Abstract
Interleukin-1α (IL-1α) and IL-1β are potent inflammatory cytokines that activate local and systemic inflammatory processes and are involved in protective immune responses against infections. However, their dysregulated production and signaling can aggravate tissue damage during infection, inflammatory diseases, and chemotherapy-induced intestinal mucositis. Additionally, cytokines of the IL-1 family play an important role in homeostatic as well as “emergency” hematopoiesis and are involved in the pathogenesis of several myeloid and lymphoid hematological malignancies. In the pathogenesis of intestinal mucositis and graft-versus-host disease (GVHD), these cytokines are considered pivotal during the initiation as well as propagation phase, and insights from animal studies suggest that targeting the IL-1 pathway can significantly ameliorate mucositis and GVHD. Moreover, IL-1α and IL-1β might prove to be valuable targets for both prevention and treatment of cancer and cancer therapy–related complications, and the first clinical studies have already been performed in the setting of hematological malignancies. In this review, we will discuss the role of cytokines of the IL-1 family in hematological malignancies, chemotherapy-induced intestinal mucositis, and GVHD, and speculate on possibilities of therapeutically targeting the IL-1 pathway in hematological patients.
Introduction
Interleukin-1 (IL-1) has been called “the master cytokine of inflammation,”1 and was discovered in the late 1970s. Since then, this cytokine has been studied extensively in a wide range of physiological processes and diseases.1,2 Notable members of the IL-1 family include 2 cytokines that bind the same receptor: IL-1α and IL-1β. The most important function of IL-1 is mediation of local and systemic inflammatory responses that contribute to protective immunity against infections, but, when dysregulated, result in increased tissue damage and inflammation.1 In addition, IL-1 is crucial for homeostatic as well as “emergency” hematopoiesis, facilitating adjusted responses depending on the demand, for instance during infection.3,4
The IL-1 pathway has been shown to be involved in patients suffering from cancer in roughly 3 contexts. First, both IL-1α and IL-1β are implicated in the pathogenesis of several solid tumors and hematological malignancies. Different pathophysiological mechanisms are involved and include the evolution of a proinflammatory bone marrow microenvironment, increased and aberrant innate cytokine signaling, for example, in leukemic stem cells (LSCs), and disruption of antitumor immunity. However, emphasizing the complexity of the situation, IL-1 can also play a protective role by the induction of potentially beneficial immune responses during anticancer treatment (“immunogenic cell death”).1,5 Second, IL-1 is involved in the occurrence of cancer cachexia and treatment-related complications, such as cardiotoxicity and intestinal mucositis, which often accompany the use of intensive chemotherapy and radiotherapy. Last, inflammatory complications occurring postallogeneic stem cell transplantation (SCT) such as graft-versus-host disease (GVHD), acute lung injury, and sepsis also rely on dysregulated proinflammatory responses with a central role for IL-1.
In recent years, the value of therapeutic targeting of the IL-1 pathway (with, eg, the recombinant human IL-1 receptor antagonist [IL-1Ra] anakinra and the neutralizing monoclonal anti-IL-1β antibody canakinumab) has increasingly been explored in hematological patients. Successes have been achieved with amelioration of cancer cachexia,6 anthracycline-induced cardiotoxicity,7 and intestinal mucositis,8-13 but importantly also with reduced induction and progression of hematological malignancies, especially multiple myeloma (MM).14,15 However, not all studies were successful, such as the trials on IL-1 inhibition in human acute GVHD,16 suggesting that timing, dosing, and the specific context might be crucial. Because IL-1 has dual and sometimes contradictory roles, and inhibition of IL-1 may be beneficial in one context but disadvantageous in the other, careful design of studies is necessary to exploit targeting of the IL-1 pathway in the setting of hematology (Figure 1).
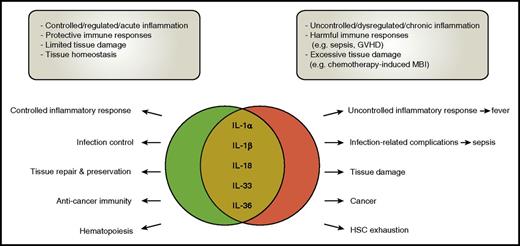
The dichotomous roles of the IL-1 cytokine family members. Cytokines of the IL-1 family are involved in many processes during health and disease. Their role is, however, often dichotomous, with the balance between benefit and harm quite fragile. In many diseases, including infections, GVHD, MBI, and cancer, in general “too much” cytokine release seems harmful, emphasizing the “Goldilocks principle” which states that the immune response should be “just right.” HSC, hematopoietic stem cell.
The dichotomous roles of the IL-1 cytokine family members. Cytokines of the IL-1 family are involved in many processes during health and disease. Their role is, however, often dichotomous, with the balance between benefit and harm quite fragile. In many diseases, including infections, GVHD, MBI, and cancer, in general “too much” cytokine release seems harmful, emphasizing the “Goldilocks principle” which states that the immune response should be “just right.” HSC, hematopoietic stem cell.
In this review, we will summarize recent findings on the role of IL-1 in hematology, with an emphasis on the pathogenesis of hematological malignancies and anticancer therapy. Also, we will discuss the role of IL-1 in chemotherapy-induced complications, particularly intestinal mucositis. Finally, we will explore the possibilities and challenges of targeting the IL-1 pathway in the context of hematological malignancies.
The IL-1 cytokine family
The IL-1 family consists of 7 cytokines with agonist activity (IL-1α and IL-1β, IL-18, IL-33, IL-36α, IL-36β, IL-36γ), 3 receptor antagonists (IL-1Ra, IL-36Ra, IL-38), and an anti-inflammatory cytokine (IL-37).17 The innate proinflammatory cytokines IL-1α and IL-1β affect a large array of organs and cells, including those of the hematopoietic system. Besides facilitating protective immunity during infection and tissue homeostasis, they are major pathogenic mediators of autoinflammatory, autoimmune, infectious, and degenerative diseases. Therefore, tight regulation via receptor antagonists, decoy receptors, and signaling inhibitors normally ensures a balance between amplification of innate immunity and uncontrolled inflammation (Figure 1).
IL-1α
IL-1α is constitutively expressed in several types of cells at steady state, especially in epithelial cells, whereas its expression is increased in response to proinflammatory and stress-associated stimuli. This intracellular cytokine exerts its function in 3 ways. First, IL-1α can translocate from the cytosol onto the cell surface where membrane-bound IL-1α activates IL-1 receptor type 1 (IL-1R1) signaling in an intra- and paracrine manner, contributing to local inflammatory responses. Second, by extracellular release after loss of membrane integrity resulting from necrotic-type cell death, IL-1α functions as an alarmin or danger-associated molecular pattern (DAMP) initiating a cascade of inflammatory responses.18 Third, IL-1α can migrate from the cytosol into the cell nucleus and exert intracellular functions, similar to transcription factors.19 Major biological functions of IL-1α include induction of sterile and pathogen-induced inflammation, as well as T helper 17 (Th17) responses.17,20
IL-1β
Contrary to IL-1α, IL-1β is produced by a limited number of cells, primarily monocytes, macrophages, and dendritic cells. The biology of IL-1β is far more complex with the release of functional IL-1β requiring 2 steps. The first rate-limiting step is the transcription of its precursor, pro-IL-1β. The stimulus can be a microbe-associated molecular pattern (MAMP) or DAMP, but cytokines such as tumor necrosis factor-α (TNFα), IL-18, or IL-1β itself can also induce pro-IL-1β transcription. The second step involves the conversion of the inactive precursor into the biologically active IL-1β. This process is mediated by inflammasomes that activate the cysteine protease caspase-1, such as NOD-like receptor protein 3, or by caspase-1–independent cleavage that is mediated by neutrophil-derived serine proteases.19,21
IL-1β mediates inflammation not only at the tissue level, but also systemically, where one of its actions is inducing the acute-phase response, including fever. It propagates inflammation by inducing other proinflammatory cytokines, such as IL-6 and TNFα, and bridges innate and adaptive immunity by activation of Th1 and Th17 cells. IL-1β plays an important role in the host defense against infections, and in tissue homeostasis and repair.
IL-33
IL-33 is constitutively expressed in the nucleus during health. Upon cell injury and cell necrosis, bioactive IL-33 is released, functioning as an alarmin. IL-33 signals through the suppression of tumorigenicity 2 receptor, inducing the production of Th2-associated cytokines. It plays a role in allergic type 2 immunity, maintenance of epithelial barrier functions, and tissue repair.17,22
IL-1Ra
The IL-1 pathway in hematological malignancies
Because inflammation is an important driver of carcinogenesis, not surprisingly, cytokines of the IL-1 family, and cytokines induced by IL-1β such as IL-6, are involved in the pathogenesis of solid tumors and hematological malignancies.24-26 In numerous solid tumors, including colon, breast, and renal cancers and melanomas, upregulation of IL-1 family cytokines has been documented.27-29
In hematology, IL-1 is implicated in both lymphoid and myeloid malignancies. Most straightforward is its role in chronic inflammation and infection- or antigen-induced lymphoproliferative diseases, including Helicobacter pylori–associated gastric marginal zone lymphoma and Epstein-Barr virus–associated lymphoproliferative diseases.30 In MM, the role of the cytokines IL-1 and IL-6 has also been firmly established. Moreover, several therapies, such as the immunomodulatory drugs, have been designed to target the proinflammatory tumor microenvironment.31 Furthermore, IL-1 plays an important role in several histiocytic disorders, such as Erdheim-Chester disease (ECD) and macrophage activation syndrome–hemophagocytic lymphohistiocytosis (MAS-HLH).32,33 The role of IL-1 in myeloid malignancies is less clear, although it has been suggested in myelodysplastic syndromes (MDSs), acute myeloid leukemia (AML) and chronic myeloid leukemia (CML), and the myeloproliferative neoplasms, including primary myelofibrosis.34-39
Pathophysiological mechanisms
Hematopoietic stem and progenitor cells acquire somatic mutations with aging, resulting in the emergence of (sub)clonal hematopoiesis. An example includes clonal hematopoiesis of indeterminate potential, which can eventually evolve to MDS and AML. Similarly, acquisition of mutations in post–germinal center B lymphocytes can result in monoclonal gammopathy of undetermined significance and monoclonal B-cell lymphocytosis that precede overt malignant lymphomas, chronic lymphocytic leukemia, and MM.40 It is appreciated that clonal dominance precedes the development of overt hematological malignancies, but it remains unknown why most people never develop disease whereas a small fraction does. Recently, cell-extrinsic factors such as infection and inflammation have been proposed important mechanisms that contribute to selective competitive advantage of mutant cells to expand and survive.41 Data support this “hypothesis” by showing that a proinflammatory bone marrow microenvironment, aberrant innate immune and cytokine signaling, and perturbed antitumor immunity are pivotal in the pathogenesis of hematological malignancies.
The proinflammatory microenvironment.
Cellular senescence and the senescence-associated secretory phenotype.
Cellular senescence is a process that protects damaged cells by inducing a stable growth arrest in response to cellular stresses. As these stresses are mostly mitogenic or oncogenic stimuli, and senescence provides resistance to malignant transformation, it is recognized as a potent tumor-suppressive mechanism.42 However, senescence can also have detrimental effects that increase the risk of cancer. In part, this is thought to be caused by the senescence-associated secretory phenotype, which promotes chronic inflammation. This complex response includes secretion of the proinflammatory cytokines IL-1α, IL-6, and IL-8, and other signaling molecules such as vascular endothelial growth factor and matrix metalloproteinases.42-44 It is thought that the accumulation of senescent cells during aging creates a proinflammatory microenvironment within the bone marrow that stimulates age-related clonal hematopoiesis and ultimately leukemogenesis (Figure 2).45
Role for IL-1 in the leukemogeneic bone marrow microenvironment. Hematopoietic stem cells acquire mutations during aging, which results in clonal hematopoiesis, and in some persons, in the development of hematological malignancies. This process is promoted by chronic inflammation, during which proinflammatory cytokines (eg, IL-1, IL-6, and IL-8), as well as other signaling molecules (eg, vascular endothelial growth factor [VEGF] and matrix metalloproteinases) stimulate leukemogenesis in the bone marrow microenvironment.
Role for IL-1 in the leukemogeneic bone marrow microenvironment. Hematopoietic stem cells acquire mutations during aging, which results in clonal hematopoiesis, and in some persons, in the development of hematological malignancies. This process is promoted by chronic inflammation, during which proinflammatory cytokines (eg, IL-1, IL-6, and IL-8), as well as other signaling molecules (eg, vascular endothelial growth factor [VEGF] and matrix metalloproteinases) stimulate leukemogenesis in the bone marrow microenvironment.
Cross-talk bone between marrow stromal cells and malignant cells.
In MM, increased IL-1 and IL-6 signaling in the bone marrow microenvironment is crucial in the pathogenesis of the disease and occurrence of osteolytic lesions. IL-1β messenger RNA expression increases in the bone marrow of patients upon progression of monoclonal gammopathy of undetermined significance to smoldering and symptomatic MM.46 Sources of IL-6 are bone marrow stromal cells and myeloma-associated macrophages, and IL-6 production is mainly driven by IL-1β which originates from monocytes/macrophages activated through Toll-like receptor 2 (TLR2).47,48 IL-6 functions as a survival and proliferation factor for neoplastic plasma cells, thus creating an amplification loop. Interestingly, it has been shown that this process can be effectively inhibited by IL-1Ra and abrogation of TLR2 signaling through tumor-promoting locus 2 inhibition.31,47,48
Similar pathophysiological mechanisms have been uncovered in myeloproliferative neoplasms, AML, CML, and MDS.34,49 Close cross-talk between leukemic cells (including LSCs) and osteoblasts, endothelial cells, sympathetic nerves, and mesenchymal stromal cells induces remodeling of the bone marrow niche that again facilitates leukemogenesis.49,50 These processes are mainly regulated by cytokines, chemokines, and growth factors, including IL-1β, IL-6, CCL3, and fibroblast growth factor.
Chronic inflammation and infection in lymphoproliferative disease.
Antigen-driven lymphoproliferation is a hallmark of chronic lymphocytic leukemia and malignant lymphomas.30 Characteristic features are chronic innate and acquired immune activation, with antigen-presenting cells producing prosurvival factors such as IL-1β and IL-6 for lymphocytes, and dysregulated chronic B-cell receptor signaling.
Mutations in epigenetic regulators and inflammation.
Loss-of-function mutations in the epigenetic regulator enhancer of zeste homolog 2, which are seen in myeloid malignancies, result in propagation of MDS clones by selectively activating proinflammatory cytokine responses in the bone marrow.51 Similarly, the epigenetic regulators ten-eleven translocation 1 (TET1) and TET2 are negative regulators of IL-1β. Therefore, loss-of-function mutations in TET2, which are reported in leukemia, MDS, and malignant lymphomas, lead to increased IL-1β secretion, thus creating a proinflammatory bone marrow microenvironment that stimulates clonal hematopoiesis.52
Aberrant innate immune and cytokine signaling.
IL-1/IL-17/G-CSF pathway and myeloid cell proliferation.
IL-1β is crucial for homeostatic as well as “emergency” hematopoiesis.3,4,53 It induces the production and secretion of IL-17 and subsequently of granulocyte colony-stimulating factor (G-CSF), which drives proliferation of hematopoietic stem cells and myeloid cells.20,54 Under pathological conditions, increased IL-1/IL-17/G-CSF signaling promotes the growth of leukemic cells facilitating the progression to AML.55 Increased cell proliferation itself can have untoward effects, as increased cell division makes cells more susceptible for acquisition of additional gene mutations.56
Pattern recognition receptor/IL-1R/IRAK1 pathway.
Innate immune signaling by pattern recognition receptors that are activated by MAMPs and DAMPs, and IL-1 cytokine signaling through the IL-1R, converge on a shared common pathway, being that of the immune-modulating IL-1R–associated kinase 1 (IRAK1) (Figure 3).36 IRAK1 signaling in hematopoietic cells results in the activation of NF-κB, release of proinflammatory cytokines, increased cell proliferation, and decreased apoptosis.57 IRAK1 signaling renders leukemic cells more resistant to cell death and gives them a growth advantage over normal hematopoietic cells. Aberrant innate immune activation through TLRs, and hyperactivation and overexpression of the IL-1R accessory protein (IL1RAP) and IRAK1 have all been described in leukemic cells and LSCs in CML, MDS, and AML.36,58,59 IRAK1 and IL1RAP are therefore suggested to be promising therapeutic targets in these diseases.
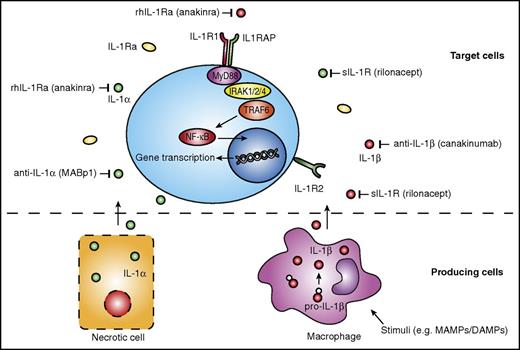
Potential therapeutic targets on the IL-1 pathway and available IL-1–targeting therapies. Production of IL-1β, mainly by monocytes, macrophages, and dendritic cells, requires a stimulus such as MAMPs or DAMPs. IL-1α does not require a stimulus, and is released upon cell necrosis (bottom panel). IL-1α and IL-1β bind to the IL-1R1 and induce further intracellular signaling pathways, whereas IL-1R2 functions as a decoy receptor for IL-1. Various agents are available that target specific components of the IL-1 pathway. rhIL-1Ra anakinra targets both IL-1α and IL-1β, as does the sIL-1R rilonacept. Specific antibodies targeting IL-1α or IL-1β are MABp1 and canakinumab, respectively. Both IL1RAP, a coreceptor of the IL-1R1, and IRAK1, a kinase downstream of the IL-1R1, have also been suggested potential targets for treatment of hematological malignancies. MyD88, myeloid differentiation primary response 88; rhIL-1Ra, recombinant human IL-1Ra; sIL-1R, soluble IL-1R; TRAF6, TNF receptor–associated factor 6.
Potential therapeutic targets on the IL-1 pathway and available IL-1–targeting therapies. Production of IL-1β, mainly by monocytes, macrophages, and dendritic cells, requires a stimulus such as MAMPs or DAMPs. IL-1α does not require a stimulus, and is released upon cell necrosis (bottom panel). IL-1α and IL-1β bind to the IL-1R1 and induce further intracellular signaling pathways, whereas IL-1R2 functions as a decoy receptor for IL-1. Various agents are available that target specific components of the IL-1 pathway. rhIL-1Ra anakinra targets both IL-1α and IL-1β, as does the sIL-1R rilonacept. Specific antibodies targeting IL-1α or IL-1β are MABp1 and canakinumab, respectively. Both IL1RAP, a coreceptor of the IL-1R1, and IRAK1, a kinase downstream of the IL-1R1, have also been suggested potential targets for treatment of hematological malignancies. MyD88, myeloid differentiation primary response 88; rhIL-1Ra, recombinant human IL-1Ra; sIL-1R, soluble IL-1R; TRAF6, TNF receptor–associated factor 6.
S100A8-9/TLR4/NLRP3 pathway.
One important hallmark of MDS is the activation of the NLRP3 inflammasome. Through TLR4 signaling and reactive oxygen species (ROS) formation, DAMPs in the bone marrow microenvironment, including S100A8 and S100A9, induce NLRP3 expression in MDS hematopoietic stem and progenitor cells. This results in the activation of caspase-1, generation of IL-1β, and pyroptotic cell death which contributes to the MDS phenotype.60,61 Interestingly, inhibition of NLRP3 or neutralization of DAMPs restores effective hematopoiesis, underscoring the importance of this pathway as a valuable therapeutic target in MDS.60
Impaired anticancer immunity.
Many hematological malignancies are associated with the development of an immunosuppressive microenvironment. A considerable part thereof consists of functionally altered innate immune cells, including myeloid-derived suppressor cells (MDSCs).62
Somewhat paradoxically, IL-1β, released during chronic inflammation, impairs anticancer immunity by promoting the accumulation of these MDSCs.63 MDSCs have different mechanisms by which they inhibit immune responses, and interestingly, one involves the NLRP3 inflammasome-mediated release of IL-1β that results in the secretion of IL-17 by CD4+ T cells.64 Although most available data come from studies on solid tumors, both MDSCs and IL-17 have also been shown to be involved in the pathogenesis of MM and AML.65-67
Targeting IL-1 in hematological malignancies
Prevention and treatment of hematological malignancies.
In murine xenograft models of various solid tumors, IL-1–targeting therapies were effective in decreasing tumor growth, angiogenesis, and metastases.25,27 More importantly, these findings have recently resulted in a phase 3 trial involving a monoclonal antibody targeting IL-1α, MABp1, which revealed promising results in patients with advanced colorectal cancer.6 In hematology, data on potential therapeutic efficacy of IL-1 inhibition are less mature, but preclinical and even clinical data provide a strong rationale for further exploration of anti-IL-1 strategies in predominantly CML, MM, and histiocytic disorders.
Multiple studies using xenograft models and human data have underscored the pivotal role of IL-1 signaling in CML and AML. In both diseases, LSCs show increased expression of the IL-1R1 and its coreceptor IL1RAP.37,39,59 Monoclonal antibodies targeting IL1RAP suppress IL-1–induced proliferation of LSCs.37,38,59 In addition, IL-1Ra also inhibits the growth of CML LSCs and, when combined with tyrosine kinase inhibitors (TKIs), leads to a greater inhibition than compared with TKI alone.39 These insights are useful as clinical studies are designed on IL-1 inhibition as means to overcome TKI resistance and eradicate the LSCs in CML.37,39,68
The strong evidence on IL-1 and IL-6 signaling in the tumor microenvironment has generated interest in IL-1 inhibition in the clinical setting of MM. Lust et al were the first to perform a proof-of-concept trial on IL-1 inhibition in patients diagnosed with a hematological malignancy.14 Patients with smoldering or indolent MM were treated with anakinra, or anakinra in combination with dexamethasone. Achieving a reduction in high-sensitivity C-reactive protein, a surrogate marker of IL-6, was correlated with the occurrence of a clinical response that consisted mostly of partial remissions or absence of disease progression. Long-term follow-up data confirmed that patients who achieved a ≥40% decrease in high-sensitivity C-reactive protein had significantly longer progression-free and overall survival.15
Clinical successes have also been achieved with IL-1 inhibition in histiocytic disorders. In ECD, treatment with anakinra significantly reduces constitutional symptoms and bone pain. Current guidelines consider anakinra as one of the first-line treatment options.32,69 Nevertheless, anakinra does not seem to be the most effective treatment in ECD because it has only a limited effect on the tumor itself.70 More efficacy of anakinra is reported in MAS-HLH, especially in the context of rheumatoid disorders, such as systemic juvenile idiopathic arthritis. This might be explained by the fact that MAS is primarily a disease of hyperinflammation, and not a neoplastic disorder.33
Cancer cachexia and paraneoplastic phenomena.
IL-1 and IL-6 are involved in the pathophysiology of cancer cachexia, and in patients with advanced cancer, increased IL-1β plasma levels are associated with the clinical features of cancer cachexia, such as anorexia and weight loss.71 In a phase 3 clinical study, treatment with the IL-1α antibody MABp1 resulted in increased lean body mass, as well as in symptom relief (pain, anorexia, fatigue), in a substantial number of patients with advanced colorectal cancer.6
Additionally, paraneoplastic manifestations, including B symptoms, Sweet syndrome, and autoinflammatory disorders such as Schnitzler syndrome are mediated by release of proinflammatory cytokines. Anakinra and canakinumab have been used successfully in the treatment of Sweet syndrome and Schnitzler syndrome.72,73
The IL-1 pathway and cancer therapy–induced complications
Increased release of IL-1α and IL-1β is implicated in the occurrence of several cancer therapy–related complications, including acute lung injury, neurotoxicity, anthracycline-induced cardiotoxicity, and mucosal barrier injury (MBI) resulting from chemotherapy and radiotherapy.7,74,75 Interestingly, data from animal studies have suggested that by using IL-1 inhibitors, some of these complications might be prevented and treated.7,74 Currently, however, the most promising settings for exploring the potential benefits of IL-1 inhibition in ameliorating treatment side effects in patients with hematological malignancies seem to be chemotherapy-induced MBI and GVHD.
Mucosal barrier injury
MBI, or oral and intestinal mucositis, is a frequent complication of intensive treatment with chemotherapy and radiotherapy.76,77 The occurrence of MBI is associated with impaired patient outcome, reduced quality of life, and increased health care costs.78,79 MBI causes physical complaints such as diarrhea, oral and abdominal pain, and contributes to the occurrence of febrile neutropenia, acute lung injury, and infections, such as bloodstream infections with gut-residing organisms.78-82 Moreover, intestinal damage that results from conditioning therapy for an allogeneic SCT is the crucial first step in the pathogenesis of acute gastrointestinal GVHD (GI-GVHD).83-85 Currently, no effective drugs exist that can be used for the prevention and treatment of MBI.
Pathogenesis MBI and role of IL-1 cytokines.
The pathogenesis of MBI, which consists of 5 phases,86 has been extensively described by Sonis86 and is summarized in Figure 4.
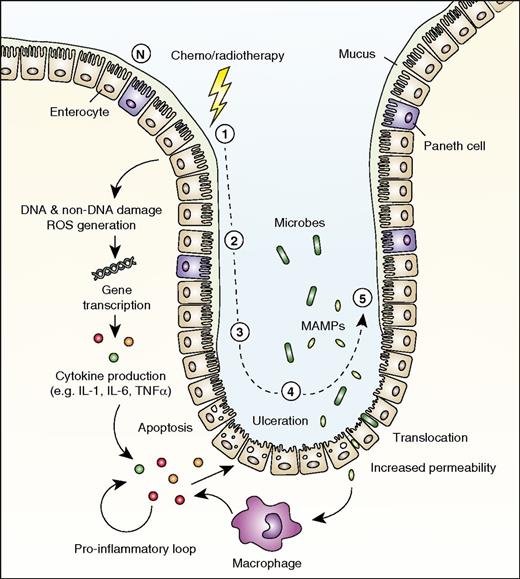
Role for IL-1 in mucosal barrier injury. The development of mucosal barrier injury (mucositis) is traditionally described as consisting of 5 stages. (1) DNA and non-DNA damage is initiated by chemotherapy or radiotherapy, and ROS are generated. (2) Transduction pathways are activated, stimulating transcription factors such as NF-κB. Many genes are upregulated, resulting in the production of proinflammatory cytokines (eg, TNFα, IL-1β, and IL-6), after which epithelial cell death and injury occur. (3) Primary damage is amplified by a positive-feedback loop of proinflammatory cytokines. (4) Ulceration occurs, and increased permeability with impaired tight junctions results in bacterial translocation. (5) Repair and healing. N indicates normal mucosa in the small intestine.
Role for IL-1 in mucosal barrier injury. The development of mucosal barrier injury (mucositis) is traditionally described as consisting of 5 stages. (1) DNA and non-DNA damage is initiated by chemotherapy or radiotherapy, and ROS are generated. (2) Transduction pathways are activated, stimulating transcription factors such as NF-κB. Many genes are upregulated, resulting in the production of proinflammatory cytokines (eg, TNFα, IL-1β, and IL-6), after which epithelial cell death and injury occur. (3) Primary damage is amplified by a positive-feedback loop of proinflammatory cytokines. (4) Ulceration occurs, and increased permeability with impaired tight junctions results in bacterial translocation. (5) Repair and healing. N indicates normal mucosa in the small intestine.
Most members of the IL-1 cytokine family participate in gut health and disease.87 However, the situation is rather complex, with most cytokines having dichotomous functions which depend on the specific context (time, location, and quantity). So, the consequences, either protective or damaging, of inhibiting or stimulating the IL-1 pathway can change during the different stages of infection, inflammation, and MBI. Indeed, several members of the IL-1 cytokine family implicated in the pathogenesis of MBI possess contradicting functions, similar to different roles attributed to these cytokines in the pathogenesis of inflammatory bowel disease (IBD).87 Low levels of IL-1β seem to protect the gut mucosa from damage during the early stages of inflammation.88 Levels of IL-1 increase significantly with ongoing and progressive mucosal inflammation in MBI, GI-GVHD, and IBD, contributing to a state of uncontrolled inflammation and progressive tissue damage.11,89-91 The impact of related cytokines including IL-18, IL-33, and IL-36 has been studied less extensively, but most data also suggest a dichotomous role for these cytokines in epithelial barrier homeostasis.
The role for IL-1β, IL-6, and TNFα in MBI initiation and propagation has been extensively studied and confirmed. In rat models of intestinal mucositis induced by several chemotherapeutic agents, such as 5-fluorouracil, methotrexate, and irinotecan, increased levels of these proinflammatory cytokines have been consistently measured in the mucosa and peripheral blood, underpinning a role in local as well as systemic inflammation.89,92-94 Early induction of IL-1β in the gut epithelium after chemotherapy results from DNA damage and formation of ROS, which then results in activation of inflammasomes and caspase-1.12,13 Importantly, IL-1β is responsible for the increased mucosal barrier permeability that occurs early in the course of MBI due to dysregulation of intestinal epithelial tight junctions.13,95 Subsequently, the increased gut permeability facilitates translocation of microbes and MAMPs further inducing inflammation.13 The most solid proof for the pivotal role of IL-1β, and probably IL-1α, in MBI pathogenesis comes from studies in animals where IL-1 inhibition was successfully used in reducing the incidence and severity of intestinal mucositis. Although less supported by evidence, a role for IL-1α and IL-33 in MBI is a rational hypothesis because epithelial cell damage will inevitably result in the release of these alarmins. Moreover, in an animal model of irinotecan-induced mucositis, the role of IL-33 was demonstrated by showing that administration of recombinant IL-33 exacerbated mucositis, whereas blockade of IL-33 attenuated mucositis.96
IL-1 inhibition in MBI.
The impact of IL-1 pathway inhibition with anakinra and anti-IL-1β antibodies on chemotherapy-induced intestinal mucositis has been studied extensively in mouse models.8-13,97 In these studies, a number of different chemotherapeutic regimens were used and various treatment schedules of IL-1–targeting agents were examined. It was demonstrated that IL-1β expression in intestinal tissue followed a similar course over time as epithelial cell apoptosis. Treatment with IL-1Ra resulted in reduced p53-dependent crypt cell apoptosis, reduced expression of proapoptotic factors, and less downregulation of antiapoptotic factors.11 Diarrhea and weight loss were significantly reduced in the mice, and histological examination of the intestine revealed attenuated damage with preservation of crypts and villi.8-13,97 The antitumor effects of the chemotherapy were shown not to be compromised,8,9,11 and the overall survival of mice treated with IL-1 inhibition improved.8,10 Mechanisms that contribute to the protective effect of IL-1 inhibition are various and predominantly consist of preservation of the mucosa and dampening of inflammation.
Graft-versus-host disease
In allogeneic SCT, intestinal mucositis resulting from conditioning therapy plays an important role in the initiation phase of acute GI-GVHD where the inflamed gut mucosa aggravates the activation of alloreactive T cells. The cytokine IL-1β is crucial herein and, recently, the close interrelatedness between intestinal barrier damage, gut microbiota composition, NLRP3-inflammasome–mediated IL-1β secretion, and Th17 has been established.85,98,99 In addition, inflammasome activation occurring within the inflamed tissues leads to MDSC-derived IL-1 secretion that subsequently abrogates the immune-suppressive effects of MDSCs, again aggravating GVHD.100
In the past, clinical trials were performed to explore the efficacy of IL-1 inhibition in the prevention and treatment of GVHD in allogeneic SCT recipients with, however, conflicting results. In 2 studies in patients with steroid-resistant acute GI-GVHD, which used anakinra and recombinant human IL-1R, respectively, improvement of GVHD was seen.101,102 However, in a randomized controlled trial that studied whether administration of anakinra during conditioning could prevent acute GI-GVHD, no effect on acute GVHD could be proven.16 Recent insights into the mechanisms of hyperacute and breakthrough GVHD have provided clues for these conflicting outcomes showing that IL-1β and Th/T cytotoxic 17 responses dominate during “late” GVHD.103
Downsides of targeting IL-1 after chemotherapy
The dual and context-dependent roles of the IL-1 family cytokines pose problems and warrant caution when exploring IL-1 inhibition as a new therapeutic strategy for treating hematological malignancies and cancer therapy–related complications such as MBI. These problems consist of infections, dysregulated gut homeostasis and tissue repair, anticancer immunity, and drug side effects.
Infections
IL-1β plays an important role in the host defense against fungi and bacteria, including intracellular bacteria such as Mycobacterium tuberculosis.104 Clinical studies on IL-1–targeting therapies demonstrated an increase in the occurrence of conventional bacterial infections, as well as a higher incidence of viral respiratory tract infections.1,23 However, virtually no increase in the rate of opportunistic infections occurred.105 While the rapid kinetics of anakinra in the blood may be responsible for its advantageous side-effect profile, the much longer circulation half-life of currently developed anti-IL-1 antibodies may lead to a different (and presumably more severe) side-effect profile.
Negative impact on mucosal tissue repair
Although most animal studies showed a protective role for IL-1 inhibition, caution should be exercised when applying IL-1 inhibition after chemotherapy and in the presence of MBI and GVHD. Controversy has arisen from studies on IL-1 inhibition in IBD, the pathogenesis of which has striking similarities with those of MBI and GI-GVHD. Although several investigators studying animal models of experimental colitis showed that inhibition of IL-1 decreased inflammation and necrosis, others demonstrated reduced inflammation after the administration of low-dose IL-1β, and some found only little or no effect of IL-1 inhibition at all.88,106,107
Opposing effects in IBD and MBI animal models might in part be caused by differences in timing and dosing of IL-1–targeting therapies, as well as by differences in animal models.
Negative impact on immune-mediated anticancer responses
Several cytotoxic drugs, such as anthracyclines and oxaliplatin, as well as radiotherapy, are able to induce immunogenic cell death, which contributes to their anticancer effects.108,109 Moreover, cyclophosphamide has been described to elicit a systemic anticancer Th17 immune response via IL-1, the release of which results from the induction of MBI and subsequently translocation of gut microbiota.110 Therefore, when used in conjunction with chemotherapy or radiotherapy, IL-1 neutralization might exert a negative effect on the efficacy of anticancer therapy. However, tumor-bearing mouse models have not shown a negative impact of IL-1 inhibition on tumor growth or chemosensitivity.8,9,11 Moreover, IL-1 neutralization during GI-GVHD had no negative impact on the graft-versus-leukemia responses in patients post-SCT.111 One possible explanation lies in the fact that during anticancer therapy, IL-1 has multiple roles, including the promotion of cancer. Hence, the net effect will ultimately depend on the specific context and timing of IL-1 inhibition.64
Drug side effects
Several side effects have been documented for the currently available IL-1–blocking therapies, but most involve injection site reactions. One particular side effect of anakinra is neutropenia, which occurs in ∼2% of patients and resolves on drug cessation. Although this side effect might raise concerns in hematological patients, studies in the SCT setting have not revealed a negative impact on the time to engraftment.16,101
Future directions and conclusion
Cytokines of the IL-1 family, especially IL-1α and IL-1β, play pivotal roles in both the pathogenesis of hematological malignancies as well as the complications that arise from anticancer therapies. Clearly, additional clinical studies must be designed to further investigate the clinical effects of IL-1–targeting therapies in hematological diseases in order to determine if there is a role for this new strategy at all. The most promising data thus far come from studies on MM, and indeed, recently a study has been initiated that investigates the combination of anakinra with lenalidomide and dexamethasone in patients with early-stage MM (NCT02492750). Future research should also elucidate the role of IL-1 inhibition in other hematological malignancies where the proinflammatory microenvironment and aberrant cytokine signaling are contributing to disease initiation and propagation, such as MDS, AML, CML, and lymphoproliferative malignancies. Several other drugs that target the proinflammatory milieu, such as IRAK1 inhibitors (pacritinib) and tumor-promoting locus 2 inhibitors, are also attractive candidates for further exploration in these diseases.
The potential beneficial effects of IL-1 inhibition on cancer therapy–related complications and post-SCT complications deserve more study; perhaps a revival in interest in the GI-GVHD setting can be envisioned. Clear evidence supports at least the further exploration of IL-1 inhibition in chemotherapy-induced intestinal mucositis. An ideal setting could be the setting of autologous SCT in patients with MM because the incidence of MBI and febrile neutropenia with this treatment is high (∼70%-80%)81,82 and there seems little risk of compromising the antimyeloma effect by IL-1 inhibition.
Crucial in the design of these studies is the acknowledgment of the complexity of the diseases and complications under study, and the realization that all IL-1 cytokine family members have dual roles necessitating careful considerations on timing and dosing of administration of IL-1 inhibitors.
Considering the accumulating evidence on the role of IL-1 in hematological malignancies and cancer therapy–related complications, and the availability of effective and safe IL-1–targeting drugs, IL-1 may prove to be a very promising and useful target in hematology in the near future.
Acknowledgment
The authors thank Dorothea Evers for critical review of the manuscript.
Authorship
Contribution: C.E.M.d.M., W.J.F.M.v.d.V., N.M.A.B., and M.G.N. wrote and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Charlotte E. M. de Mooij, Radboud University Medical Center, Department of Hematology, PO Box 9101, 6500 HB Nijmegen, The Netherlands; e-mail: c.demooij@radboudumc.nl.

![Figure 2. Role for IL-1 in the leukemogeneic bone marrow microenvironment. Hematopoietic stem cells acquire mutations during aging, which results in clonal hematopoiesis, and in some persons, in the development of hematological malignancies. This process is promoted by chronic inflammation, during which proinflammatory cytokines (eg, IL-1, IL-6, and IL-8), as well as other signaling molecules (eg, vascular endothelial growth factor [VEGF] and matrix metalloproteinases) stimulate leukemogenesis in the bone marrow microenvironment.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/129/24/10.1182_blood-2016-12-754994/4/m_blood754994f2.jpeg?Expires=1765897543&Signature=D62J4Mkzo6UeMmQcKnyJK1PwUmkh9gjiQuzSMvxpRPMdXDKfeBoYn-GCX5Nc3TJcU4s57c0EIt9QfNZMyFoP0RInDPWUIpvPCwd5Sf7AqiBpKvnRqxmDKO2JGJwpBkpKrBU~l0EsSscSUGmYSKXujt3oSyZwem5AHyx8pMsj1RFLIRFaLzLTZFdximfBIDdInk0~xpuZ9t5mFwmy8cj233LdDJgZlq-L18PGus6ve--1JEhEMYZUXre2nWNiYK8CnRofOFJxEU4MjSrfhmXvcmnVSBpbYOl5estTJLwjhhXxGOrO86upYHle2BnWutritFlgNXopF9f9iK3Jb29kRA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)