Key Points
No overall clinical benefit was seen after the addition of lestaurtinib to standard chemotherapy for newly diagnosed FLT3-mutated AML.
Lower rates of relapse and improved overall survival were seen in patients who achieved sustained levels of FLT3 inhibitory activity.
Abstract
The clinical benefit of adding FMS-like tyrosine kinase-3 (FLT3)-directed small molecule therapy to standard first-line treatment of acute myeloid leukemia (AML) has not yet been established. As part of the UK AML15 and AML17 trials, patients with previously untreated AML and confirmed FLT3-activating mutations, mostly younger than 60 years, were randomly assigned either to receive oral lestaurtinib (CEP701) or not after each of 4 cycles of induction and consolidation chemotherapy. Lestaurtinib was commenced 2 days after completing chemotherapy and administered in cycles of up to 28 days. The trials ran consecutively. Primary endpoints were overall survival in AML15 and relapse-free survival in AML17; outcome data were meta-analyzed. Five hundred patients were randomly assigned between lestaurtinib and control: 74% had FLT3-internal tandem duplication mutations, 23% FLT3–tyrosine kinase domain point mutations, and 2% both types. No significant differences were seen in either 5-year overall survival (lestaurtinib 46% vs control 45%; hazard ratio, 0.90; 95% CI 0.70-1.15; P = .3) or 5-year relapse-free survival (40% vs 36%; hazard ratio, 0.88; 95% CI 0.69-1.12; P = .3). Exploratory subgroup analysis suggested survival benefit with lestaurtinib in patients receiving concomitant azole antifungal prophylaxis and gemtuzumab ozogamicin with the first course of chemotherapy. Correlative studies included analysis of in vivo FLT3 inhibition by plasma inhibitory activity assay and indicated improved overall survival and significantly reduced rates of relapse in lestaurtinib-treated patients who achieved sustained greater than 85% FLT3 inhibition. In conclusion, combining lestaurtinib with intensive chemotherapy proved feasible in younger patients with newly diagnosed FLT3-mutated AML, but yielded no overall clinical benefit. The improved clinical outcomes seen in patients achieving sustained FLT3 inhibition encourage continued evaluation of FLT3-directed therapy alongside front-line AML treatment. The UK AML15 and AML17 trials are registered at www.isrctn.com/ISRCTN17161961 and www.isrctn.com/ISRCTN55675535 respectively.
Introduction
Activating mutations of the receptor tyrosine kinase FMS-like tyrosine kinase-3 (FLT3) are present at diagnosis in approximately one-third of patients with acute myeloid leukemia (AML), the majority of whom have a normal karyotype.1-3 Internal tandem duplication (ITD) mutations of the FLT3 juxtamembrane domain account for approximately three-quarters of these mutations and are associated with proliferative disease phenotype, increased relapse rate, and shortened overall survival (OS).4-6 The prognostic implications of the FLT3-ITD mutation vary according to mutation burden, with a high allelic ratio predicting higher relapse risk,5 and according to presence of coexisting mutations, the most frequent of these being NPM1c, which is present in 60% of younger FLT3-ITD mutated cases and appears to lessen the adverse prognostic effect.7 Tyrosine kinase domain point mutations make up the remaining 25% of FLT3 mutations and have less clearly established prognostic associations.8
Given the high incidence and clear deleterious prognostic effect of FLT3-ITD mutations, there has been a great deal of clinical interest in FLT3 as a therapeutic target, and a number of small molecule inhibitors with inhibitory activity against FLT3 have entered clinical trials.9 Although many of the patient responses seen in the early FLT3 monotherapy trials were limited in both depth and duration,10-14 there have been more recent reports of deeper, sustained remissions from newer, more potent FLT3 inhibitory compounds.15,16
Lestaurtinib (previously CEP-701), one of the so-called “first generation” of FLT3 inhibitors, is an orally available indolocarbazole alkaloid compound that was identified as a potent inhibitor of FLT3 (in both its ITD- and point-mutated configurations) at low nanomolar in vitro concentrations17 after originally being developed as a tropomyosin receptor kinase A neurotropin receptor inhibitor18 ; it is also a potent inhibitor of JAK2.19,20 Lestaurtinib is orally bioavailable and was generally well-tolerated in 2 monotherapy trials in patients with relapsed/refractory AML and in older patients considered unsuitable for intensive therapy, in which transient clinical responses, characterized by reductions in peripheral blood or bone marrow blasts or decreased transfusion requirements, were observed primarily in patients harboring FLT3-activating mutations.13,14 Crucially, in both of these monotherapy studies, clinical activity of lestaurtinib correlated closely with evidence of achievement of sustained reduction of FLT3 phosphorylation by more than 85%, as determined by plasma inhibitory activity (PIA) assay.21
Synergistic cytotoxicity to FLT3-mutated AML cells was observed in the laboratory when lestaurtinib was administered sequentially after chemotherapeutic agents.22 On this basis, the combination of lestaurtinib with chemotherapy (either mitozantrone, etoposide, cytarabine [MEC] or high-dose cytarabine [AraC]) was assessed in the Cephalon 204 trial, a randomized phase 3 study in patients with relapsed FLT3-mutated AML.23 Although no significant improvements in second complete remission (CR) rate or OS were demonstrated with the addition of lestaurtinib, correlation was again observed between in vivo FLT3 inhibition and achievement of clinical response; however, a disappointing proportion of Cephalon 204 study patients failed to achieve free drug levels sufficient to achieve optimal FLT3 inhibitory activity.
The published randomized clinical trial experience of FLT3-targeted kinase inhibitors has so far been limited to the difficult-to-treat population of patients with AML with relapsed or refractory disease. The potential clinical benefit of combining FLT3-targeted therapy with first-line intensive chemotherapy in patients with previously untreated AML has not yet been formally established. We undertook the first prospective randomized assessment of the addition, or not, of oral lestaurtinib, given sequentially after each cycle of chemotherapy, to patients with newly diagnosed AML presenting with a FLT3-ITD or FLT3–tyrosine kinase domain (TKD) mutation. This intervention was part of the UK MRC AML15 (ISRNCTN17161961) and carried forward, with the data blinded, into the UK NCRI AML17 (ISRNCTN55675535) trial.
Methods
Study design and participants
The UK MRC AML15 and NCRI AML 17 studies (ISRCTN 17161961 and 55675535) were large, prospective phase 3 multicenter trials for patients with newly diagnosed AML or high-risk myelodysplastic syndrome (MDS; >10% marrow blasts) that ran consecutively between May 2002 and December 2014 at more than 130 centers in the United Kingdom, Denmark, and New Zealand and addressed several randomized questions (supplemental Table 1, available on the Blood Web site). During 2007 to October 2012, patients with a FLT3 mutation could be randomly assigned to lestaurtinib or not. Patients were generally younger than 60 years, although older patients could be entered if considered suitable for intensive chemotherapy. Patients with acute promyelocytic leukemia or blast transformation of chronic myeloid leukemia were not eligible for randomization.
Both trials were sponsored by Cardiff University and approved by Wales REC3 on behalf of all UK investigators, by the Danish Medicines Agency for sites in Denmark, and by MEDSAFE for sites in New Zealand. The trials were conducted in accordance with the Declaration of Helsinki, with written consent being required for each randomization.
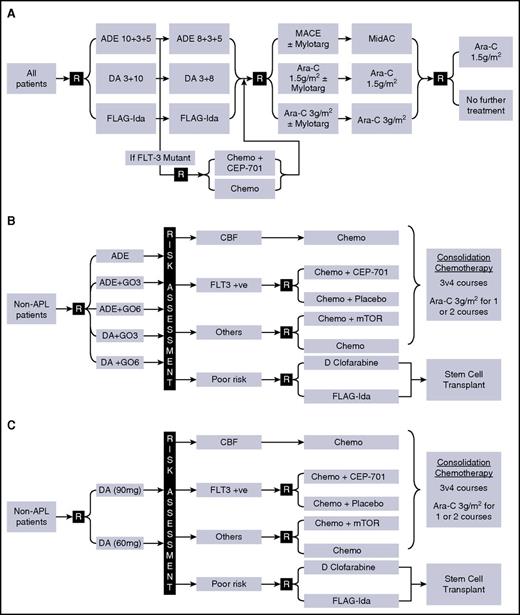
The trial designs of AML15 and AML17 involved a number of randomized interventions (Figure 1). Induction chemotherapy (courses 1-2) was with ADE, DA, or FLAG-Ida, with or without GO in course 1; consolidation (courses 3-4) comprised high-dose cytarabine (1.5 g/m2 or 3 g/m2) or MACE/MidAC. Allogeneic stem cell transplantation was permitted for patients with intermediate- or poor-risk disease with a recommendation of myeloablative conditioning for patients younger than 35 years and reduced-intensity conditioning for patients older than 45 years, with investigator/patient choice in the intermediate age group in AML15, but was recommended only for poor-risk patients in AML17. In neither trial was FLT3 status an indication for transplant.
Trial designs and treatment plan. (A) AML15 (2007-2009); (B) AML17 (2009-2011); (C) AML17 (2011-2014). ADE, cytarabine/daunorubicin/etoposide; APL, acute promyelocytic leukemia; CBF, core binding factor leukemia; DA, daunorubicin/cytarabine; D Clofarabine, daunorubicin/clofarabine; FLAG-Ida, fludarabine/cytarabine/G-CSF/idarubicin; GO, gemtuzumab ozogamicin; MACE, amsacrine/cytarabine/etoposide; MidAC, mitozantrone/cytarabine; mTOR: mTOR inhibition with everolimus.
Trial designs and treatment plan. (A) AML15 (2007-2009); (B) AML17 (2009-2011); (C) AML17 (2011-2014). ADE, cytarabine/daunorubicin/etoposide; APL, acute promyelocytic leukemia; CBF, core binding factor leukemia; DA, daunorubicin/cytarabine; D Clofarabine, daunorubicin/clofarabine; FLAG-Ida, fludarabine/cytarabine/G-CSF/idarubicin; GO, gemtuzumab ozogamicin; MACE, amsacrine/cytarabine/etoposide; MidAC, mitozantrone/cytarabine; mTOR: mTOR inhibition with everolimus.
Patients entered the allocated first induction chemotherapy course, during which investigators were informed of the FLT3 mutation status, which was centrally ascertained for all patients in 1 of 2 reference laboratories. Patients confirmed to harbor a FLT3 mutation (FLT3 ITD or TKD mutation quantified at 5% or more of total FLT3 alleles) were able to enter the lestaurtinib randomization and to start the allocated treatment 48 hours after completion of course 1 of induction treatment.
Lestaurtinib randomization and treatment schedule
In AML15, eligible patients were randomly assigned in a 1:1 ratio to receive lestaurtinib, or not, after each of 4 courses of chemotherapy. In AML17, this randomization was placebo controlled, with an allocation ratio of 2:1 lestaurtinib to placebo. In both studies, treatment allocation was by web-based computer minimization hosted by Cardiff University. Minimization parameters were age (0-15, 16-29, 30-39, 40-49, 50-59, or 60 years and older), World Health Organization performance status (0-4), induction treatment, and de novo vs secondary disease vs high-risk MDS.
Lestaurtinib (Cephalon Inc, Frazer, PA) was commenced 2 days after completion of each course of chemotherapy and administered in cycles of up to 28 days, for a maximum of 4 cycles, being stopped at least 2 days before commencing the next course of chemotherapy (Figure 1). The initial dose was 80 mg orally twice daily (bd; 12 hours between doses); if well-tolerated, an increase to a maximum dose of 100 mg bd was permitted from cycle 2 onward. In case of additional toxicity, which was anticipated with the coadministration of azole antifungal drugs (which have CYP3A4 inhibitory activity), provision was made for a reduced dose of 40-60 mg bd. There was no maintenance therapy with lestaurtinib. Patients receiving allogeneic stem cell transplant continued lestaurtinib until 28 days after their last pretransplant course of chemotherapy, but did not receive further lestaurtinib after transplant.
Correlative studies
Whole-blood samples were requested to be sent to the central UK laboratory on day 14 (±2 days) of each cycle of lestaurtinib. The samples were to be taken 12 hours after the patient’s most recent dose to enable assessment of trough FLT3 PIA, trough plasma concentration of lestaurtinib, and FLT3 ligand (FL) levels. Samples were separated by centrifugation and plasma stored frozen at −80°C before batch shipment.
The PIA assay was performed at Johns Hopkins University, Baltimore, Maryland, as previously described.21 Briefly, frozen plasma samples were thawed and clarified by centrifugation at 16 000g for 2 minutes. For each point, 2 × 106 TF/ITD cells (human AML TF-1 cell line expressing a FLT3-ITD construct) were incubated with 1 mL patient plasma at 37°C for 1 hour. Cells were then washed twice with ice-cold phosphate-buffered saline and lysed. After immunoblotting for phosphorylated FLT3, densitometric analysis was performed, and the FLT3 PIA for each plasma sample was calculated by expressing the density of its corresponding band as a percentage of that obtained from baseline untreated plasma.
Day 14 trough plasma concentrations of lestaurtinib were quantified by Cephalon Inc (West Chester, PA), using a validated high-performance liquid chromatography method, as previously described.23 FL concentrations in plasma samples were determined using an ELISA kit obtained from R&D Systems (Minneapolis, MN).
Statistical analysis
All study endpoints were defined according to the Revised International Working Group Criteria.24 The primary outcome measure for the AML15 trial was OS, which was amended to relapse-free survival (RFS) when the randomization rolled over into AML17. Secondary endpoints were achievement of CR, CR with incomplete peripheral blood count recovery (CRi), OS from lestaurtinib randomization, and relapse and death in remission (for patients achieving either CR or CRi), together with hematological recovery times, toxicity (scored using the National Cancer Institute Common Toxicity Criteria Version 3.025 ), and resource usage. Remission status was determined locally in participating centers.
All analyses are by intention-to-treat. Categorical endpoints (eg, CR rates) were compared using Mantel-Haenszel tests to give Peto odds ratios and confidence intervals. Continuous/scale variables were analyzed by nonparametric (Wilcoxon rank sum) tests. Time-to-event outcomes were analyzed using the log-rank test, with Kaplan-Meier survival curves. Odds/hazard ratios (OR/HR) lower than 1 indicate benefit for lestaurtinib. All survival percentages are at 5 years unless otherwise stated. Because of the change of design between AML15 and AML17, the 2 trials have been meta-analyzed, using standard methodology,26 and meta-analytic survival curves have been plotted.
In addition to overall analyses, exploratory analyses were performed stratified by the randomization stratification parameters and other important variables, with suitable tests for interaction. Because of the well-known dangers of subgroup analysis, these were interpreted cautiously.
Analyses of correlative laboratory studies were carried out using log rank tests and Cox proportional hazards regression for multivariable analyses. Repeated measures analyses were carried out using multilevel model repeated measure analyses.
Follow-up is complete until March 1, 2015, with a median follow-up for survival of 50.5 months (range, 1.3-97 months) and 288 events.
Results
Patients
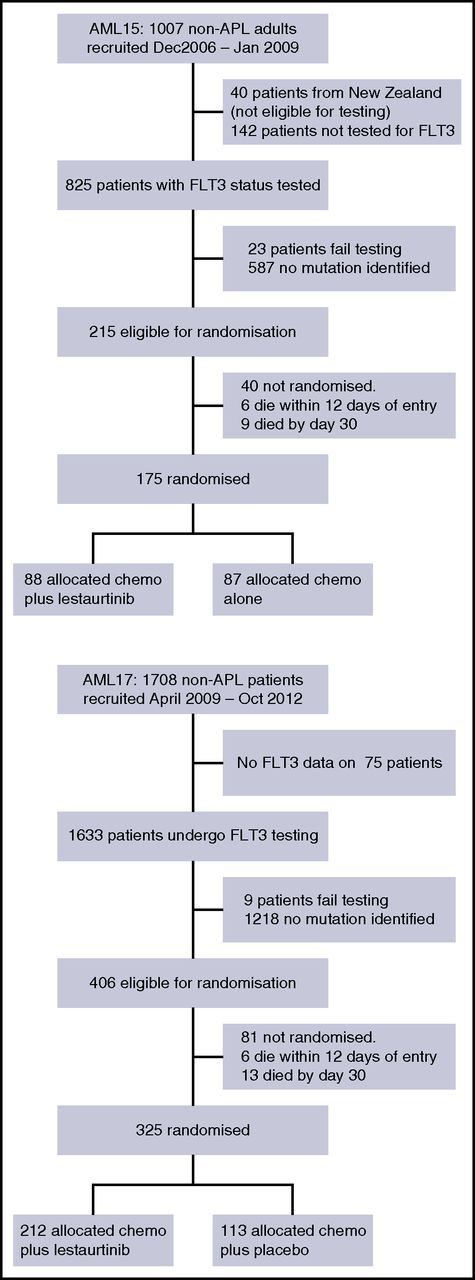
Between January 2007 and January 2009, 967 adult patients who did not have APL entered the AML15 trial and were eligible for FLT3 testing, of whom 215 had a FLT3 mutation (ITD alone, n = 156; TKD point mutation alone, n = 45; both, n = 3; mutation type undetermined, n = 7). Between April 2009 and October 2012, 1708 patients entered AML17, of whom 406 were identified as having a FLT3 mutation (ITD alone, n = 297; TKD alone, n = 94; both, n = 12; mutation type undetermined, n = 3). In total, 500 FLT3-mutated patients (AML15, n = 175; AML17, n = 325; 370 (74%) who had ITD alone, 115 (23%) with TKD alone, and 11 [2%] who had both; median ITD mutant percentage, 30.9% [range, 3%-98.4%]; 57 patients with allelic ratio ≥50%) entered the randomization; there were 4 patients for whom the mutation type was not determined; for 2 patients, the ITD allelic ratio was found to be below 5%, but these are included in the above mutated patients. The characteristics of patients, which were balanced between the groups, are shown in Table 1. The median age of FLT3-randomized patients was 49 years (range, 5-68 years); 5 patients younger than 16 years were included. Ninety-four percent of patients had de novo AML, 5% secondary AML, and 1% high-risk MDS. The majority of patients (89%) had cytogenetically intermediate-risk disease, with 6% favorable and 5% adverse risk. Median presenting WBC was 28 × 109/L (range, 0.2-363). Two hundred seventy patients (54%) had concomitant mutated NPM1c. All disease characteristics were balanced between the lestaurtinib and control groups, as were the other treatment interventions.
Patient characteristics
| Characteristics . | AML15 . | AML17 . | ||
|---|---|---|---|---|
| Lestaurtinib . | Control . | Lestaurtinib . | Placebo . | |
| Number randomized | 88 | 87 | 212 | 113 |
| Age group, years | ||||
| 0-15 | 0 | 0 | 3 | 2 |
| 16-29 | 9 | 10 | 22 | 10 |
| 30-39 | 15 | 14 | 20 | 10 |
| 40-49 | 24 | 26 | 57 | 31 |
| 50-59 | 30 | 28 | 83 | 44 |
| 60+ | 10 | 9 | 27 | 16 |
| Median (range) | 48 (16-66) | 46 (16-65) | 50 (5-68) | 50 (6-65) |
| Sex | ||||
| Female | 47 | 51 | 113 | 57 |
| Male | 41 | 36 | 99 | 56 |
| Type of disease | ||||
| De novo | 84 | 84 | 198 | 104 |
| Secondary | 3 | 4 | 10 | 6 |
| High-risk MDS | 0 | 0 | 4 | 3 |
| Performance status* | ||||
| 0 | 54 | 51 | 127 | 64 |
| 1 | 30 | 31 | 69 | 38 |
| 2 | 3 | 2 | 10 | 6 |
| 3 | 1 | 3 | 5 | 4 |
| 4 | 0 | 0 | 0 | 0 |
| WBC | ||||
| 0-9.9 | 17 | 25 | 48 | 29 |
| 10-49.9 | 33 | 37 | 100 | 42 |
| 50-99.9 | 19 | 10 | 31 | 20 |
| 100+ | 18 | 15 | 20 | 22 |
| Median (range) | 38.4 (0.2-363) | 26.0 (1.2-308.0) | 25.9 (0.8-360.0) | 30.0 (0.8-285.8) |
| Cytogenetics | ||||
| Favorable | 5 | 6 | 11 | 5 |
| Intermediate | 64 | 69 | 190 | 97 |
| Adverse | 7 | 5 | 6 | 5 |
| Unknown | 12 | 7 | 5 | 6 |
| Induction treatment | ||||
| AML15 | ||||
| ADE | 41 | 43 | ||
| DA | 43 | 40 | ||
| FLAG-Ida | 4 | 4 | ||
| AML17 | ||||
| ADE† | 38 | 21 | ||
| ADE + GO3 | 17 | 9 | ||
| ADE + GO6 | 26 | 13 | ||
| DA + GO3 | 21 | 11 | ||
| DA + GO6 | 26 | 14 | ||
| DA60 | 41 | 24 | ||
| DA90 | 44 | 21 | ||
| SCT | ||||
| Any | 47 | 39 | 89 | 51 |
| In 1st CR | 33 | 29 | 46 | 25 |
| Allograft | 41 | 37 | 73 | 47 |
| Allo in CR1 | 32 | 27 | 40 | 23 |
| FLT3 mutation status | ||||
| ITD alone | 65 | 65 | 155 | 85 |
| TKD alone | 22 | 18 | 52 | 23 |
| ITD+TKD | 1 | 2 | 4 | 4 |
| Not assessable | 0 | 2 | 1 | 1 |
| FLT3 ITD mutant percentage | ||||
| <25% | 18 | 22 | 55 | 31 |
| 25-50% | 38 | 22 | 77 | 47 |
| 50%+ | 5 | 14 | 27 | 11 |
| Unknown | 5 | 9 | 0 | 0 |
| Median | 32.5 | 36.5 | 29.5 | 31 |
| Range | 5.8-92.5 | 3-98.4 | 5-98 | 3.5-96 |
| NPM1c status | ||||
| WT | 42 | 34 | 83 | 52 |
| Mutant | 43 | 45 | 124 | 58 |
| Not known | 3 | 8 | 5 | 3 |
| Characteristics . | AML15 . | AML17 . | ||
|---|---|---|---|---|
| Lestaurtinib . | Control . | Lestaurtinib . | Placebo . | |
| Number randomized | 88 | 87 | 212 | 113 |
| Age group, years | ||||
| 0-15 | 0 | 0 | 3 | 2 |
| 16-29 | 9 | 10 | 22 | 10 |
| 30-39 | 15 | 14 | 20 | 10 |
| 40-49 | 24 | 26 | 57 | 31 |
| 50-59 | 30 | 28 | 83 | 44 |
| 60+ | 10 | 9 | 27 | 16 |
| Median (range) | 48 (16-66) | 46 (16-65) | 50 (5-68) | 50 (6-65) |
| Sex | ||||
| Female | 47 | 51 | 113 | 57 |
| Male | 41 | 36 | 99 | 56 |
| Type of disease | ||||
| De novo | 84 | 84 | 198 | 104 |
| Secondary | 3 | 4 | 10 | 6 |
| High-risk MDS | 0 | 0 | 4 | 3 |
| Performance status* | ||||
| 0 | 54 | 51 | 127 | 64 |
| 1 | 30 | 31 | 69 | 38 |
| 2 | 3 | 2 | 10 | 6 |
| 3 | 1 | 3 | 5 | 4 |
| 4 | 0 | 0 | 0 | 0 |
| WBC | ||||
| 0-9.9 | 17 | 25 | 48 | 29 |
| 10-49.9 | 33 | 37 | 100 | 42 |
| 50-99.9 | 19 | 10 | 31 | 20 |
| 100+ | 18 | 15 | 20 | 22 |
| Median (range) | 38.4 (0.2-363) | 26.0 (1.2-308.0) | 25.9 (0.8-360.0) | 30.0 (0.8-285.8) |
| Cytogenetics | ||||
| Favorable | 5 | 6 | 11 | 5 |
| Intermediate | 64 | 69 | 190 | 97 |
| Adverse | 7 | 5 | 6 | 5 |
| Unknown | 12 | 7 | 5 | 6 |
| Induction treatment | ||||
| AML15 | ||||
| ADE | 41 | 43 | ||
| DA | 43 | 40 | ||
| FLAG-Ida | 4 | 4 | ||
| AML17 | ||||
| ADE† | 38 | 21 | ||
| ADE + GO3 | 17 | 9 | ||
| ADE + GO6 | 26 | 13 | ||
| DA + GO3 | 21 | 11 | ||
| DA + GO6 | 26 | 14 | ||
| DA60 | 41 | 24 | ||
| DA90 | 44 | 21 | ||
| SCT | ||||
| Any | 47 | 39 | 89 | 51 |
| In 1st CR | 33 | 29 | 46 | 25 |
| Allograft | 41 | 37 | 73 | 47 |
| Allo in CR1 | 32 | 27 | 40 | 23 |
| FLT3 mutation status | ||||
| ITD alone | 65 | 65 | 155 | 85 |
| TKD alone | 22 | 18 | 52 | 23 |
| ITD+TKD | 1 | 2 | 4 | 4 |
| Not assessable | 0 | 2 | 1 | 1 |
| FLT3 ITD mutant percentage | ||||
| <25% | 18 | 22 | 55 | 31 |
| 25-50% | 38 | 22 | 77 | 47 |
| 50%+ | 5 | 14 | 27 | 11 |
| Unknown | 5 | 9 | 0 | 0 |
| Median | 32.5 | 36.5 | 29.5 | 31 |
| Range | 5.8-92.5 | 3-98.4 | 5-98 | 3.5-96 |
| NPM1c status | ||||
| WT | 42 | 34 | 83 | 52 |
| Mutant | 43 | 45 | 124 | 58 |
| Not known | 3 | 8 | 5 | 3 |
NPM1c, nucelophosmin mutation; SCT, stem cell transplant; WBC, white blood cell count ×109/L.
Two children did not complete the WHO performance status,
Includes people who were not eligible for GO in AML17 and 2 patients mistakenly originally believed to be APL.
The disposition of the patients is shown in Figure 2.
Overall response
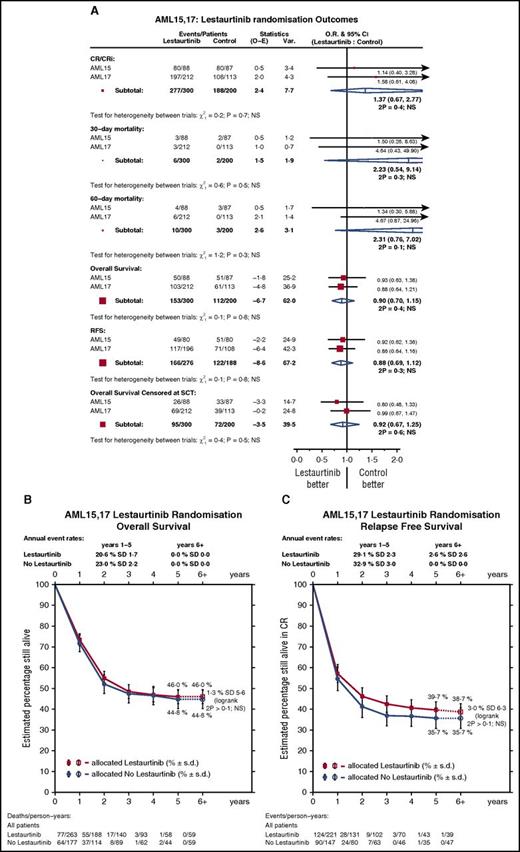
Patients received a median of 3 cycles of lestaurtinib (range, 0-4 cycles). With median follow-up of 50.5 months (range, 1.3-97.8 months), 5-year OS is 45% for all patients randomized: Outcomes were stratified by treatment group and trial and are summarized in Table 2. There was no overall difference in remission rate (combined CR/CRi at any time) between treatment groups (lestaurtinib, 92%; control, 94%; OR, 1.37 95% CI [0.68-2.78]; P = .4).
Outcomes after lestaurtinib randomization
| . | AML15 . | AML17 . | Overall HR/OR, 95% CI; P value . | P value for heterogeneity by trial . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lestaurtinib, % . | Control, % . | HR/OR, 95% CI . | P value . | Lestaurtinib, % . | Placebo, % . | HR/OR, 95%CI . | P value . | |||
| ORR (CR+CRi) | 91 | 92 | 1.14 (0.40-3.28) | .8 | 93 | 96 | 1.58 (0.61-4.08) | .3 | 1.37 (0.67-2.77); P = .4 | .7 |
| 30-d mortality | 3 | 2 | 1.50 (0.26-8.63) | .7 | 1 | 0 | 4.64 (0.43-49.9) | .2 | 2.23 (0.54-9.14); P = .3 | .5 |
| 60-d mortality | 5 | 3 | 1.34 (0.30-5.88) | .7 | 3 | 0 | 4.67 (0.87-25.0) | .07 | 2.31 (0.76-7.02); P = .1 | .3 |
| 5-y OS | 43 | 41 | 0.93 (0.63-1.38) | .7 | 50 | 45 | 0.88 (0.64-1.21) | .4 | 0.90 (0.70-1.15); P = .4 | .8 |
| 5-y OS censored at SCT | 51 | 41 | 0.80 (0.48-1.33) | .4 | 53 | 47 | 0.99 (0.67-1.47) | 1.0 | 0.92 (0.67-1.25); P = .6 | .5 |
| 5-y CIR | 50 | 50 | 0.98 (0.63-1.15) | .9 | 52 | 62 | 0.79 (0.57-1.09) | .15 | 0.85 (0.66-1.10); P = .2 | .4 |
| 5-y CIDCR | 10 | 14 | 0.70 (0.28-1.71) | .4 | 9 | 5 | 1.78 (0.69-4.57) | .2 | 1.08 (0.58-2.03); P = .8 | .18 |
| 5-y RFS | 40 | 36 | 0.92 (0.62-1.36) | .7 | 39 | 34 | 0.85 (0.64-1.16) | .3 | 0.88 (0.69-1.12); P = .3 | .8 |
| . | AML15 . | AML17 . | Overall HR/OR, 95% CI; P value . | P value for heterogeneity by trial . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lestaurtinib, % . | Control, % . | HR/OR, 95% CI . | P value . | Lestaurtinib, % . | Placebo, % . | HR/OR, 95%CI . | P value . | |||
| ORR (CR+CRi) | 91 | 92 | 1.14 (0.40-3.28) | .8 | 93 | 96 | 1.58 (0.61-4.08) | .3 | 1.37 (0.67-2.77); P = .4 | .7 |
| 30-d mortality | 3 | 2 | 1.50 (0.26-8.63) | .7 | 1 | 0 | 4.64 (0.43-49.9) | .2 | 2.23 (0.54-9.14); P = .3 | .5 |
| 60-d mortality | 5 | 3 | 1.34 (0.30-5.88) | .7 | 3 | 0 | 4.67 (0.87-25.0) | .07 | 2.31 (0.76-7.02); P = .1 | .3 |
| 5-y OS | 43 | 41 | 0.93 (0.63-1.38) | .7 | 50 | 45 | 0.88 (0.64-1.21) | .4 | 0.90 (0.70-1.15); P = .4 | .8 |
| 5-y OS censored at SCT | 51 | 41 | 0.80 (0.48-1.33) | .4 | 53 | 47 | 0.99 (0.67-1.47) | 1.0 | 0.92 (0.67-1.25); P = .6 | .5 |
| 5-y CIR | 50 | 50 | 0.98 (0.63-1.15) | .9 | 52 | 62 | 0.79 (0.57-1.09) | .15 | 0.85 (0.66-1.10); P = .2 | .4 |
| 5-y CIDCR | 10 | 14 | 0.70 (0.28-1.71) | .4 | 9 | 5 | 1.78 (0.69-4.57) | .2 | 1.08 (0.58-2.03); P = .8 | .18 |
| 5-y RFS | 40 | 36 | 0.92 (0.62-1.36) | .7 | 39 | 34 | 0.85 (0.64-1.16) | .3 | 0.88 (0.69-1.12); P = .3 | .8 |
CIR, cumulative incidence of relapse; CIDCR, cumulative incidence of death in remission.
RFS and OS
No significant differences were seen in either 5-year RFS (lestaurtinib, 40% vs control, 36%; HR, 0.88 95% CI [0.69-1.12]; P = .3) or OS (lestaurtinib, 46% vs control, 45%; HR, 0.90 95% CI [0.70-1.15]; P = .3) (Figure 3). Analyses stratified by trial (AML15 vs AML17) showed no heterogeneity of effect of lestaurtinib on any endpoint (Figure 3; Table 2).
Outcomes by treatment. (A) Forest plot stratified by trial. (B) OS. (C) RFS.
Transplant
A total of 226 (45%) patients received a stem cell transplant (45% in each group) at some stage, with 198 of these being allografts (control, 42%; lestaurtinib, 38%), and 122 allografts being delivered in first remission (25% vs 24%) (Table 1). Censoring survival at the time of stem cell transplant did not materially change the results (HR, 0.92; 95% CI [0.67-1.25]; P = .6) (Figure 3A).
Safety and toxicity
Overall, across AML15 and AML17, only marginal differences in toxicity were seen between the lestaurtinib and control groups, and there was no significant difference in early (30- or 60-day) mortality (supplemental Figure 1). There were moderate increases in nausea and diarrhea with lestaurtinib in the first 2 courses of treatment, and a slightly higher grade of bilirubin in course 1. More antibiotics were required by lestaurtinib-treated patients in courses 1 and 2, and there were also slightly higher supportive care needs during course 2, associated with a 2-day increase in median time to platelet recovery (P = .01) (supplemental Table 2; supplemental Figure 1). In the AML17 study, where comparisons could be made, no significant differences were noted between compliance with lestaurtinib (91%) and placebo (95%) therapy during course 1.
Exploratory subgroup analysis
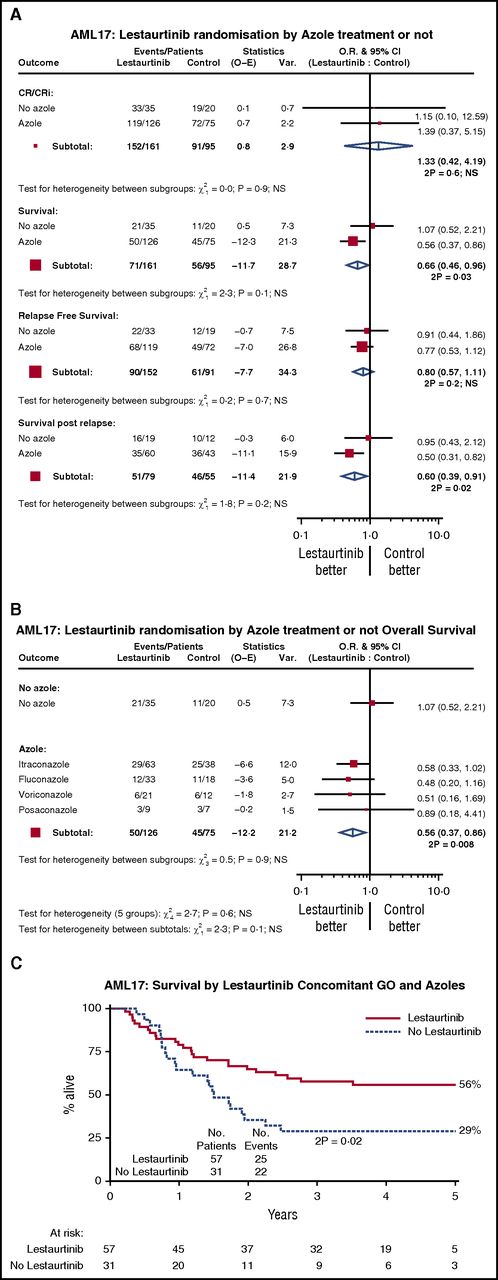
Exploratory subgroup analyses were performed by age, sex, diagnosis (de novo/secondary/MDS), cytogenetics, risk group, performance status, type of FLT3 mutation, FLT3 mutant allelic burden, and NPM1 mutation status. No significant interactions were found (supplemental Figure 2), so we explored potential interaction with treatments in the trial, including the use of concomitant antifungal prophylaxis (Figure 4A), and with the individual azole drugs (fluconazole, itraconazole, posaconazole, or voriconazole) (Figure 4B). We noted that although there was no significant interaction with azole therapy, there appeared to be a significantly superior survival in recipients of lestaurtinib who were receiving azole prophylaxis (HR, 0.57; 95% CI [0.36-0.92]; P = .02); this appears to be because of better survival after relapse for which there is no obvious explanation; there was no evidence of azole-related reduction in relapse itself, or benefit on CR rate. No other significant treatment interactions were seen, and in particular, the type of azole prophylaxis did not seem to affect the benefit, although for patients in the AML17 trial who received both GO and an azole, the addition of lestaurtinib provided additional benefit (Figure 4C), which resulted from a combination of a nonsignificant reduction in relapse (HR, 0.62; 95% CI [0.35-1.12]; P = .11) and significantly better survival post relapse (HR, 0.49; 95% CI [0.25-0.97]; P = .04).
Interaction with azole prophylaxis in AML17. (A) Azole vs not; (B) by type of azole; (C) survival in patients given concomitant GO and azoles. CR: complete remission; CRi: complete remission with incomplete peripheral count recovery; GO: gemtuzumab ozogamicin
Interaction with azole prophylaxis in AML17. (A) Azole vs not; (B) by type of azole; (C) survival in patients given concomitant GO and azoles. CR: complete remission; CRi: complete remission with incomplete peripheral count recovery; GO: gemtuzumab ozogamicin
Correlative pharmacodynamic/pharmacokinetic studies
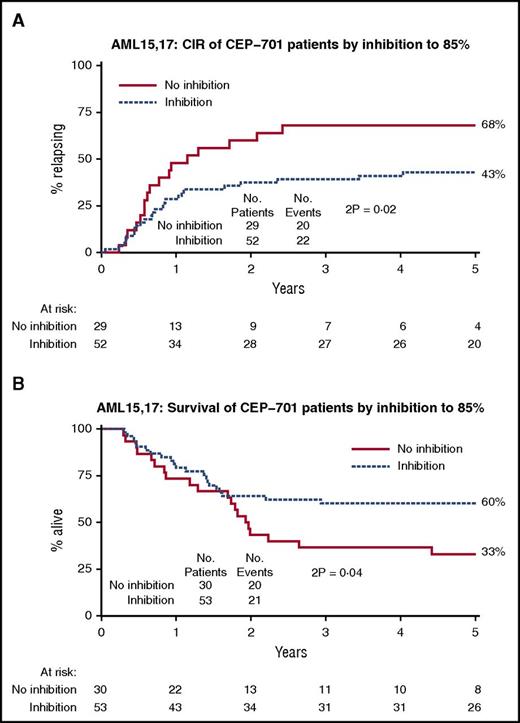
To estimate the degree of FLT3 inhibition achieved in vivo, trough FLT3 PIA was measured at day 14 of each cycle of lestaurtinib. The PIA assay uses FLT3-dependent cell line TF1-ITD as a “surrogate tissue,” allowing FLT3-inhibitory activity to be assessed after clearance of leukemia cells from the blood/marrow. It has previously been hypothesized, based on data from preclinical and early-phase monotherapy studies of lestaurtinib, that sustained inhibition of FLT3 phosphorylation by more than 85% (ie, to <15% of its baseline activity) is required to achieve a cytotoxic and clinically relevant response to the drug.11,12
Plasma inhibitory assays at trough were carried out on 83 patients, at a total of 161 points; a FLT3 PIA higher than 85% was seen at 118/161 (73%) of all evaluated times, and 82% of the patients (68/83) achieved at least 1 FLT3 PIA measurement in excess of 85%, with 64% (53/83) showing greater than 85% inhibition at all assayed points. Although no relationship was seen between FLT3 PIA and the successful induction of remission, rates of relapse were significantly lower in patients who achieved sustained FLT3 inhibition (FLT3 PIA > 85% at all evaluated points; 43% in inhibited vs 68% in noninhibited patients; HR, 0.44; 95% CI [0.23-0.86]; P = .02; Figure 5A), leading to a significantly better OS (60% vs 33%; HR, 0.50; 95% CI [0.26-0.97]; P = .04; Figure 5B). Although FLT3 inhibition appeared to be greater in patients with NPM1c mutations (81% vs 39% inhibited; P = .003) the relationship between PIA and clinical outcome remained significant after adjusting for NPM1 mutation status. Although there was some evidence of a beneficial effect of coadministration of azoles on survival, this was attributable to better postrelapse survival, rather than relapse itself, and was not explained by a difference in the PIA levels in azole-treated patients (44/64 inhibited with concomitant azole; 13/18 inhibited without; P = .8). Day 14 trough plasma lestaurtinib levels were measured in 155 patients after course 1. The median plasma level of lestaurtinib in course 1 was 3996 ng/mL. Patients who were inhibited according to the FLT3 PIA tended to have higher levels of lestaurtinib during course 1 (median, 5663 vs 3092 ng/mL; P = .002).
Analysis by plasma inhibition. (A) Cumulative incidence of relapse; (B) OS.
Among the 83 patients in whom PIA measurements were carried out, mean day 14 FL concentrations rose through successive courses of lestaurtinib treatment, going from 496 pg/mL during course 1 to 1467, 2565, and 2720 pg/mL during courses 2, 3, and 4, respectively (P < .0001 by repeated measures analysis). Despite these rising FL levels, no apparent fall off in the proportion of patients successfully achieving optimal levels of FLT3 inhibition was observed; a day 14 FLT3 PIA level in excess of 85% was achieved in 73% of assayed patients during course 1 (47/64), 76% during course 2 (38/50), 80% during course 3 (24/30), and 53% during course 4 (9/17). In addition, no significant correlation was seen between PIA values and FL concentrations in a repeated measures analysis across all points (P = .14).
Discussion
In this prospective randomized assessment, we sought to establish whether the FLT3-targeted inhibitor lestaurtinib, added sequentially to standard front-line chemotherapy, would improve the clinical outcome for newly diagnosed younger patients with AML with FLT3-mutated disease. By intention-to-treat analysis, no statistically significant evidence of benefit was seen: lestaurtinib failed to reach its primary endpoints of improving OS or RFS, and there was no improvement in remission rate or evidence of subgroup benefit restricted according to type of FLT3 mutation or FLT3-ITD mutant allelic burden, or accompanying NPM1 mutation. Unplanned subgroup analysis did suggest potential benefit with lestaurtinib when combined with azoles and GO in induction.
In the wider context of FLT3-directed therapy, the most encouraging aspect of our results was the demonstration that achievement of sustained levels of in vivo FLT3 inhibition, quantified using the FLT3 PIA assay, correlated with significantly improved patient outcome in terms of reduced relapse rate and improved OS; these findings augment those of the Cephalon 204 trial, in which 39% of relapsed patients with FLT3-AML with more than 85% FLT3 inhibition during their first course of lestaurtinib plus chemotherapy achieved a second CR compared with only 9% of suboptimally inhibited patients.23 Such data appear to re-emphasize the validity of FLT3 as a therapeutic target in previously untreated and relapsed AML, but underline that lestaurtinib is unlikely to be the best drug for future clinical exploitation. Although the number of patients with a full set of assays is limited, 27% of assayed AML15/AML17 cases (compared with 42% in Cephalon 204) failed to maintain adequate sustained FLT3 inhibition, and as in that trial, large interpatient variations were observed in steady state plasma lestaurtinib concentrations. We were unable to explain the observed azole benefit in terms of any effect of azoles on PIA levels. Lestaurtinib is known to be highly plasma protein-bound; it has previously been suggested that levels of free, biologically active drug fall as levels of plasma proteins rise during chemotherapy.23 This combination of pharmacokinetic limitations makes it unlikely to be possible to dose lestaurtinib in a schedule that delivers sustained FLT3 inhibition while maintaining tolerability.
Progressively rising levels of FL, measured as patients with relapsed AML receive chemotherapy, but seemingly independent of FLT3 inhibitor exposure, have been hypothesized as 1 mechanism of resistance to FLT3 inhibition; adding FL to in vitro assays significantly blunted the efficacy of a panel of FLT3 inhibitors against cell lines and primary AML blasts.27 In AML15/AML17, we demonstrated that rising FL levels, again evident as patients progressed through chemotherapy, failed to impede target inhibition; no fall off was seen in the proportion of patients achieving adequate FLT3 PIA through successive treatment cycles, no inverse correlation was observed between FL concentration and FLT3 PIA, and there was no association between FL level and clinical outcome. These data provide encouragement that rising FL levels may not prove an insurmountable obstacle to successful combination of FLT3 inhibition with chemotherapy.
The clinical benefit seen in the azole recipients may reflect the general benefit of azole therapy in AML treatment, although we saw no difference in 30- and 60-day mortality with azole treatment. The additional clinical benefit observed with the concomitant use of GO in induction is especially interesting in the context of our recently published extended follow-up data from AML17, which identified FLT3-ITD patients as the only subgroup to benefit from increasing the course 1 daunorubicin dose from 60 to 90 mg/m2; late benefits were seen in terms of relapse reduction and improved RFS and OS.28 This potential benefit of intensified induction therapy in FLT3-ITD cases was also highlighted in extended follow-up data from the ECOG E1900 study.29
Over the period of recruitment of AML15/AML17, another large, international study, RATIFY, has prospectively assessed the addition of first-generation FLT3-targeted TKI therapy to standard chemotherapy in a broadly similar population of younger adults with newly diagnosed FLT3-mutated AML. Midostaurin (PKC412) is an indolocarbazole compound that has considerable structural homology with lestaurtinib and an inhibitory profile that includes FLT3, cellular KIT, platelet-derived growth factor receptor beta, vascular endothelial growth factor receptor 2, and protein kinase C. In contrast to AML15/AML17, results of the RATIFY study, so far published in abstract form, point to improvement in both OS and EFS in Midostaurin-treated patients (51% vs 43% 5-year OS; P = .007).30 In the absence of any correlative in vivo data from RATIFY to suggest differences in the degrees of FLT3 inhibition achieved by midostaurin and lestaurtinib, the reasons for the apparent discrepancies in clinical outcome between the studies remain a matter of speculation; the incorporation of maintenance FLT3 inhibition on completion of chemotherapy in RATIFY (not permitted in AML15/AML17) could be relevant, as could the greater proportion of patients receiving allogeneic SCT in RATIFY (57% vs 43% in AML15/AML17), or the differences in “non-FLT3” kinase inhibitory profiles of the compounds. Certainly, the incorporation of formal prospective randomized assessment of the value of maintenance FLT3-directed therapy, including posttransplant, will be pertinent to the design of future FLT3 inhibitor plus chemotherapy studies.
The longer-term future of this first generation of FLT3 inhibitors, relatively nonselective compounds that were originally developed to target other kinases, is uncertain. During the lifetime of the AML15/AML17 study, a second generation of more selective FLT3 inhibitors with more restricted “off target” activity and the apparent capability of achieving sustained profound FLT3 inhibition in a tolerable fashion have achieved deeper, longer-lasting remissions in the setting of monotherapy of relapsed/refractory FLT3-AML15,16 and are moving into combination with chemotherapy. Differences are well documented between the biology of FLT3/ITD AML at initial diagnosis and at relapse, however. In vitro data support that, whereas relapsed FLT3-driven disease may be particularly vulnerable to highly selective FLT3 inhibition as a result of the effect of higher FLT3 mutant allelic burden and greater “addiction” to FLT3 signaling; in contrast, at the time of initial AML diagnosis, there is far less “FLT3-dependency,” and selective inhibition of FLT3 alone is usually insufficient to induce in vitro cytotoxicity.31 Continuing exploration of the role of multikinase inhibition may still, therefore, be biologically justified in the setting of newly diagnosed FLT3-mutated AML. The mixed clinical experiences with lestaurtinib in AML15/AML17 have, however, reemphasized the necessity of optimizing pharmacokinetics when combining kinase inhibition with chemotherapy and have underlined the importance of continuing to correlate clinical response with laboratory evidence of target inhibition in future studies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the staff of the Cardiff University Haematology Trials Unit for supervision of the trials. The authors are grateful to Cephalon Inc (Peter Brown and Debra Kennedy) and, subsequently, Teva for provision of lestaurtinib.
The AML15 and AML17 trials were supported, respectively, by research funding from the United Kingdom Medical Research Council and Cancer Research UK. Correlative pharmacodynamic laboratory studies at Johns Hopkins University (FLT3 PIA and FL assays) were supported by the US National Cancer Institute (M.D. Anderson Leukemia SPORE P50 CA100632). Pharmacokinetic analysis was funded and hosted by Cephalon Inc.
Authorship
Contribution: A.K.B. was the lead investigator, designed the study, and wrote the manuscript; S.K. coordinated the study, oversaw correlative studies, and wrote the manuscript; N.R. designed and coordinated the study; A.G. and R.E.G. designed and oversaw molecular analysis; R.K.H. provided statistical input to the study design, analyzed data, and wrote the manuscript; J.D.C., G.J., and L.K. provided patients to the study; M.J.L. designed, coordinated, and performed correlative studies; M.R.G. and H.K. performed and analyzed correlative studies; and I.T. coordinated the conduct of the study and data collection. All authors reviewed the manuscript before submission.
Conflict-of-interest disclosure: A.K.B., S.K., and M.J.L. served on the Clinical Advisory Board of Cephalon Inc. A.K.B. is currently an employee of CTI Life Sciences. The remaining authors declare no competing financial interests.
Correspondence: Steven Knapper, Division of Cancer & Genetics, School of Medicine, Cardiff University, Heath Park, Cardiff, CF14 4XN, United Kingdom; e-mail: knappers@cf.ac.uk.