Key Points
Platelet-activating, but not nonactivating, human HIT antibodies bind to and activate PF4-treated platelets.
Activating antibodies may recognize subtle conformational changes induced in PF4 by chondroitin-4-sulfate, the major platelet glycosaminoglycan.
Abstract
Antibodies specific for platelet factor 4 (PF4)/heparin complexes are the hallmark of heparin-induced thrombocytopenia and thrombosis (HIT), but many antibody-positive patients have normal platelet counts. The basis for this is not fully understood, but it is believed that antibodies testing positive in the serotonin release assay (SRA) are the most likely to cause disease. We addressed this issue by characterizing PF4-dependent binding of HIT antibodies to intact platelets and found that most antibodies testing positive in the SRA, but none of those testing negative, bind to and activate platelets when PF4 is present without any requirement for heparin (P < .0001). Binding of SRA-positive antibodies to platelets was inhibited by chondroitinase ABC digestion (P < .05) and by the addition of chondroitin-4-sulfate (CS) or heparin in excess quantities. The findings suggest that although all HIT antibodies recognize PF4 in a complex with heparin, only a subset of these antibodies recognize more subtle epitopes induced in PF4 when it binds to CS, the major platelet glycosaminoglycan. Antibodies having this property could explain “delayed HIT” seen in some individuals after discontinuation of heparin and the high risk for thrombosis that persists for weeks in patients recovered from HIT.
Introduction
Heparin-induced thrombocytopenia and thrombosis (HIT), a major cause of morbidity and mortality in patients treated with heparin,1-3 is caused by immunoglobulins (“HIT antibodies”) that recognize platelet factor 4 (PF4) in a complex with heparin.1,2,4-6 HIT is typically characterized by a more than 50% decrease in platelet counts occurring 5 to 10 days after initiating heparin therapy, sometimes accompanied by thrombosis.7,8 It is widely thought that HIT antibodies bind to heparin/PF4 complexes formed on or near the platelet membrane when heparin is infused, and that the resulting immune complexes induce platelet activation, release of platelet granule contents, and formation of procoagulant microparticles when immunoglobulin G (IgG) Fc domains engage platelet FcγRIIa (CD32) receptors.3,9 The half-life of unfractionated heparin administered intravenously is only about 2 hours,10 yet thrombotic risk persists for at least several weeks after an episode of HIT,7,11,12 and occasional patients with “delayed HIT” develop acute thrombocytopenia and thrombosis a week or more after their last exposure to heparin.13-16 These manifestations of heparin sensitivity, occurring long after heparin has been cleared from the circulation, are not well understood.
Rauova et al found that a murine monoclonal antibody (KKO) with specificity closely resembling that of a human HIT antibody binds to and activates human platelets in the absence of heparin when optimal quantities of PF4 are present and obtained evidence suggesting that the target recognized by KKO is PF4 in a complex with chondroitin-4 sulfate (CS), the major platelet glycosaminoglycan.17 In the same report, Rauova et al showed that 3 of 4 sera from patients who experienced HIT caused heparin-independent platelet activation (as measured by annexin V binding) when PF4 was present, but they did not directly demonstrate binding of the human antibodies to the target platelets. Because of its potential relevance to HIT pathogenesis, we further characterized the physical interaction of HIT antibodies with platelets in the presence and absence of PF4 and heparin, respectively. In this report, we provide the first direct demonstration of PF4-dependent, heparin-independent binding of human HIT antibodies to platelets and show that only antibodies capable of activating platelets (serotonin release and p-selectin expression) recognize platelets in the presence of PF4 alone. The findings are consistent with the possibility that, although both platelet activating and nonactivating antibodies bind to heparin/PF4 complexes, only the former antibodies recognize epitopes induced in PF4 when it binds to CS on the platelet surface, and suggest new approaches to identify antibodies that are most likely to be pathogenic.
Methods
Patient samples, HIT antibody testing
Samples from patients suspected of having HIT were obtained from the Platelet and Neutrophil Immunology Laboratory of the BloodCenter of Wisconsin (Milwaukee, WI), and were initially tested by that laboratory as part of a HIT evaluation. Three to 5 normal donor sera were pooled for use as a normal control and were confirmed negative for HIT antibodies in the polyvinylsulfonate PF4 enzyme-linked immunosorbent assay (PF4 ELISA). IgG PF4 ELISA was performed as previously described.18 The serotonin release assay (SRA) was performed as described by Sheridan et al,19 with slight modifications. A total of 57 identity-blinded patient samples were selected on the basis of testing positive in the PF4 ELISA (optical density [OD] > 0.4 and being inhibited by at least 49% with high dose [HD; 100 U/mL] unfractionated heparin). Of these patients, 24 were SRA-negative (serotonin release of less than 20% with low dose [LD; 0.1 U/mL] heparin), and 33 were SRA-positive (serotonin release of more than 20% with LD heparin and less than 20% release with HD heparin with a greater than 5% difference between values). SRA testing was performed at least twice on each sample, and a sample was designated SRA-positive if one or more unequivocally positive results was obtained.
Reagents
Reagents used were phycoerythrin-labeled mouse anti-human CD62p (p-selectin) (BD Biosciences, Franklin Lakes, NJ); unfractionated porcine heparin (Sagent Pharmaceuticals, Schaumburg, IL); Dylight649 goat anti-mouse IgG (Fc) and allophycocyanin (APC)-labeled goat anti-human IgG (Fc) (Jackson Immunology, West Grove, PA); phosphate-buffered saline (PBS) (Corning, Corning, NY); heparinase III, chondroitinase ABC, CS (from bovine trachea), bovine serum albumin (BSA), and mouse IgG isotype control (Sigma-Aldrich, St. Louis, MO); murine monoclonal antibody KKO (a generous gift from Gowthami Arepally, MD, Duke University Medical Center, Durham, NC); and Alexafluor 647 labeled anti-GPIIb antibody (290.5; BloodCenter of Wisconsin). Human PF4 was purified from normal donor platelets, as previously described.4 For PF4:glycosaminoglycan (GAG) ratio calculations, the average molecular weight of unfractionated heparin was assumed to be 15 kD.10 An average molecular weight of 31 kD was used for CS (determined by polyacrylamide gel electrophoresis and Alcian blue stain, data not shown).
Binding of HIT antibodies to normal platelets
Normal platelets were isolated from pooled citrated platelet-rich plasma of 2 to 3 group O blood donors. Prostaglandin E1 (50 ng/mL) was added, and platelet-rich plasma was centrifuged at 150 × g for 15 minutes. The supernatant was centrifuged at 1000 × g for 15 minutes to pellet platelets. The platelet button was resuspended in phosphate-buffered isotonic saline at pH 7.2, 1% BSA (PBS-BSA). Next, 1.0 × 106 platelets suspended in PBS-BSA were treated with serum samples (2.5 µL) or 10 µg/mL of murine monoclonal antibody, with and without 100 µg/mL PF4, HD heparin, or excess CS (as described in Results) to produce a final reaction mixture of 50 µL. After incubation for 15 minutes at room temperature, platelets were centrifuged once, and the pellet was resuspended in 50 µL PBS-BSA. APC-labeled goat anti-human IgG or Dylight649 goat anti-mouse IgG (both at 1:300 dilution) were added, and the mixture was incubated in the dark for 30 minutes. The samples were diluted to 250 μL, the platelet population was gated by forward and side scatter in an Accuri C6 flow cytometer, and median fluorescent intensity (MFI) of APC or Dylight649-labeled platelet events was recorded. Results were expressed as the ratio of APC/Dylighth649 MFI obtained with test sample to MFI obtained with pooled normal serum.
P-selectin expression
1.0 × 106 platelets suspended in PBS-BSA were treated with serum samples (5 µL) or 10 µg/mL murine monoclonal antibody, with and without 75 µg/mL PF4, HD heparin, or excess CS (as described in Results) to produce a final reaction mixture of 50 µL. After incubation for 45 minutes at 37°C, phycoerythrin-labeled anti-p-selectin and Alexafluor 647-labeled anti-GPIIb antibodies were added. After 15 minutes, platelet fluorescence was analyzed in an Accuri C6 flow cytometer. Platelet events were gated by GPIIb positivity (Alexafluor 647), and p-selectin expression (phycoerythrin-positive events) was recorded. Results were expressed as the ratio of p-selectin expression (MFI) obtained with test sample to MFI obtained with pooled normal serum.
Chondroitinase ABC and heparinase III digestion
Statistics
The Mann-Whitney test was used to compare the 2 groups of SRA-positive and SRA-negative samples, and Pearson coefficients were calculated for correlation testing (Prism; Graphpad, La Jolla, CA). All other analyses used Student’s t-test (Microsoft Excel; Microsoft Corp., Redmond, WA). A P value of <.05 was considered significant.
Institutional review board approval
Studies involving human subjects were approved by the institutional review board of the BloodCenter of Wisconsin. Research was conducted in accordance with the Declaration of Helsinki.
Results
PF4 promotes binding to platelets of IgG in SRA-positive, but not SRA-negative, patient samples
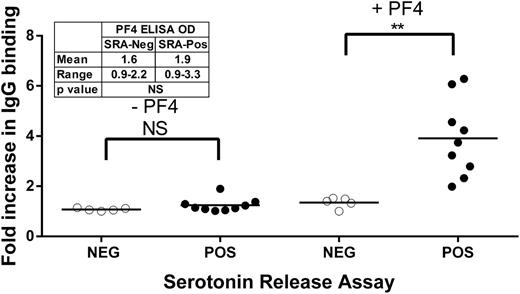
Preliminary studies confirmed the finding of Rauova et al,17 that the HIT-like monoclonal antibody KKO binds to platelets in the absence of heparin when PF4 (100 µg/mL) is present, but not when PF4 is absent (data not shown). Similar studies were conducted with 9 human HIT antibodies previously shown to produce strong positive reactions in the PF4 ELISA (mean OD, 1.9; range, 0.9-3.3) and in the SRA (all >70% release), and with 5 antibodies that were strongly positive in PF4 ELISA (mean OD, 1.6; range, 0.9-2.2; P = not significant compared with the SRA-positive group) but negative in the SRA. Under the experimental conditions employed, the SRA-positive sera behaved similar to KKO in binding to platelets when PF4 was present, but the SRA-negative samples did not (Figure 1). Mean IgG platelet binding values for SRA-positive vs SRA-negative samples were 1.2-fold vs 1.1-fold (no PF4; P = not significant) and 3.9-fold vs 1.3-fold (added PF4; P < .01), respectively, relative to normal serum (Figure 1).
Platelet binding of IgG in selected SRA-positive (filled circles) and SRA-negative (open circles) HIT samples in the presence and absence of PF4. Ordinate depicts fold increase in IgG binding relative to value obtained with normal serum. Each circle depicts the average of triplicate measurements. Horizontal bars depict the mean of each group. Asterisks indicate significance of the difference between means (**P < .01). Insert summarizes the results of PF4 ELISA testing done with the same sample set. SRA-negative and SRA-positive samples did not differ from each other in the PF4 ELISA.
Platelet binding of IgG in selected SRA-positive (filled circles) and SRA-negative (open circles) HIT samples in the presence and absence of PF4. Ordinate depicts fold increase in IgG binding relative to value obtained with normal serum. Each circle depicts the average of triplicate measurements. Horizontal bars depict the mean of each group. Asterisks indicate significance of the difference between means (**P < .01). Insert summarizes the results of PF4 ELISA testing done with the same sample set. SRA-negative and SRA-positive samples did not differ from each other in the PF4 ELISA.
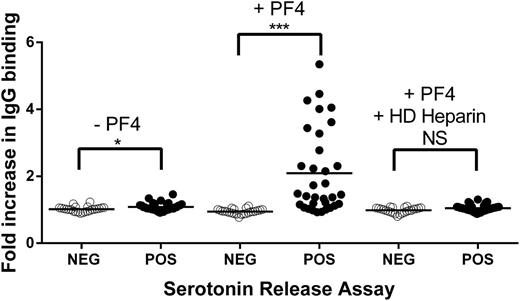
Samples used to obtain results shown in Figure 1 had been selected on the basis of their prior strong positive reactions in HIT testing, and the technologist performing the studies was aware of these results. We therefore performed a similar, independent study using 57 serum samples that had produced a wider range of test results in initial testing and were coded so that their identity was unknown to those performing the assays. Each of the 57 samples had tested positive in PF4 ELISA, and 33 had also tested positive in the SRA. As shown in supplemental Figure 1 (available on the Blood Web site), SRA-positive samples reacted more strongly in PF4 ELISA (mean OD, 2.5; range, 1.2-5.9) than SRA-negative samples (mean OD, 1.1; range, 0.5-3.6; P < .0001). However, there was considerable overlap of OD values between the 2 groups. As shown in Figure 2, SRA-positive and SRA-negative samples behaved similarly when they were incubated with platelets in the absence of added PF4, although slightly more IgG binding (mean fold increase, 1.08 vs 1.02, relative to normal serum; P < .05) was observed with the SRA-positive samples. The addition of PF4 produced a marked increase in IgG binding to platelets only in SRA-positive samples (mean fold increase, 2.1 vs 0.95, relative to normal serum; P < .0001) that was completely inhibited by HD heparin.
Platelet binding of IgG in 57 identity-blinded HIT antibody samples in the presence and absence of PF4 and HD heparin. (Left) In the absence of added PF4, IgG from SRA-negative samples (open circles) failed to bind, and SRA-positive samples (filled circles) bound very weakly. (Center) When PF4 was present, binding of IgG in SRA-positive samples, but not SRA-negative samples, was markedly enhanced. (Right) PF4-dependent IgG binding was completely inhibited by HD heparin (100 U/mL). Ordinate depicts fold increase in IgG binding relative to value obtained with normal serum. Each circle depicts the average of duplicate determinations. Horizontal bars depict the mean of each group. Asterisks indicate significance of the difference between means (***P < .001; *P < .05).
Platelet binding of IgG in 57 identity-blinded HIT antibody samples in the presence and absence of PF4 and HD heparin. (Left) In the absence of added PF4, IgG from SRA-negative samples (open circles) failed to bind, and SRA-positive samples (filled circles) bound very weakly. (Center) When PF4 was present, binding of IgG in SRA-positive samples, but not SRA-negative samples, was markedly enhanced. (Right) PF4-dependent IgG binding was completely inhibited by HD heparin (100 U/mL). Ordinate depicts fold increase in IgG binding relative to value obtained with normal serum. Each circle depicts the average of duplicate determinations. Horizontal bars depict the mean of each group. Asterisks indicate significance of the difference between means (***P < .001; *P < .05).
SRA-positive, but not SRA-negative, patient samples induced platelet p-selectin expression when PF4 was present
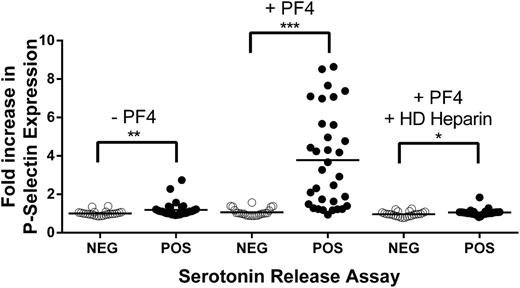
In preliminary experiments, using 2 SRA-positive HIT samples that were available in large amounts, we demonstrated that platelet activation can be reliably measured by examining surface p-selectin expression in PF4-treated platelets to which SRA-positive HIT sera were added, and showed that p-selectin expression is fully inhibited by FcγRIIa receptor blockade with murine monoclonal antibody IV.3 (supplemental Figure 2). The ability of the 57-member HIT antibody panel to induce p-selectin expression in the presence and absence of PF4 was then characterized. As shown in Figure 3, in the absence of added PF4, SRA-negative samples failed to induce p-selectin expression, and SRA-positive samples produced only a slight, but significant, p-selectin increase (mean fold increase, 1.19 vs 1.01, relative to normal serum; P < .01). The addition of PF4 had no effect on the SRA-negative samples but greatly augmented p-selectin expression induced by the SRA-positive samples (mean fold increase, 3.78 vs 1.07 for SRA-negative samples; P < .0001). As in the IgG binding studies, p-selectin expression was significantly inhibited by HD heparin.
Platelet activation induced by 57 identity-blinded HIT antibody samples in the presence and absence of PF4 and HD heparin. (Left) In the absence of added PF4, minimal, but significant, p-selectin expression was induced by SRA-positive samples (filled circles) only. (Center) When PF4 was present, p-selectin expression induced by SRA-positive samples was markedly enhanced. (Right) PF4-dependent p-selectin expression was significantly inhibited by HD heparin (100 U/mL). Ordinate depicts fold increase in p-selectin expression relative to value obtained with normal serum. Values shown are the means of duplicate determinations. Horizontal bars depict the mean of each group. Asterisks indicate significance of the difference between means (***P < .001; **P < .01; *P < .05).
Platelet activation induced by 57 identity-blinded HIT antibody samples in the presence and absence of PF4 and HD heparin. (Left) In the absence of added PF4, minimal, but significant, p-selectin expression was induced by SRA-positive samples (filled circles) only. (Center) When PF4 was present, p-selectin expression induced by SRA-positive samples was markedly enhanced. (Right) PF4-dependent p-selectin expression was significantly inhibited by HD heparin (100 U/mL). Ordinate depicts fold increase in p-selectin expression relative to value obtained with normal serum. Values shown are the means of duplicate determinations. Horizontal bars depict the mean of each group. Asterisks indicate significance of the difference between means (***P < .001; **P < .01; *P < .05).
Although patient samples were used at a final dilution of 1/10 to 1/20 in these studies, we considered the possibility that traces of residual heparin might somehow have been responsible for IgG binding and platelet activation seen with the SRA-positive samples only. To address this possibility, the studies were repeated using IgG purified from 3 SRA-positive samples with protein G Sepharose, and with these samples preabsorbed with Ecteola Cellulose to remove traces of heparin.4 Findings made were essentially identical to those obtained with untreated samples (data not shown).
PF4-dependent IgG binding to platelets correlated with PF4-dependent p-selectin expression
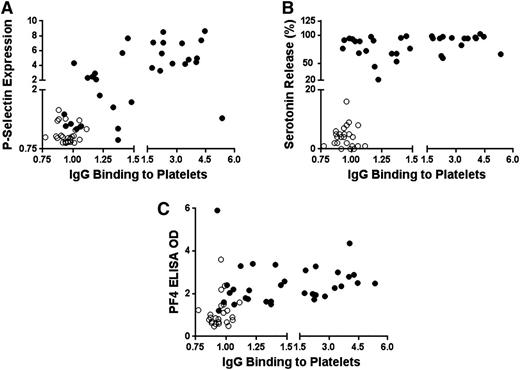
The relationship of PF4-dependent IgG binding to p-selectin expression, serotonin release, and performance in PF4 ELISA is shown for individual patient samples in Figure 4A-C. When SRA-positive (filled circles) and SRA-negative (open circles) samples were analyzed together, there was a significant correlation (Pearson correlation, r = 0.64; P < .0001) between PF4-dependent IgG binding to platelets and p-selectin expression (Figure 4A). PF4-dependent IgG binding also correlated with the degree of serotonin release (r = 0.55; P < .0001) (Figure 4B) and performance in the PF4 ELISA (r = 0.42; P < .01) (Figure 4C). Because statistical outcomes were obviously skewed by clustering of the SRA-negative sample results in the lower left-hand sector of each figure, the analysis was repeated using only data obtained with the SRA-positive samples (Figure 4A-C, filled circles). The correlation between IgG binding and p-selectin expression persisted in this subset (r = 0.48; P < .01), while there was no significant relationship between IgG binding and performance in the PF4 ELISA or in SRA (Figure 4B-C).
Relationships between PF4-dependent IgG binding to platelets and p-selectin expression, serotonin release in SRA, and OD in PF4 ELISA observed in studying 57 identity-blinded HIT samples. (A-C) Ordinate depicts fold increase in PF4-dependent p-selectin expression relative to value obtained with normal control serum, percentage serotonin release, and PF4 ELISA OD, respectively. In addition, abscissa depicts fold increase in PF4-dependent IgG binding relative to value obtained with normal serum. SRA-positive and SRA-negative HIT sera are depicted by filled and open circles, respectively.
Relationships between PF4-dependent IgG binding to platelets and p-selectin expression, serotonin release in SRA, and OD in PF4 ELISA observed in studying 57 identity-blinded HIT samples. (A-C) Ordinate depicts fold increase in PF4-dependent p-selectin expression relative to value obtained with normal control serum, percentage serotonin release, and PF4 ELISA OD, respectively. In addition, abscissa depicts fold increase in PF4-dependent IgG binding relative to value obtained with normal serum. SRA-positive and SRA-negative HIT sera are depicted by filled and open circles, respectively.
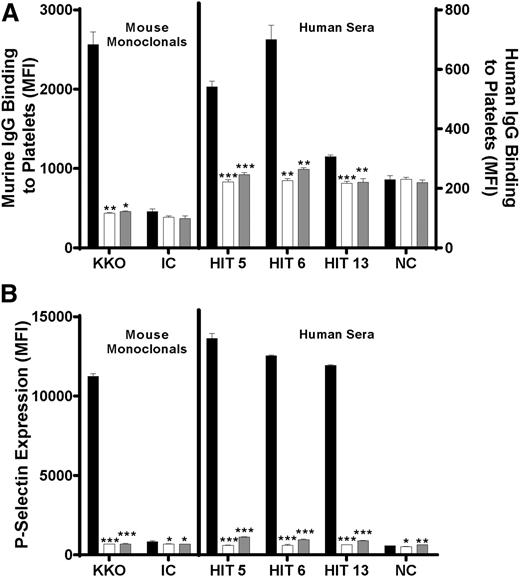
PF4-dependent HIT antibody binding was reduced after treatment of platelets with chondroitinase ABC, but not heparinase III
These findings suggest that platelet-activating HIT antibodies recognize PF4 in a complex with some component of the platelet surface and that the resulting interaction is sufficiently stable for the Fc domains of bound antibodies to activate platelets via the platelet IgG receptor, FcγRIIa. Potential candidate molecules that could form complexes with PF4 recognized by HIT antibodies include CS and heparan sulfate, both GAGs and known to be present in human platelets.21 Therefore, the effect of treating platelets with chondroitinase ABC (which degrades CS) and heparinase III (which degrades heparan sulfate) on HIT antibody-platelet binding was studied. Chondroitinase ABC treatment led to a ∼60% decrease in PF4-dependent KKO binding, but heparinase III treatment had no effect (Figure 5, left), confirming the previous report of Rauova et al.22 When 3 SRA-positive HIT antibodies were similarly studied, it was found that chondroitinase ABC treatment reduced PF4-dependent IgG binding by 32% (P < .01), 32% (P < .05), and 22% (P < .01), but heparinase III treatment only reduced binding slightly (7%; P < .05) in 1 sample (Figure 5, right). Neither chondroitinase ABC nor heparinase III digestion affected binding of a human antibody specific for the platelet-specific antigen HPA-1a (Figure 5, right). Previous work indicates that bovine and human CS are similar in chemical composition.21 Both CS from a bovine source and unfractionated heparin, when added in molar excess to PF4 (10:1, GAG:PF4), completely inhibited PF4-dependent IgG binding (Figure 6A) and platelet p-selectin expression (Figure 6B) induced by KKO and the 3 SRA-positive HIT antibodies.
Effect of chondroitinase ABC and heparinase III treatment on PF4-dependent IgG binding to platelets. Chondroitinase ABC treatment (filled bar) significantly reduced PF4-dependent binding of the HIT-mimetic monoclonal KKO (left) and IgG in 3 SRA-positive HIT sera (right). Ordinate depicts IgG binding to platelets relative to control digestion (no enzyme). Treatment with heparinase III (open bar) was without effect except for a 7% reduction in IgG binding observed with a single HIT sample. Values shown are the means of triplicate determinations +1.0 standard deviation. HPA-1a is a serum sample containing antibody to platelet-specific antigen HPA-1a. NC, pooled normal serum. Asterisks indicate significance of differences between enzyme-treated and untreated samples (***P < .001; **P < .01; *P < .05).
Effect of chondroitinase ABC and heparinase III treatment on PF4-dependent IgG binding to platelets. Chondroitinase ABC treatment (filled bar) significantly reduced PF4-dependent binding of the HIT-mimetic monoclonal KKO (left) and IgG in 3 SRA-positive HIT sera (right). Ordinate depicts IgG binding to platelets relative to control digestion (no enzyme). Treatment with heparinase III (open bar) was without effect except for a 7% reduction in IgG binding observed with a single HIT sample. Values shown are the means of triplicate determinations +1.0 standard deviation. HPA-1a is a serum sample containing antibody to platelet-specific antigen HPA-1a. NC, pooled normal serum. Asterisks indicate significance of differences between enzyme-treated and untreated samples (***P < .001; **P < .01; *P < .05).
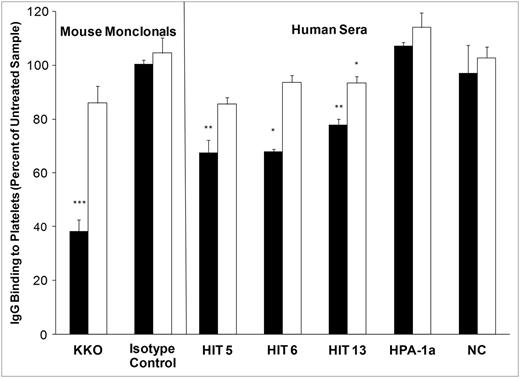
Excess CS and heparin (relative to PF4) inhibit PF4-dependent binding of KKO and HIT IgG to platelets and resultant platelet activation. Platelets were treated with PF4 alone (black bar), PF4 and excess heparin (white bar), or PF4 and excess CS (gray bar), and with KKO (left) or HIT sera (right). Values shown are the average of triplicate determinations + 1.0 standard deviation. The ordinate depicts median fluorescence intensity of IgG binding to platelets (A) and platelet p-selectin expression (B). IC, isotype control; NC, pooled normal serum. Asterisks indicate significance of differences between means of samples treated with excess GAG vs no GAG (***P < .001; **P < .01; P < .05).
Excess CS and heparin (relative to PF4) inhibit PF4-dependent binding of KKO and HIT IgG to platelets and resultant platelet activation. Platelets were treated with PF4 alone (black bar), PF4 and excess heparin (white bar), or PF4 and excess CS (gray bar), and with KKO (left) or HIT sera (right). Values shown are the average of triplicate determinations + 1.0 standard deviation. The ordinate depicts median fluorescence intensity of IgG binding to platelets (A) and platelet p-selectin expression (B). IC, isotype control; NC, pooled normal serum. Asterisks indicate significance of differences between means of samples treated with excess GAG vs no GAG (***P < .001; **P < .01; P < .05).
Discussion
Several previous studies have demonstrated heparin-dependent binding of HIT antibodies to intact platelets in the presence or absence of PF4,4,9,23,24 but PF4-dependent heparin-independent binding has not been previously reported. Newman et al showed that PF4 released from platelets by HIT antibodies in the presence of heparin binds to the platelet surface and promotes antibody binding and platelet activation, but concluded that heparin is required for this process.9 Rauova et al showed that PF4 alone at a concentration of 50 to 100 µg/mL enables binding to platelets and platelet activation by KKO,17 a monoclonal antibody developed by Arepally et al that closely mimics the behavior of platelet-activating antibodies from patients with HIT.25 In the same study, Rauova et al showed that HIT antibodies from 3 of 4 HIT patients induced platelet activation (annexin V binding) when PF4 was present but did not directly demonstrate IgG-platelet binding,17 Findings described here show that PF4, at the same concentration that is optimal for KKO binding, promotes binding of SRA-positive HIT antibodies to platelets that can be detected using flow cytometry, provided platelets are washed only once in buffer before platelet-bound IgG is measured. In preliminary studies, PF4 in the range of 75 to 100 µg/mL was found to be optimal for the demonstration of IgG-platelet binding and p-selectin expression under the experimental conditions used (data not shown). Failure to demonstrate PF4-dependent binding of HIT antibodies to platelets in previous studies may have been the result of washing the targeted platelets multiple times and/or using PF4 at a concentration that is not optimal.4
HIT antibodies that are positive in SRA are known to react more strongly in the PF4 ELISA than SRA-negative antibodies;26,27 this was true of the antibodies evaluated in our study with identity-blinded HIT samples. However, there was a significant overlap in OD values obtained in PF4 ELISA with SRA-positive and SRA-negative samples in the 57 sera tested (supplemental Figure 1). Moreover, studies performed with an initial set of antibodies composed of SRA-positive and SRA-negative samples that reacted equally well in the PF4 ELISA also showed that only SRA-positive samples bind to platelets treated with PF4 (Figure 1). P-selectin expression studies performed with this same group of antibodies showed that SRA-positive samples induce far more p-selectin expression than SRA-negative samples (mean, 8-fold and 1.9-fold, respectively; P < .01, data not shown), relative to normal serum. PF4-dependent binding of HIT antibodies to platelets was not an experimental artifact lacking functional significance, as there was a strong positive correlation between IgG binding and induction of p-selectin expression (Figure 4A). In contrast, there was no correlation between platelet binding and the strength of reactions obtained in the PF4 ELISA when only SRA-positive samples were considered (Figure 4B-C). These observations suggest that although all HIT antibodies bind to PF4-heparin complexes, yielding positive results in the PF4 ELISA, only the subset of antibodies that is positive in SRA binds to platelets when PF4 is present, likely by recognizing conformational changes induced in PF4 when it binds to platelet surface GAGs. Reduction of this interaction after treatment of platelets with chondroitinase ABC, but not heparinase III, suggests that CS is the likely binding partner for PF4. Failure of chondroitinase ABC treatment to totally abolish PF4-dependent IgG binding may reflect physical inaccessibility of much of the membrane GAG to enzyme.
Platelet GAGs have been only partially characterized. Okayama et al28 and Nader21 identified CS as the major platelet GAG; lesser quantities of heparan sulfate are also present.21 Both CS and heparin are sulfated GAGs that carry a net negative charge; however, CS carries fewer than half as many sulfates as heparin (0.97 vs 2.33 sulfates per disaccharide subunit, respectively).29 Consistent with this difference, the avidity of heparin for PF4 is much greater than that of CS.29 A commonly used diagnostic maneuver to confirm the specificity of antibodies that test positive in the PF4 ELISA and the SRA is the demonstration that a reaction is significantly inhibited when excess heparin is added.18,19 Similarly, addition of excess CS, despite its weaker negative charge, also inhibits PF4-dependent IgG binding and platelet activation by SRA-positive HIT antibodies (Figure 6).
The molecular structure of PF4 in a complex with heparin or a heparin fragment has not yet been resolved, but evidence suggests that HIT antibodies recognize at least 2 and probably more distinct conformational epitopes created in PF4 when sulfate groups of heparin bind to positively charged lysine residues in α helices of the PF4 tetramer.30-33 Because of its lower charge density, CS would be expected to induce more subtle structural changes in PF4 than heparin or other strongly charged linear polyanions such as polyvinylsulfonate.34 Our findings are consistent with the possibility that HIT antibodies capable of recognizing PF4-CS complexes on the platelet surface differ qualitatively from their nonactivating counterparts in recognizing epitopes induced in PF4 by CS (Figure 7). Our attempts to confirm this by using complexes of commercially available CS (from bovine trachea, average molecular weight 31 kDa) and PF4 as targets for antibody in ELISA have been unsuccessful (data not shown), making it likely that to induce structural changes in PF4 recognized by this antibody subset, CS, and possibly the structural proteins on which it is arrayed, must be in a configuration comparable to that found naturally in the platelet plasma membrane. Alternatively, bovine and human CS could differ structurally in ways not yet defined. A recent report by Cuker et al also suggests that platelet-activating and nonactivating HIT antibodies recognize different targets, in that the former antibodies partially block binding of monoclonal KKO to PF4-heparin complexes, whereas the latter do not.27 Further studies are needed to define the relationship between their findings and the results described here.
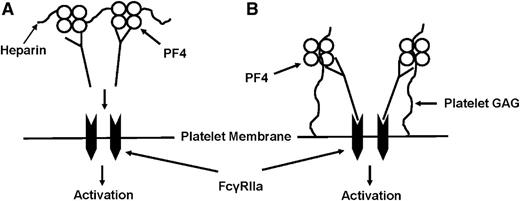
Proposed model of PF4-dependent, heparin-independent HIT antibody-mediated platelet binding/activation. (A) According to a widely held model, HIT antibodies bind to complexes of PF4 and heparin formed on or near the platelet surface; IgG Fc domains clustered in this way cross-link platelet FcγRIIa, triggering platelet activation. Because all HIT antibodies bind to heparin/PF4 complexes, this model does not explain why only a subset of antibodies is platelet-activating or why thrombotic risk persists for several months in patients recovered from HIT. (B) An alternative model suggested by findings described here proposes that platelet-activating antibodies differ qualitatively from those that are nonactivating, in being able to recognize subtle conformational changes induced in PF4 when it binds to chondroitin sulfate (CS) normally displayed on the platelet membrane. Small quantities of PF4 may always be present on the platelet surface in a complex with CS and can be recognized by these antibodies. If bound IgG is clustered sufficiently, IgG Fc domains cross-link FcγRIIa, leading to platelet activation. Resulting release of PF4 could further increase levels of CS/PF4 on the platelet surface, leading to additional antibody binding and acceleration of the activation process.
Proposed model of PF4-dependent, heparin-independent HIT antibody-mediated platelet binding/activation. (A) According to a widely held model, HIT antibodies bind to complexes of PF4 and heparin formed on or near the platelet surface; IgG Fc domains clustered in this way cross-link platelet FcγRIIa, triggering platelet activation. Because all HIT antibodies bind to heparin/PF4 complexes, this model does not explain why only a subset of antibodies is platelet-activating or why thrombotic risk persists for several months in patients recovered from HIT. (B) An alternative model suggested by findings described here proposes that platelet-activating antibodies differ qualitatively from those that are nonactivating, in being able to recognize subtle conformational changes induced in PF4 when it binds to chondroitin sulfate (CS) normally displayed on the platelet membrane. Small quantities of PF4 may always be present on the platelet surface in a complex with CS and can be recognized by these antibodies. If bound IgG is clustered sufficiently, IgG Fc domains cross-link FcγRIIa, leading to platelet activation. Resulting release of PF4 could further increase levels of CS/PF4 on the platelet surface, leading to additional antibody binding and acceleration of the activation process.
Significant quantities of PF4 are normally present on endothelial cells, presumably the result of PF4 released from circulating platelets in the course of their lifespan,35 and it is likely that small quantities of PF4 are normally present on the surface of platelets themselves in amounts that could differ significantly from time to time, depending on vascular pathology, low-grade activation of clotting, platelet PF4 content,17,36 and other variables. A report by Rauova et al17 that concentrations of PF4 found to be optimal for activation of platelets by HIT antibodies can be achieved “in the immediate environ of activated platelets” (unpublished observations) and by their demonstration that injection of KKO alone causes moderate thrombocytopenia in FcγRIIa transgenic mice expressing low-range levels of PF4 and severe thrombocytopenia in animals expressing high or mid-range levels of human PF417 suggest our findings may be directly relevant to HIT pathogenesis. A subset of HIT antibodies that recognizes PF4 on the platelet surface in the absence of heparin and activates platelets when it binds to this target could account for heparin-independent serotonin release seen with some HIT antibodies15,37 and the significantly increased thrombotic risk that persists for several weeks to months in patients recovered from HIT.7,11,38 High-titer antibodies of this type might also be capable of inducing thrombocytopenia and thrombosis typical of “delayed HIT” long after heparin has been discontinued.13-15
The SRA is claimed to have sensitivity and specificity greater than 98% and 95%, respectively, for a clinical diagnosis of HIT,39 and it has been suggested that a positive result in this test can serve as a surrogate for the clinical condition.40,41 Our study was performed retrospectively on samples tested initially because of a suspicion of HIT, but the clinical status of the individual patients is unknown. Considering the strong correlation that exists between SRA positivity and clinical disease, we anticipate that antibodies from patients with clinical HIT are also likely to exhibit PF4-dependent, heparin-independent platelet binding and activation. A prospective study to examine this issue is now being planned.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Gowthami Arepally (Duke University Medical Center) for providing KKO antibody for our studies and for critically reviewing the manuscript.
This study was supported in part by funds from the National Blood Foundation and the Clinical & Translational Science Institute of Southeastern Wisconsin (A.P.) and by National Institutes of Health National Heart, Lung, and Blood Institute grant HL-13629 (R.H.A.).
Authorship
Contribution: All authors were involved in study design; B.R.C. and J.G.M. provided the study materials; A.P. and C.G.J. performed experiments and analyzed the data; R.H.A. provided close oversight; A.P. wrote the manuscript; and all authors edited the manuscript and approved the final version.
Conflict-of-interest disclosure: A provisional US patent (61/896 951) covering some aspects of the HIT antibody assays described in the paper has been filed.
Correspondence: Anand Padmanabhan, BloodCenter of Wisconsin, 8733 Watertown Plank Rd, Milwaukee, WI 53226-3548; e-mail: anand.padmanabhan@bcw.edu.