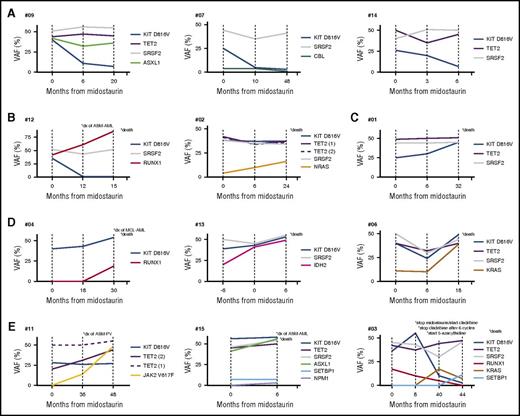
Serial NGS analyses of PB samples in a cohort of multimutated patients (n = 16) were performed at baseline and up to 3 times per patient (median 2, range [1-3]) during midostaurin treatment at a median interval of 14 months (range 6-48). In total, 70 serial NGS (including baseline NGS) analyses were performed. The dynamics of VAF of recurrently mutated genes are shown in Figure 4.
Schematic presentation of the VAF changes of KIT D816V and additional mutations in 12 patients during treatment with midostaurin. Five clusters were identified (A-E): (A) Significant reduction of KIT D816V but without significant VAF changes of the additional mutations. All patients were maintained on midostaurin. (B) Significant reduction of KIT D816V but expansion of RUNX1 (#12) or NRAS (#02) with subsequent disease progression and death (in #12 after progression into secondary acute myeloid leukemia). (C) Significant increase of KIT D816V but stable VAF of additional mutations with subsequent disease progression and death. (D) Significant increase of KIT D816V in combination with increase of VAF of additional mutations (RUNX1, #04; IDH2, #13; KRAS, #06) followed by disease progression and rapid death. Patient #04 progressed into sMCL and then sAML. (E) Acquisition of new mutations (JAK2 V617F, #11; NMP1, #15; KRAS/SETBP1, #03) with disease progression into ASM and associated polycythemia vera (ASM-PV, #11) or ASM-AML (#15) followed by death (#15, #03). Patient #11 with transfusion-dependent anemia at baseline became transfusion-independent. *, Clinical end points, including type of secondary neoplasm observed at the time of progression and death. dx, diagnosis.
Schematic presentation of the VAF changes of KIT D816V and additional mutations in 12 patients during treatment with midostaurin. Five clusters were identified (A-E): (A) Significant reduction of KIT D816V but without significant VAF changes of the additional mutations. All patients were maintained on midostaurin. (B) Significant reduction of KIT D816V but expansion of RUNX1 (#12) or NRAS (#02) with subsequent disease progression and death (in #12 after progression into secondary acute myeloid leukemia). (C) Significant increase of KIT D816V but stable VAF of additional mutations with subsequent disease progression and death. (D) Significant increase of KIT D816V in combination with increase of VAF of additional mutations (RUNX1, #04; IDH2, #13; KRAS, #06) followed by disease progression and rapid death. Patient #04 progressed into sMCL and then sAML. (E) Acquisition of new mutations (JAK2 V617F, #11; NMP1, #15; KRAS/SETBP1, #03) with disease progression into ASM and associated polycythemia vera (ASM-PV, #11) or ASM-AML (#15) followed by death (#15, #03). Patient #11 with transfusion-dependent anemia at baseline became transfusion-independent. *, Clinical end points, including type of secondary neoplasm observed at the time of progression and death. dx, diagnosis.
Among KIT-responders, a significant increase in the VAF of additional mutations was identified in patients #06 (KRAS, 11% to 39%) and #12 (RUNX1, 42% to 86%). In patient #12, progression of ASM-MDS/MPN to ASM-AML was observed although KIT D816V had virtually disappeared (VAF, 1%). Both patients died, 22 months (patient #09) and 15 months (patient #12) after start of midostaurin, respectively. In the remaining 6 patients, the appearance of new mutations was observed in 2 patients (#10 and #11, JAK2 V617F [VAF, 3% and 52%, respectively] in both cases) and the virtual disappearance (VAF ≤ 1%) of mutations in 4 patients (KIT D816V in patients #16 and #12; CBL in patient #07; TET2 in patient #11, baseline VAF [7%, 48%, 4%, 8%, respectively]). In patient #11 (acquisition of JAK2 V617F, VAF 52%), progression of ASM to ASM-polycythemia vera was observed. All 6 patients are alive and on midostaurin at a median of 40 months (range 22-79).
Among the 8 KIT-nonresponders, 7 (88%) patients died at a median of 21 months (range 6-54) after start of midostaurin. An increase in the KIT D816V EAB was observed in 7/8 patients (88%) and a significant increase of the VAF of additional mutations already present at start of midostaurin was observed in patients #13 (IDH2, 20% to 49%) and #02 (KRAS, 4% to 16%), respectively. The appearance of new mutations was seen in patients #15 (NPM1, VAF 3%) and #04 (RUNX1, VAF 19%), and was associated with progression to secondary AML (sAML) after 6 (patient #15) and 25 (patient #4) months on midostaurin, respectively. Both patients failed to achieve a response to intensive chemotherapy and died 1 and 12 months, respectively, after diagnosis of sAML. In patient #03, midostaurin was stopped after 8 months because of progression. After 6 cycles of cladribine, a significant reduction of AP and serum tryptase levels were observed, which was associated with a reduction of the KIT D816V EAB from > 50% to 5%. Relapse occurred 6 months later and 5-azacytidine was initiated. The patient remained in SD with a significant reduction of the KIT D816V EAB after 11 cycles of 5-azacytidine. Acquisition of KRAS (VAF, 17%) and SETBP1 (VAF, 11%) mutations was associated with rapid progression and death at 12 months after start of 5-azacytidine (Figure 4).
Overall, serial NGS analysis of 7 deceased patients revealed acquisition of additional mutations in RUNX1 (n = 2), K/NRAS (n = 3), IDH2 (n = 1), or NPM1 (n = 1) and/or increasing VAF of additional mutations, whereas KIT D816V EAB was increasing (n = 4), stable (n = 1), or even low (n = 2). In contrast, 2 patients acquired a JAK2 V617F mutation but remained in durable PR on midostaurin (Figure 4).
Discussion
In addition to the known beneficial effects of midostaurin on BM MC infiltration, serum tryptase, and C-findings,12,21 we demonstrate here that midostaurin also reduces the KIT D816V allele burden. In patients who had been treated for at least 6 months, a significant reduction ≥25% at month 6 was the strongest on-treatment predictor for improved survival in multivariate analysis and was even superior to Valent response criteria. Because all patients with KIT D816V positive advSM have at diagnosis a measurable allele burden in PB,9 regular measurements of the KIT D816V allele burden should be performed in the routine follow-up for residual disease on or after potentially effective treatment regimens, including midostaurin, chemotherapy and/or allogeneic stem cell transplantation (SCT).22-25
Disparate mechanisms may be responsible for progression on midostaurin. In addition to the known negative effects on phenotype and prognosis,6,11 we also show that response rates because of early or late progression and consequently survival were significantly inferior in S/A/Rpos patients (Figure 3A) suggesting persistence and/or outgrowth of an aggressive and multimutated KIT D816V positive clone. Almost all patients who stopped because of early progression, who lost the KIT-response or who progressed to sMCL or sAML had at least 1 mutation in the S/A/R gene panel. However, the median OS of midostaurin-treated S/A/Rpos patients is better than the median OS of S/A/Rpos patients without midostaurin treatment whereas the rate of progression to sAML during follow-up is not different.
There is a strong link between AHN and additional mutations, however, no significant differences were observed between advSM with or without AHN because advSM with AHN was also associated with other mutations than in S/A/R, eg, JAK2 V617F. It is therefore not the presence of AHN per se but the molecular background of AHN that impacts on response and prognosis. Of note, the majority of patients who progressed on midostaurin also failed to respond to subsequent chemotherapy (eg, cladribine) and even allogeneic SCT in 1 patient. These data are reminiscent of the treatment of myelofibrosis (MF) with ruxolitinib for which the presence of mutations in ASXL1 or SRSF2 was also inversely correlated with spleen response, time to treatment discontinuation and shortened OS.26 We found no evidence for additional KIT mutations that might be conferring resistance to midostaurin (data not shown).
A second mechanism leading to progression was associated with increasing VAF or new emergence of mutations in RUNX1, K/NRAS or IDH2, despite otherwise durable response of C-findings and significant reduction of the KIT D816V allele burden. In this regard it is noteworthy that the first published patient with advSM receiving midostaurin also relapsed with a KIT D816V negative subclone resembling AML.27 The data are also reminiscent to other myeloid neoplasms and their clonal diversity, in which mutations in RUNX1, N/KRAS or IDH2 were also identified as late events and drivers for disease progression.19,28-31 Of interest, no significant increase in clone size was observed for mutations in TET2 or SRSF2, although they represent early mutations in the multistep pathogenesis of SM,8 chronic myelomonocytic leukemia,32 or MDS.19 These results suggest that the extensive clinical heterogeneity of advSM is most likely because of the presence and dynamic evolution of several molecularly disparate subclones, which have a variable impact on clinical characteristics and response to treatment.
Dysregulated KIT and JAK2 signaling are certainly pivotal for the pathogenesis of SM and MF. However, it is not yet fully understood how 1 mutation can cause distinct phenotypes, eg, KIT D816V in indolent SM and advSM or JAK2 V617F in classical MPN. In addition, it has also become apparent that patients do not experience complete hematologic remissions on targeted treatment with midostaurin or ruxolitinib.33 But there is now accumulating evidence that complex mutation profiles are strongly associated with primary and secondary progression on targeted treatment of these neoplasms. Whether additional mutations cause progression directly or whether they are simply markers for other factors remains yet unknown. Based on promising in vitro studies,34,35 the combination of midostaurin with other effective anti-SM therapies such as chemotherapy with or without allogeneic SCT22-25 may become a reasonable approach to overcome this frequently occurring scenario.
In conclusion, we have found that the absence of mutations in the S/A/R gene panel at baseline and a reduction of the KIT D816V allele burden ≥ 25% at month 6 are the most favorable predictors for improved survival in midostaurin-treated advSM patients. Serial sequencing of relevant mutations at multiple time points during treatment has led to a more detailed understanding of the clonal dynamics for response assessment. Progression may be caused by expansion of subclones exhibiting new mutations in critical target genes independent of KIT D816V. Prospective clinical trials are warranted which integrate NGS for clinical decision-making in advSM and use combinations of midostaurin with drugs, which are able to also suppress KIT D816V-negative subclones.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the ‘Deutsche José Carreras Leukämie-Stiftung e.V.’ (grant no. DJCLS R 13/05) and by the SEED program of the Mannheim Medical Faculty, Heidelberg University.
Authorship
Contribution: M.J., J.S., N.N., T.H., A.F., N.C.P.C., M.M., and A.R. performed the laboratory work for the study; M.J., J.S., K.S., G.M., M.M., and A.R. provided patient material and information; H.-P.H. and K.S. reviewed the bone marrow biopsies; and M.J., J.S., N.N., T.H., G.M., A.F., P.V., W.-K.H., N.C.P.C., M.M., and A.R. wrote the manuscript.
Conflict-of-interest disclosure: H.-P.H., K.S., P.V., and A.R. served as consultants in a global phase 2 study examining the effects of midostaurin in advanced systemic mastocytosis. H.-P.H., K.S., P.V., N.C.P.C., and A.R. received honoraria and/or travel support from Novartis Pharmaceuticals. M.J. and J.S. received travel support from Novartis Pharmaceuticals. T.H. has equity ownership of MLL Munich Leukemia Laboratory. M.M. is employed by MLL Munich Leukemia Laboratory. The remaining authors declare no competing financial interests.
Correspondence: Andreas Reiter, Department of Hematology and Oncology, Mannheim University Medical Center, Mannheim Medical Faculty, Heidelberg University, Theodor-Kutzer-Ufer 1-3, 68167 Mannheim, Germany; e-mail: andreas.reiter@medma.uni-heidelberg.de.
References
Author notes
M.M. and A.R. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal