To the editor:
Heparin-induced thrombocytopenia (HIT) is a prothrombotic adverse drug reaction caused by platelet-activating immunoglobulin G (IgG) that recognizes PF4 in complexes with heparin or certain platelet-derived polyanions (chondroitin sulfate and polyphosphates).1,-3 HIT occurs in ∼0.2% of hospitalized patients who undergo any heparin exposure,4 although most cases occur with prophylactic- or therapeutic-dose unfractionated heparin (UFH) given for ≥5 days, usually in postoperative or proinflammatory settings (frequency, 1% to 5%).5
Rarely, HIT has been reported in patients whose sole exposure consisted of heparin “flushes.”6,-8 The paucity of these reports makes it challenging to determine how such low amounts of heparin could cause HIT. For example, UFH concentrations of ∼0.1 to 0.3 U/mL (using washed platelets9 ) or ∼0.5 to 1.0 U/mL (using plasma-rich plasma10 ) in vitro are optimal for HIT antibody-induced platelet activation. However, to achieve these heparin concentrations in vivo, a patient must receive at least systemic prophylactic-dose UFH.11
Recently, “autoimmune HIT” has been recognized12,-14 ; here, HIT antibodies activate platelets strongly, even in the absence of heparin. Clinical syndromes linked to autoimmune HIT include delayed-onset HIT (where HIT begins or worsens after stopping heparin),15 persisting HIT (where thrombocytopenia continues for several weeks despite stopping heparin),15,16 and spontaneous HIT syndrome (clinical/serological picture of HIT without proximate heparin exposure).17,18 We now report 4 multiple myeloma (MM) patients who developed HIT exclusively through exposure to UFH flushes (for apheresis catheter management) prior to autologous stem cell transplantation (aSCT), implicating autoimmune HIT antibodies. These patients were identified during a 44-month period (beginning May 2013) during which 101 MM patients underwent aSCT without receiving stem cell mobilization with cyclophosphamide 10 days preharvest (this routine practice was stopped in April 2013).
All 101 MM patients had previously received standard induction chemotherapy (cyclophosphamide, bortezomib, and dexamethasone).19 Each patient received a Palindrome apheresis catheter (via the right internal jugular vein), followed by a 5-day course of granulocyte colony-stimulating factor (10 μg/kg per day), after which stem cells were harvested (performed only with citrate anticoagulation). UFH was added directly to the stem cell product (final concentration, ≥50 IU/mL) prior to cryopreservation (this did not result in patient heparin exposure). All patients received daily UFH (BD PosiFlush Heparin Lock Flush Syringes, 10 USP units/mL) for catheter maintenance, ∼3 to 5 mL solution drawn from each lumen, and a 20-mL flush of normal saline, followed by a 3-mL flush of heparin lock solution into each lumen (total, 60 U/day). For the 4 patients with HIT, no other heparin exposure was identified within the preceding 100 days. Although their platelet counts were normal at catheter insertion and ∼1 week later at stem cell harvesting, severe thrombocytopenia was subsequently found at time of scheduled aSCT.
Figure 1A compares the platelet counts of these 4 patients with those of 97 control subjects at 3 key time points: (1) time of catheter placement (when heparin flushes were commenced), (2) time of stem cell harvesting, and (3) time of scheduled aSCT. In all 4 patients, thrombocytopenia was evident only at the time of scheduled aSCT. In contrast, the platelet counts were unremarkable (>100 × 109/L) at scheduled aSCT in all 97 controls.
Four MM patients with HIT complicating heparin flush exposure associated with stem cell harvesting for planned aSCT. (A) Platelet counts for 101 MM patients undergoing stem cell harvesting (without cyclophosphamide stem cell mobilization) pre-aSCT; box and whisker plots indicate the platelet count values (median, interquartile range [IQR], 1.5 × IQR, and outliers [open circles]) for 97 non-HIT MM controls at 3 time points: (1) apheresis catheter placement (start of heparin flushes), (2) immediately prior to stem cell harvesting, and (3) and time of scheduled aSCT. The corresponding platelet count values for the 4 patients with HIT are shown as colored solid circles (green, patient 1; purple, patient 2; red, patient 3; and blue, patient 4). The outlier data point indicated by an asterisk (*) represents a control patient whose platelet count was only 40 × 109/L at time of stem cell harvesting; the thrombocytopenia, which was attributed to possible line infection, had resolved by the time of aSCT 2 weeks later (platelet count, 369 × 109/L). For the 97 controls, there was a median (IQR) interval of 6 (5, 9) days between apheresis catheter insertion and stem cell harvesting and a median (IQR) interval of 23 (22, 29) days between catheter insertion and admission for subsequent aSCT. (B) Clinical courses of 4 MM patients who developed HIT. Shown for each patient are the sequential platelet counts (including nadir values), thrombosis occurrence (3 of 4 patients developed right upper-limb deep vein thrombosis [DVT]), and anticoagulants given. Patient age (in years) is given prior to designation of patient sex (F, female; M, male). (C) Summary of HIT assay results. The 4 patients had strongly positive results in all 4 HIT assays: (1) SRA (maximal percent serotonin release at 0.1 and/or 0.3 IU/mL UFH; for all patients, serotonin release was <10% at 100 IU/mL UFH and in the presence of the Fc receptor–blocking monoclonal antibody IV.3), (2) in-house IgG-specific enzyme immunoassay (EIA-IgG), (3) commercial polyspecific enzyme immunoassay that detects antibodies of IgG, IgA, and/or IgM istotypes (EIA-GAM), and (4) latex immunoturbidimetric assay (LIA). AL amyl., amyloid light-chain amyloidosis; F, female; Fx, fondaparinux; LMWH, low-molecular-weight heparin; M, male; OD, optical density; Pos, positive; RUL DVT, right upper-limb deep-vein thrombosis.
Four MM patients with HIT complicating heparin flush exposure associated with stem cell harvesting for planned aSCT. (A) Platelet counts for 101 MM patients undergoing stem cell harvesting (without cyclophosphamide stem cell mobilization) pre-aSCT; box and whisker plots indicate the platelet count values (median, interquartile range [IQR], 1.5 × IQR, and outliers [open circles]) for 97 non-HIT MM controls at 3 time points: (1) apheresis catheter placement (start of heparin flushes), (2) immediately prior to stem cell harvesting, and (3) and time of scheduled aSCT. The corresponding platelet count values for the 4 patients with HIT are shown as colored solid circles (green, patient 1; purple, patient 2; red, patient 3; and blue, patient 4). The outlier data point indicated by an asterisk (*) represents a control patient whose platelet count was only 40 × 109/L at time of stem cell harvesting; the thrombocytopenia, which was attributed to possible line infection, had resolved by the time of aSCT 2 weeks later (platelet count, 369 × 109/L). For the 97 controls, there was a median (IQR) interval of 6 (5, 9) days between apheresis catheter insertion and stem cell harvesting and a median (IQR) interval of 23 (22, 29) days between catheter insertion and admission for subsequent aSCT. (B) Clinical courses of 4 MM patients who developed HIT. Shown for each patient are the sequential platelet counts (including nadir values), thrombosis occurrence (3 of 4 patients developed right upper-limb deep vein thrombosis [DVT]), and anticoagulants given. Patient age (in years) is given prior to designation of patient sex (F, female; M, male). (C) Summary of HIT assay results. The 4 patients had strongly positive results in all 4 HIT assays: (1) SRA (maximal percent serotonin release at 0.1 and/or 0.3 IU/mL UFH; for all patients, serotonin release was <10% at 100 IU/mL UFH and in the presence of the Fc receptor–blocking monoclonal antibody IV.3), (2) in-house IgG-specific enzyme immunoassay (EIA-IgG), (3) commercial polyspecific enzyme immunoassay that detects antibodies of IgG, IgA, and/or IgM istotypes (EIA-GAM), and (4) latex immunoturbidimetric assay (LIA). AL amyl., amyloid light-chain amyloidosis; F, female; Fx, fondaparinux; LMWH, low-molecular-weight heparin; M, male; OD, optical density; Pos, positive; RUL DVT, right upper-limb deep-vein thrombosis.
Figure 1B summarizes the clinical courses of the 4 patients with HIT, including serial platelet counts, thrombosis occurrence, and treatment. aSCT was cancelled in all 4 patients because of the severe thrombocytopenia (mean platelet count nadir, 23 × 109/L). Three of the patients with HIT also developed symptomatic upper-extremity DVT in the same limb as the apheresis catheter. This frequency of HIT-associated thrombosis (6-week follow-up) was markedly greater in these patients than in the non-HIT controls (3 out of 4 patients [75%] vs 2 out of 97 controls [2.1%]; odds ratio, 142; 95% confidence interval, 10-2040; P = .0002), consistent with a previously reported association between HIT and upper-limb DVT with upper-extremity venous catheter use.20
The diagnosis of HIT was not immediately made in patients 1 and 2 (resulting in some treatment with low-molecular-weight heparin or UFH), whereas the HIT diagnosis was made promptly in patients 3 and 4. Following diagnosis, patient 1 was enrolled into a prospective single-arm interventional study of rivaroxaban for HIT (reported elsewhere21 ). Although this patient’s serotonin-release assay (SRA) result became negative (day 141), he never underwent aSCT and died of unrelated complications (day 176). Patient 2 was treated successfully with fondaparinux/warfarin; patients 3 and 4 were treated successfully with rivaroxaban.22 Patient 2 became SRA negative by day 225, at which point aSCT with the stored (heparin-containing) stem cell product was theoretically possible23 (although not performed). Patient 3 did not have follow-up SRA testing performed. However, patient 4 (who remained strongly SRA positive) underwent repeat stem cell harvest (without heparin use), followed by aSCT with heparin-free product.
Figure 1C summarizes the HIT antibody test results. All 4 patients had strongly positive results in 4 different tests: the SRA,9 the latex immunoturbidimetric assay [HemosIL HIT-Ab(PF4-H)],24 and 2 PF4-dependent enzyme-immunoassays (in-house IgG-specific [McMaster Platelet Immunology Laboratory] and polyspecific [PF4 Enhanced; Immucor GTI Diagnostics, Waukesha, WI]).
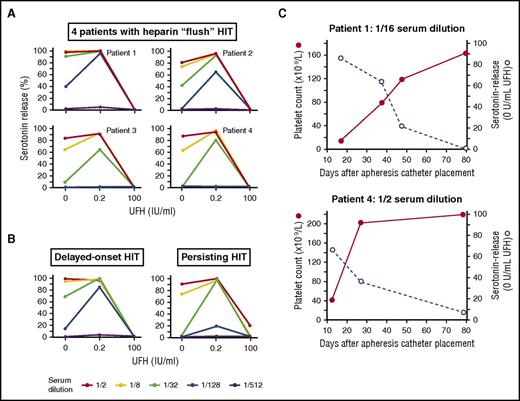
Further, all 4 patients’ acute sera showed strong heparin-independent platelet activation (>80% serotonin-release at 0 U/mL UFH) and (upon serum dilution) heparin-dependent platelet activation (Figure 2A). The detection of serum-independent platelet activation in all 4 patient sera was significantly more frequent than expected based on a comparison with 100 consecutive HIT patients with a positive SRA identified in local Hamilton hospitals (4 out of 4 patients vs 34 out of 100 patients; P = .0161). Indeed, the patients’ strong serum-induced serotonin release, which was even evident using sera diluted 1/128 (patient 1), 1/32 (patient 2), and 1/8 (patients 3 and 4), was comparable to that seen in controls with autoimmune HIT disorders such as delayed-onset HIT15 and persisting HIT16 (Figure 2B). Since our patients were receiving only low doses of UFH (maximum, 60 U/day), it appears likely that HIT occurred because of the unusual autoimmune-like pathogenicity of these antibodies rather than because of formation of PF4/heparin complexes. Further supporting a pathogenic role of the heparin-independent platelet-activating properties is the inverse correlation we observed for patients 1 and 4 between platelet count recovery and waning of serum-induced serotonin release at buffer control (0 IU/mL UFH) (Figure 2C), a phenomenon previously observed during recovery from persisting HIT.16,22 Highly pathogenic HIT antibodies implicated in autoimmune HIT disorders can bind to PF4 without the need for heparin,13,14 perhaps facilitated by endogenous platelet-associated chondroitin sulfate2 and/or polyphosphates.3
Heparin-independent serum-induced serotonin release. (A) SRA results using serial fourfold serum dilutions for 4 MM patients who developed HIT. All 4 patient sera showed strong serum-induced serotonin release (>80%) using 1/2 diluted serum (heparin-independent serotonin release); at higher dilutions, as expected, typical heparin-dependent serotonin release (at 0.2 IU/mL UFH) was shown, with serotonin-release inhibited at 100 IU/mL UFH. (B) SRA results using serial fourfold serum dilutions for 2 positive autoimmune HIT controls. Both sera were available from previously reported patients with delayed-onset HIT (patient 115 ) and persisting HIT.16 (C) Relationship of platelet count recovery with decrease of heparin-independent serum-induced serotonin release. For 2 patients (1 and 4) for whom follow-up sera were available, platelet count recovery occurred in association with decrease in heparin-independent serum-induced serotonin release.
Heparin-independent serum-induced serotonin release. (A) SRA results using serial fourfold serum dilutions for 4 MM patients who developed HIT. All 4 patient sera showed strong serum-induced serotonin release (>80%) using 1/2 diluted serum (heparin-independent serotonin release); at higher dilutions, as expected, typical heparin-dependent serotonin release (at 0.2 IU/mL UFH) was shown, with serotonin-release inhibited at 100 IU/mL UFH. (B) SRA results using serial fourfold serum dilutions for 2 positive autoimmune HIT controls. Both sera were available from previously reported patients with delayed-onset HIT (patient 115 ) and persisting HIT.16 (C) Relationship of platelet count recovery with decrease of heparin-independent serum-induced serotonin release. For 2 patients (1 and 4) for whom follow-up sera were available, platelet count recovery occurred in association with decrease in heparin-independent serum-induced serotonin release.
The frequency of HIT in our aSCT population is unusually high. The 4 cases were identified among 101 (4.0% [95% CI, 1.1% to 9.8%]) patients who have undergone aSCT since May 2013. Such a high frequency of HIT is striking, given that heparin flush-induced HIT is rare. Perhaps the use of granulocyte colony-stimulating factor, which resulted in marked leukocytosis in our 4 patients (peak white blood count, 52.2 × 109/L [mean]), together with platelet activation associated with apheresis/stem cell collection, increases the risk of anti-PF4/heparin immunization, even with small heparin doses. It is known that HIT occurs more often in surgical patients (rather than medical) and in patients with major (vs minor) trauma,25 consistent with the idea that proinflammatory cofactors raise the risk of HIT. Another potential factor is the discontinuation of routine cyclophosphamide for stem cell mobilization at our center since 2013; perhaps routine administration of this immunosuppressive agent prevents anti-PF4/heparin immunization that might otherwise have occurred in this clinical setting.
In conclusion, exposure to small doses of heparin through flushing of the apheresis catheter during stem cell collection for aSCT for MM can be associated with HIT, including catheter-associated DVT. Moreover, this complication will contraindicate stem cell infusion during acute HIT if heparin is added to the stem cell product. Finally, the observation of strong heparin-independent serum-induced platelet activation suggests that heparin flush-associated HIT can be considered a form of autoimmune HIT.
Authorship
Contribution: T.E.W. and H.M. reviewed the cases, analyzed the data, and interpreted the results; T.E.W. and J.-A.I.S. designed and/or performed the serological studies, and interpreted the results; A.B., R.F., and L.-A.L. oversaw the clinical issues raised by the cases; A.M. provided data regarding the stem cell transplants; T.E.W. and H.M. coauthored the initial draft of the manuscript; all authors reviewed and approved the final version of the manuscript.
Conflict-of-interest disclosure: T.E.W. has received lecture honoraria from Instrumentation Laboratory, royalties from Taylor & Francis (Informa), and consulting fees and research funding from Aspen Global, W.L. Gore, Instrumentation Laboratory, and Octapharma, and has provided expert witness testimony related to HIT. R.F. has received consulting fees (advisory board) for Celgene, Sanofi, Amgen, and Takeda. L.-A.L. has received research funding from Bayer (investigator-sponsored study) and consulting fees from Bayer for data adjudication. The remaining authors declare no competing financial interests.
Correspondence: Theodore E. Warkentin, Room 1-270B, Hamilton Regional Laboratory Medicine Program, Hamilton General Hospital, 237 Barton St East, Hamilton, ON L8L 2X2, Canada; e-mail: twarken@mcmaster.ca.

![Figure 1. Four MM patients with HIT complicating heparin flush exposure associated with stem cell harvesting for planned aSCT. (A) Platelet counts for 101 MM patients undergoing stem cell harvesting (without cyclophosphamide stem cell mobilization) pre-aSCT; box and whisker plots indicate the platelet count values (median, interquartile range [IQR], 1.5 × IQR, and outliers [open circles]) for 97 non-HIT MM controls at 3 time points: (1) apheresis catheter placement (start of heparin flushes), (2) immediately prior to stem cell harvesting, and (3) and time of scheduled aSCT. The corresponding platelet count values for the 4 patients with HIT are shown as colored solid circles (green, patient 1; purple, patient 2; red, patient 3; and blue, patient 4). The outlier data point indicated by an asterisk (*) represents a control patient whose platelet count was only 40 × 109/L at time of stem cell harvesting; the thrombocytopenia, which was attributed to possible line infection, had resolved by the time of aSCT 2 weeks later (platelet count, 369 × 109/L). For the 97 controls, there was a median (IQR) interval of 6 (5, 9) days between apheresis catheter insertion and stem cell harvesting and a median (IQR) interval of 23 (22, 29) days between catheter insertion and admission for subsequent aSCT. (B) Clinical courses of 4 MM patients who developed HIT. Shown for each patient are the sequential platelet counts (including nadir values), thrombosis occurrence (3 of 4 patients developed right upper-limb deep vein thrombosis [DVT]), and anticoagulants given. Patient age (in years) is given prior to designation of patient sex (F, female; M, male). (C) Summary of HIT assay results. The 4 patients had strongly positive results in all 4 HIT assays: (1) SRA (maximal percent serotonin release at 0.1 and/or 0.3 IU/mL UFH; for all patients, serotonin release was <10% at 100 IU/mL UFH and in the presence of the Fc receptor–blocking monoclonal antibody IV.3), (2) in-house IgG-specific enzyme immunoassay (EIA-IgG), (3) commercial polyspecific enzyme immunoassay that detects antibodies of IgG, IgA, and/or IgM istotypes (EIA-GAM), and (4) latex immunoturbidimetric assay (LIA). AL amyl., amyloid light-chain amyloidosis; F, female; Fx, fondaparinux; LMWH, low-molecular-weight heparin; M, male; OD, optical density; Pos, positive; RUL DVT, right upper-limb deep-vein thrombosis.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/130/14/10.1182_blood-2017-06-788679/4/m_blood788679f1.jpeg?Expires=1769138553&Signature=sZ-sSYZ~I7XDN8dRd7lvzAfHnK6YZetQQER2oYZvzTqNM4NG9dUayJ5nJcZe7lLAknyoOs76dhafD7UFCCeokb1xjmqkWnNHheAS7KpcSAkO8udrEYtG5YEqyaUUDzf9Y~vl4xQY34hUtD9brcUABZOXmh6P6ex8I~McqzLsolRUQ4bswVQNXRyeH2AGVAunWMKb7PGBR2ym5B4WoK0w1iBeg09De9uMu~IhCA1RFAyzdavkeVPK8dFoLWvX-aQzWJ6psyaF4aJqh8jrxw~2UipphIjjsISWkmWQSEX3FMUUg1D~zV1P26exsYIc23TSyR7Hgr6eVOqYKGrgxKczvw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)