Abstract
In hemophilia A, the most severe complication of factor VIII (FVIII) replacement therapy involves the formation of FVIII neutralizing antibodies, also known as inhibitors, in 25% to 30% of patients. This adverse event is associated with a significant increase in morbidity and economic burden, thus highlighting the need to identify methods to limit FVIII immunogenicity. Inhibitor development is regulated by a complex balance of genetic factors, such as FVIII genotype, and environmental variables, such as coexistent inflammation. One of the hypothesized risk factors of inhibitor development is the source of the FVIII concentrate, which could be either recombinant or plasma derived. Differential immunogenicity of these concentrates has been documented in several recent epidemiologic studies, thus generating significant debate within the hemophilia treatment community. To date, these discussions have been unable to reach a consensus regarding how these outcomes might be integrated into enhancing clinical care. Moreover, the biological mechanistic explanations for the observed differences are poorly understood. In this article, we complement the existing epidemiologic investigations with an overview of the range of possible biochemical and immunologic mechanisms that may contribute to the different immune outcomes observed with plasma-derived and recombinant FVIII products.
Introduction
Anti-drug antibodies represent a significant complication in the treatment of a variety of diseases, including rheumatoid arthritis, multiple sclerosis, and antibody-mediated pure red cell aplasia. The induction of such an immune response has been extensively investigated with regard to the development of anti-factor VIII (FVIII) antibodies in hemophilia A (HA). Patients with severe HA (<1% normal FVIII activity) are treated with frequent intravenous replacement of FVIII. However, this therapy is complicated in 25% to 30% of patients by the development of FVIII-neutralizing antibodies, commonly known as inhibitors, that render the protein replacement therapy ineffective.1
From an immunologic viewpoint, the development of inhibitors is not surprising considering that inhibitor risk is significantly associated with F8 mutation severity.2 Indeed, HA patients often have significant mutations in the F8 gene that either result in an absence of FVIII protein or alter the functional conformation of FVIII (eg, inversions, deletions, and missense mutations), such that infusion of the wild-type FVIII protein introduces antigenic, and potentially immunogenic, epitopes. Interestingly, 2 HA patients presenting with the same F8 mutation may be discordant for inhibitor development, which suggests that additional elements contribute to this immune response. Recently, 4 important epidemiological studies have evaluated the immunogenicity of FVIII concentrates used to treat HA.3-6 Together, these studies have highlighted an apparent differential immunogenic potential for FVIII concentrates currently in clinical use.
Since the introduction of recombinant FVIII (rFVIII) in the 1990s, there has been substantial debate about a potentially enhanced immunogenicity of these synthetic concentrates compared with plasma-derived FVIII (pdFVIII) products, which typically include the carrier protein von Willebrand factor (VWF; pdFVII refers to FVIII- and VWF-containing concentrates). Two systematic reviews from retrospective and prospective studies reported inhibitor incidences of 14.3% vs 27.4% with pdFVIII and rFVIII, respectively; however, these differences lost statistical significance upon further analysis.7,8 More recently, a retrospective study (RODIN) of 574 children also reported similar risks for inhibitor development between rFVIII and pdFVIII products.4 Collectively, this state of clinical equipoise highlighted the need for a prospective randomized study to definitively address this question. In the first randomized-controlled trial designed to answer this question (SIPPET), an 87% increase in the risk of inhibitor development was associated with 125 previously untreated patients (PUPs) treated with rFVIII compared with 126 PUPs treated with pdFVIII.3 This result has been extensively debated, and questions have been raised about the potential problems of extrapolating these results from a study population derived predominantly from India, Egypt, and Iran in clinics in which prophylactic treatment is less frequently used than in more economically advantaged countries.
In addition to differences between pdFVIII and rFVIII, a disparity between the immunogenicity of different rFVIII products has also now been documented. Three independent epidemiologic studies have each described a ∼60% increase in the incidence of inhibitors in patients treated with second-generation full-length rFVIII produced in baby hamster kidney (BHK) cells compared with third-generation rFVIII products produced in Chinese hamster ovary (CHO) cells (Table 1).4-6 Although the design and interpretation of these recent studies have been questioned, they should at least cause the hemophilia scientific community to reassess the potential biological explanations for such observations.
Categories of rFVIII
| Generation . | Trade name . | Manufacturer . | Cell line . | Protein length . | Albumin use . | Stabilizer . |
|---|---|---|---|---|---|---|
| 1 | Recombinate | Baxalta/Shire Bioscience | CHO | Full | Cell culture (human) | Bovine albumin |
| 2 | Kogenate FS | Bayer Healthcare | BHK | Full | Cell culture (human) | Sucrose |
| Helixate FS | BHK | Full | Sucrose | |||
| Refacto | Pfizer | CHO | B-domain deleted | Sucrose | ||
| 3 | Advate | Baxalta/Shire Bioscience | CHO | Full | No | No |
| Xyntha | Pfizer | CHO | B-domain deleted | No | No |
| Generation . | Trade name . | Manufacturer . | Cell line . | Protein length . | Albumin use . | Stabilizer . |
|---|---|---|---|---|---|---|
| 1 | Recombinate | Baxalta/Shire Bioscience | CHO | Full | Cell culture (human) | Bovine albumin |
| 2 | Kogenate FS | Bayer Healthcare | BHK | Full | Cell culture (human) | Sucrose |
| Helixate FS | BHK | Full | Sucrose | |||
| Refacto | Pfizer | CHO | B-domain deleted | Sucrose | ||
| 3 | Advate | Baxalta/Shire Bioscience | CHO | Full | No | No |
| Xyntha | Pfizer | CHO | B-domain deleted | No | No |
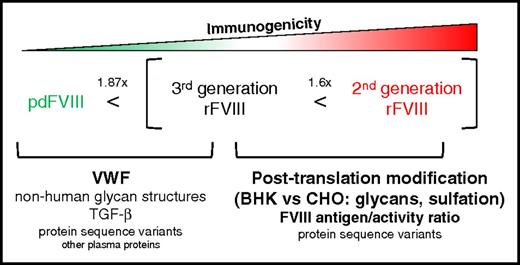
These data prompted 2 important questions concerning the mechanistic factors that differentiate the immunogenicity of pdFVIII from that of rFVIII and second-generation from third-generation rFVIII. In pdFVIII, the most obvious potential modulator of FVIII immunogenicity is VWF. However, pdFVIII represents a heterogeneous pool of plasma proteins, some of which may have influences on FVIII immunogenicity that have not yet been described. In contrast, the differences between second- and third-generation rFVIII products are likely to be quite subtle and are much more likely related to the rFVIII protein structure itself. In this article, we aim to provide plausible biological mechanisms that may explain these disparities and to offer insights that might aid in choosing an ap-propriate treatment for HA patients (Figure 1).
Proposed biological mechanisms that influence the immunogenicity of pdFVIII and rFVIII products. Recombinant FVIII products have been associated with a 1.87-fold increase in inhibitor risk compared with pdFVIII. Within the spectrum of rFVIII products, a full-length second-generation rFVIII concentrate has been reported to be 1.6-fold more immunogenic than a full-length third-generation rFVIII product. The potential immunomodulatory components of each concentrate are portrayed here in order of perceived importance. TGF-β, transforming growth factor β.
Proposed biological mechanisms that influence the immunogenicity of pdFVIII and rFVIII products. Recombinant FVIII products have been associated with a 1.87-fold increase in inhibitor risk compared with pdFVIII. Within the spectrum of rFVIII products, a full-length second-generation rFVIII concentrate has been reported to be 1.6-fold more immunogenic than a full-length third-generation rFVIII product. The potential immunomodulatory components of each concentrate are portrayed here in order of perceived importance. TGF-β, transforming growth factor β.
Immunologic considerations of pdFVIII and rFVIII
FVIII amino acid sequence variation
FVIII shows relatively limited variability in its primary structure. In a cohort of 137 healthy individuals representing 7 racial groups, 4 nonsynonymous single-nucleotide polymorphisms were identified, combinations of which resulted in 6 FVIII haplotypes.9,10 In a substantially larger cohort of 2353 individuals, the 1000 Genomes Project identified 56 nonsynonymous coding region F8 variants.11 In pooled plasma-derived products that derive from thousands of donors, there will inevitably be some FVIII sequence heterogeneity (Table 2). These protein sequence variances may introduce novel epitopes to which patients will not have central tolerance, potentially resulting in the development of cross-reactive antibodies against wild-type FVIII. This can be illustrated in the case of the Arg593Cys mutation in mild HA, in which up to 11% of pa-tients develop inhibitors.17 In this instance, patients exhibit T-cell responses against wild-type FVIII peptides that encompass the R593 amino acid, and as described in the mild A2201P mutation, also have cross-reactive T-cell responses with the endogenous mutant FVIII that ultimately converts their mild phenotype into severe FVIII deficiency.18,19 These observations suggest that single amino acid substitutions have the potential to elicit immune responses against FVIII and are particularly pertinent to treatment with pdFVIII. Although this proposition seems to be inconsistent with epidemiological findings, it is possible that noninhibitory immune responses against these amino acid variants influence the half-life of FVIII in circulation.
Major differences between pdFVIII and rFVIII products
| . | pdFVIII . | HEK293-derived rFVIII . | Second-generation BHK-derived rFVIII . | Third-generation CHO-derived rFVIII . | References . |
|---|---|---|---|---|---|
| Galα1-3Gal | Not present | Not present | Found in 3% (molar ratio) of N-linked glycans | Below limit of detection | 12, 13 |
| Neu5Gc | Not present | Not present | Below limit of detection | Present in 0.5% (molar ratio) of N-linked glycans | 12, 13 |
| ABO antigens | Present | Not present | Not present | Not present | 12, 13 |
| % nonsulfated Tyr1680 | Undetectable | Undetectable | 1.55% ± 0.07% | 6.65% ± 1.91% | 12 |
| Culture conditions | NA | No proteins of animal or human origin | Contains human plasma proteins | Co-expressed with VWF without any animal or human plasma protein additives | 14 (product monograph) |
| Additional proteins | VWF, fibronectin, fibrinogen, IgM, IgG, albumin, inter-α inhibitor, TGF-β, and others | Not reported | Not reported | Not reported | 15, 16 |
| FVIII sequence differences | ≥56 nonsynonymous variants in 2353 healthy individuals | Not reported (B-domain deleted) | Aspartic acid at amino acid 1241 | Glutamic acid at amino acid 1241 | 10, 11 |
| . | pdFVIII . | HEK293-derived rFVIII . | Second-generation BHK-derived rFVIII . | Third-generation CHO-derived rFVIII . | References . |
|---|---|---|---|---|---|
| Galα1-3Gal | Not present | Not present | Found in 3% (molar ratio) of N-linked glycans | Below limit of detection | 12, 13 |
| Neu5Gc | Not present | Not present | Below limit of detection | Present in 0.5% (molar ratio) of N-linked glycans | 12, 13 |
| ABO antigens | Present | Not present | Not present | Not present | 12, 13 |
| % nonsulfated Tyr1680 | Undetectable | Undetectable | 1.55% ± 0.07% | 6.65% ± 1.91% | 12 |
| Culture conditions | NA | No proteins of animal or human origin | Contains human plasma proteins | Co-expressed with VWF without any animal or human plasma protein additives | 14 (product monograph) |
| Additional proteins | VWF, fibronectin, fibrinogen, IgM, IgG, albumin, inter-α inhibitor, TGF-β, and others | Not reported | Not reported | Not reported | 15, 16 |
| FVIII sequence differences | ≥56 nonsynonymous variants in 2353 healthy individuals | Not reported (B-domain deleted) | Aspartic acid at amino acid 1241 | Glutamic acid at amino acid 1241 | 10, 11 |
IgM, immunoglobulin M; NA, not applicable.
Recombinant FVIII proteins do not exhibit such amino acid heterogeneity within 1 brand. However, recent epidemiological evidence suggests that different rFVIII concentrates have variable im-munogenic potential, specifically that second-generation full-length products are associated with a ∼60% higher risk of inhibitor development than third-generation full-length concentrates.4-6 These products (Table 1), respectively, Kogenate FS and Advate, differ only in their polypeptide sequences by an aspartic acid–to–glutamic acid substitution at amino acid 1241 in the B domain.10 The sequence of second-generation rFVIII matches were found in as high as ∼93% of the white population.10 It has been hypothesized that infusions with FVIII products that have a sequence mismatch with the endogenous FVIII at non–HA-associated polymorphic sites could increase inhibitor incidences (assuming that a dysfunctional hemophilic FVIII protein is expressed). Peptides spanning the 4 previously described polymorphic sequence variants (R484H, R776G, D1241E, and M2238V), however, showed different and overall limited binding affinities to HLA-DRB1 proteins.20 Importantly, CD4+ T cells isolated from HA patients treated with sequence-mismatched FVIII products showed limited binding above background levels to major histocompatibility complex (MHC) tetramers bearing core peptides containing D1241E or M2238V, whereas in vitro expansion of epitope-specific T cells was unsuccessful.20 Although peptides containing D1241 and E1241 were shown to bind to 3 of 11 tested HLA-DRB1 proteins with moderate-to-strong affinity, T-cell epitopes spanning this amino acid variant have not been reported in a DR15-restricted mouse model or in naturally processed and presented peptides by human monocyte-derived dendritic cells (DCs).20-23 Together, these findings suggest that additional factors influence the immunogenicity of rFVIII apart from the polymorphism at amino acid 1241 (and likely the other common polymorphic FVIII variants).
FVIII posttranslational modification
Posttranslational modifications (PTMs) contribute to protein folding and activity as well as receptor-mediated clearance.24 However, the role of PTM variances with regard to FVIII immunogenicity has not been explored (Table 2). If we accept that protein structure influences function, one can assume that PTMs influence protein activity and likely affect some aspect of tertiary structure. For instance, FVIII contains 6 sulfated tyrosine residues that flank thrombin cleavage sites and are necessary for optimal cofactor activity.25 The residue located at Tyr1680 is essential for the interaction with VWF.26 It has been shown that although pdFVIII is fully sulfated at this residue, there is incomplete sulfation at this site in some rFVIII products, such that up to 20% of rFVIII has been demonstrated to not associate with VWF.27,28 In terms of the increased immunogenicity of rFVIII, these findings suggest 2 paradigms in which the fraction of VWF-free rFVIII has a greater immunogenic potential than VWF-associated FVIII and/or that the nonsulfated proteins have an altered conformation that promotes antigen-presenting cell (APC) uptake and presentation. However, these theories seem unlikely to explain the immunogenicity differences between rFVIII products, given that CHO-derived rFVIII has a greater proportion of nonsulfated Tyr1680 compared with BHK-derived products.12
The most striking PTM difference between pdFVIII and rFVIII is likely glycosylation. These carbohydrate decorations are complex and diverse and reflect the cells from which FVIII originates, that is, various endothelial cell types (eg, liver sinusoidal or lymphatic endothelium) for pdFVIII, or CHO and BHK cell cultures for rFVIII.29 The FVIII glycome is particularly complex, given the total of 25 potential N-linked sites, of which only 20 have been shown to be occupied, and at least 7 O-linked glycosylation sites.30,31 One study noted that complete deglycosylation of rFVIII resulted in a 40% decrease in cofactor activity, impaired binding to phosphatidylserine, and a somewhat attenuated immune response.32 However, additional studies are necessary to firmly establish a role for FVIII glycosylation in immunogenicity.
To provide perspective for the complexities of studying FVIII glycosylation, recent analysis of the plasma-derived VWF glycome proposes more than 300 unique N-linked glycans and 18 O-linked glycans on only 12 and 10 predicted sites, respectively.33,34 It is possible that there is an optimal glycoform of FVIII that provides stable cofactor activity and limited immunogenicity. This type of detail for engineering recombinant proteins represents a substantial challenge for the therapeutic protein industry.
Given the production of rFVIII in non-human cell lines, it is not surprising that there are differences in PTMs, some of which have been shown to influence the biosynthesis of, clearance of, and immune response to FVIII.35-37 The first obvious difference from pdFVIII is the absence of ABO blood group antigens on rFVIII, which may in fact be immunoprotective via decreased formation of immune complexes against the foreign blood antigen.31 However, clinical evidence in type 3 von Willebrand disease suggests that the heterogeneity of blood group antigens in plasma-derived VWF (pdVWF) does not have a significant influence on VWF clearance, suggesting that a similar outcome may pertain to FVIII with pdFVIII replacement.38
More importantly, non-human mammalian cell lines incorporate Gal(α1-3)Galβ1-GlcNAc-R (αGal) linkages and N-glycolylneuraminic acid (Neu5Gc) residues that are absent in humans.39,40 Immunoglobulin G (IgG), IgA, and IgM antibodies against these non-human structures have been reported in healthy individuals and may contribute to the apparent increase in rFVIII immunogenicity.39,41 Specifically, it has been shown that anti-Neu5Gc antibodies form immune complexes with the recombinant therapeutic glycoprotein cetuximab and can further augment its clearance.42 In addition, patients treated with cetuximab have experienced anaphylaxis via IgE specific for the αGal linkage.43 We have previously described the overlapping processes of FVIII clearance and immunogenicity and suggest again that antibodies against these non-human carbohydrate epitopes might influence these events and contribute to the increased risk of antibodies against rFVIII products produced in non-human cell cultures.44 Interestingly, 3% of the total N-linked glycans of BHK-derived rFVIII contain the αGal epitope, whereas it is not found in CHO-derived rFVIII.13 In contrast, Neu5Gc has not been detected in BHK-derived rFVIII but is present as 0.5% of the total sialic acid content in CHO-derived rFVIII.12 Given these findings, it is possible that αGal epitopes play a stimulatory role in the increased immunogenicity of BHK-derived rFVIII. However, it should be noted that these ratios are subject to change between concentrate lots, because the sialic acid content of recombinant proteins can be modulated by the carbohydrate composition of the culture medium.45
Ultimately, considering the emergence of rFVIII products with modified glycan profiles such as Nuwiq (Octapharma AG) produced in human embryonic kidney 293 cells, and Kovaltry (Bayer), a hyper-sialylated FVIII glycoform, the role of FVIII glycosylation in immunogenicity merits further consideration.14,46 The former concentrate has been described as being devoid of the non-human glycan epitopes described above and exhibits complete sulfation of the 6 tyrosine residues.12 In turn, murine DC uptake of artificially sialylated antigens has recently been reported to inhibit Th1 and Th17 T-cell responses and promote the induction of de novo regulatory T cells (Tregs) through a mechanism dependent on the sialic acid–binding Ig-type lectin Siglec-E.47 This immunomodulatory receptor, whose human ortholog is Siglec-9, is expressed on mouse neutrophils, monocytes, macrophages, and DCs, preferentially binds to α2-8–linked sialic acids, and dampens inflammation through the downregulation of toll-like receptor 4 signaling.48,49 Targeting Siglec-E by using nanoparticles decorated with α2-8 N-acetylneuraminic acid has recently been shown to induce expression of interleukin-10 (IL-10) and downregulate the inflammatory response in a murine model of lipopolysaccharide-induced sepsis.50 This finding implies that the role of sialic acid on FVIII immunogenicity is likely more complex than simply a quantitative influence and may involve contributions from specific linkages that alter binding to inhibitory or stimulatory sialic acid receptors. Further epidemiologic evidence and fundamental immunology research are needed to assess whether these subtle changes in glycosylation are contributing to the differential immunogenicity recently described for rFVIII concentrates.
VWF
Given the intertwined life cycles of FVIII and VWF, any discussion of FVIII immunogenicity must consider the influence of the large polymeric carrier protein of FVIII. It is widely accepted that VWF exerts the most dominant influence on FVIII half-life and quite likely modulates the FVIII immune response.51 Several lines of evidence have suggested that VWF plays an immune-protective role for FVIII, predominantly through inhibition of FVIII endocytosis by murine and human DCs.52,53 Subsequent characterization of the MHC class II peptide repertoire in these cells has demonstrated that there is minimal VWF internalization and presentation by human monocyte–derived DCs and that the presence of VWF alters the peptide presentation profile of FVIII.54 However, these assay conditions may not perfectly reflect conditions in vivo, given the cell type used as well as the lack of shear forces on VWF. In an experimental murine model of HA, pdFVIII (VWF:FVIII ratio of 1) has shown a significant reduction in FVIII inhibitor titers accompanied by an increase of Foxp3+ Tregs.55 These differences were largely abolished when rFVIII and highly purified pdVWF were mixed in vitro prior to treatment (VWF:FVIII ratio of 50). It is possible that these observations are attributed to differences in the VWF:FVIII ratio, but it is also possible that there are other immunomodulatory factors in the pdFVIII concentrate or that the in vitro mixture of rFVIII and pdVWF is structurally different from the complex formed in vivo upon infusion of rFVIII.
FVIII protein structure and protein aggregates
There is conflicting evidence regarding whether or not FVIII immunogenicity is dependent on procoagulant function and by extension, tertiary structure.56,57 The structural integrity of FVIII has implications for APC uptake through the availability of endocytic epitopes. Moreover, conformational B-cell epitopes likely account for the majority of inhibitory FVIII antibodies.58 Denatured FVIII would therefore have lower affinity for B-cell receptors and thus fail to initiate B-cell maturation and proliferation. In this respect, the spontaneous aggregating nature of FVIII proteins becomes an important immunomodulatory variable.59 Aggregation of FVIII, either with itself in recombinant products or with other proteins in plasma-derived concentrates, introduces a substantial increase in the density of native epitopes, neo-epitopes, and repeating moieties, all of which have the potential to adversely influence immunogenicity.60 Interestingly, aggregated rFVIII (heat inactivated at 56°C and 80°C) bearing little native structure has been observed to be less immunogenic than native FVIII, whereas native-like aggregates (heat inactivated at 37°C), bearing some native structure result in significantly increased inhibitory titers compared with native FVIII in mice.61,62 These data reinforce the requirement for conformational B-cell epitopes of native FVIII for the development of inhibitory antibodies. Moreover, repeating epitopes in these native-like aggregates can cross-link IG and pattern recognition receptors that will further augment the immune response.63 Alternatively, these results may be attributed to decreased FVIII up-take by APCs when denatured at high temperatures, thus losing most of its tertiary structure. Ultimately, a higher tendency to form aggregates in rFVIII products may contribute to differences in immunogenicity, whereas in plasma-derived concentrates, the integrity of FVIII is stabilized by VWF and potentially by other plasma proteins.64 Overall, these findings highlight the complexities of FVIII protein immunogenicity and demonstrate a precarious balance between the formation of immunogenic microaggregates or misfolded proteins, and the possible contribution of immunogenic procoagulant properties.
Product formulations
The final differences between the 2 full-length rFVIII products involve their culture conditions and their stabilizing agents. Second-generation products use human albumin in the culture media and sucrose as a stabilizer, whereas third-generation products use neither (Table 2).10 However, there is no evidence in the literature to suggest that the stabilizing molecules influence the immune response.
Interestingly, second-generation products have been reported to have higher FVIII:C and FVIII:Ag than the advertised label content, suggesting that patients receiving these factors would be exposed to greater amounts of protein.65 However, with the exception of boosting the secondary immune response, it is unclear whether a higher dose of FVIII is more efficient at initiating immunity. In the same study, second-generation rFVIII demonstrated more rapid and complete activation by thrombin, thus amplifying further thrombin generation, an event that has been implicated, but controversial, in the initiation of the anti-FVIII immune response.56,57,65
As mentioned previously, the pdFVIII concentrates used in practice are heterogeneous in nature and contain minor protein constituents that are not removed in the purification process. One potentially critical constituent that may have biological implications for the decreased immunogenicity of pdFVIII concentrates is TGF-β.66 It has been shown in vitro that, in response to pdFVIII, phorbol 12-myristate 13-acetate (PMA)- or lipopolysaccharide-stimulated T cells, natural killer cells, and monocytes decrease synthesis of inflammatory cytokines (eg, tumor necrosis factor α and interferon-γ) and increase monocyte production of immunomodulatory IL-10.67 Furthermore, pdFVIII has been shown to dampen phytohemagglutinin-induced T-cell proliferation and inhibit Th2 cytokine production.68,69 In these studies, the addition of a TGF-β neutralizing monoclonal antibody reduced the immunomodulatory effects of pdFVIII on cytokine secretion and leukocyte proliferation.67-69 Collectively, the immune-dampening role of IL-10 and the inhibition of T-cell responses provide strong evidence in support of a potential pdFVIII-derived TGF-β–mediated immunomodulatory influence that would be absent in highly purified rFVIII concentrates. However, it is unclear whether the amount of TGF-β in pdFVIII infusions compared with native plasma levels could elicit an immunomodulatory effect. Further studies are needed to assess the role of TGF-β in vivo and to determine any adverse effects such as long-term immunosuppression.
Although the constituents of pdFVIII have not been precisely identified, an example data sheet for the pdFVIII product, Humate-P, describes that every 40 to 80 IU of FVIII may be accompanied by 72 to 224 IU of VWF, 15 to 33 mg glycine, 8 to 16 mg of human albumin, and up to 34 mg of other proteins. Proteomic characterizations of pdFVIII concentrates have identified a significant number of other proteins, including fibronectin, Ig’s, fibrinogen, galectin-3, and some complement proteins.15,16 Thus, the quantity of FVIII in these preparations is trace. Given these additional constituents, we propose here that the reduction in FVIII inhibitor incidence observed in pdFVIII-treated patients may be the result of antigen competition or distraction.
Antigen competition and bystander suppression
The phenomenon of antigen competition has been poorly described in the literature. One example is the recommended interval between multiple vaccinations, which raises the concern that simultaneous exposure to different vaccine antigens may reduce the collective protective immune response through a poorly defined mechanism of immune overload.70,71 However, multiple lines of experimental evidence have disputed these claims.72,73 Recently, it was described that concurrent influenza immunization in mice via a different anatomical compartment from rFVIII decreases the immune response against the latter, potentially through the diversion of CD4+ T-cell recruitment.74 Considering these concerns and observations, it is possible that the additional proteins in pdFVIII, and perhaps specifically exogenous VWF, influence the immune response against FVIII.
Conceptually, when a severe HA PUP is infused with rFVIII, the only perceived foreign entity is the homogeneous purified rFVIII molecule. The initiation of the anti-FVIII immune response is therefore focused primarily on the epitope discrepancies compared with any endogenous FVIII they may possess. In contrast, severe HA PUPs treated with pdFVIII are infused with FVIII and VWF from an extensive pool of donors (>10 000), as well as the additional protein constituents. As a result, these individuals are exposed to at least 2 polymorphic antigens, FVIII and VWF, which may be perceived as foreign by the recipient’s immune system. Although central immunologic tolerance to endogenous VWF is likely to be robust in these patients, VWF has recently been shown to be highly polymorphic, with 91 nonsynonymous variants and minor allele frequencies ranging from 0.03% to 3.35%.75 Indeed, patients receiving plasma-derived concentrates have been reported to develop anti-VWF immune re-sponses against their pdVWF therapies.76
Antigenic competition induced by pdFVIII concentrates can be conceptualized at 2 levels. First, there is competition in the loading of MHC class II molecules such that the frequency of immunogenic FVIII peptide-MHCs (pMHCs) can be replaced with pMHCs to which there is central tolerance and therefore no corresponding antigen-specific T cells (Figure 2). The second level occurs at the interface between APCs and CD4+ T cells as the circulating T cells sample pMHCs for cognate sequences to initiate a productive immunologic synapse. This synapse has a threshold that separates the brief serial sampling of pMHCs on APCs from the stabilized and activating APC–T-cell interaction. Initial studies using hen egg white lysozyme, have described T-cell hybridoma activation with as little as 0.03% to 0.1% of total MHC class II molecules bearing the cognate peptides.77,78 Furthermore, the kinetics of stable APC–T-cell interactions are directly correlated with the antigen dose and the clustering of pMHC molecules in lipid rafts, such that a least 4 pMHC molecules are required to reach the threshold of T-cell activation.79-81 Considering the relatively minuscule contribution of FVIII antigens to the pdFVIII peptide pool and the heterogeneity of the infused protein concentrate (particularly for VWF), it is perhaps not surprising that there is decreased FVIII immunogenicity. However, this model fails to explain how peripheral tolerance to FVIII is established with pdFVIII products, nor does it account for the fact that the total infused protein is still likely a small fraction of the total pool of peptides available for presentation. Nevertheless, we can hypothesize that there is the potential for a decrease in the statistical probability of an FVIII immune response.
Antigenic competition and bystander suppression may play roles in mediating the decreased immunogenicity of pdFVIII. Plasma-derived concentrates contain additional protein constituents that compete with FVIII for MHC II presentation. As a result, there is a decreased probability that a given antigen-presenting cell will simultaneously express FVIII-bound MHC class II cells and interact with an FVIII-specific CD4+ T cell. Similarly, Tregs against other protein components in pdFVIII may elicit bystander suppression that either inhibits FVIII-specific T cells or polarizes their differentiation into Tregs.
Antigenic competition and bystander suppression may play roles in mediating the decreased immunogenicity of pdFVIII. Plasma-derived concentrates contain additional protein constituents that compete with FVIII for MHC II presentation. As a result, there is a decreased probability that a given antigen-presenting cell will simultaneously express FVIII-bound MHC class II cells and interact with an FVIII-specific CD4+ T cell. Similarly, Tregs against other protein components in pdFVIII may elicit bystander suppression that either inhibits FVIII-specific T cells or polarizes their differentiation into Tregs.
The last mechanism we propose as a contributory factor to the potential reduced immunogenicity of pdFVIII is the bystander suppression of FVIII-specific T cells by Tregs specific to the other proteins in plasma-derived concentrates (Figure 2). These autoreactive, Foxp3-expressing Tregs originate in the thymus as a result of high avidity T-cell receptor binding to endogenous antigens.82 The double-edged role of Tregs was previously reviewed, and it has been shown that although they can limit effector immune responses to prevent autoimmunity, they have also been reported to support pathogen persistence through suppression of the required effector help.83 Although the presence of VWF-specific Tregs has not been investigated, it is plausible that Tregs specific for the other protein constituents of plasma-derived concentrates can actively suppress naïve FVIII-specific CD4+ T cells through immunomodulatory cytokine secretion or direct cell contact, as has been shown in models of allograft rejection.83,84 Activation of these Tregs and subsequent secretion of TGF-β and IL-10 may also aid in the polarization of FVIII-specific T cells to a regulatory phenotype. Ultimately, it is possible that both of these proposed mechanisms, antigen competition and bystander suppression, contribute to the apparent decrease in immunogenicity of pdFVIII concentrates.
Future perspectives
Epidemiological evidence comparing the differential immunogenicity of FVIII concentrates is now available, but studies addressing the biological basis of such differences are lacking. Today, plasma-derived concentrates are safe and effective, but they will always carry a theoretical risk of transmissible agent contamination, and thus, recombinant products are perceived as safer alternatives. However, with respect to anti-drug antibodies, recent epidemiologic evidence suggests that a hierarchy exists in which pdFVIII is less immunogenic than third-generation rFVIII, which is in turn less immunogenic than second-generation products. Here, we have highlighted potential mechanisms that may contribute to the differences observed in these epidemiologic studies. Further clinical and biological studies of the apparent differential immunogenicity of FVIII concentrates are needed, which may ultimately result in safer FVIII replacement.
Acknowledgments
This work was supported by the Canadian Institutes of Health Research. D.L. is the recipient of a Canada Research Chair in Molecular Hemostasis.
Authorship
Contribution: J.L. designed and wrote the first draft of the manuscript; and C.H., J.T., and D.L. designed and edited the manuscript.
Conflict-of-interest disclosure: D.L. received research support from Bayer, Biogen, Shire, CSL, Octapharma, and Sangamo. The remaining authors declare no competing financial interests.
Correspondence: David Lillicrap, Department of Pathology and Molecular Medicine, Richardson Laboratory, Queen’s University, 88 Stuart St, Kingston, ON K7L 3N6, Canada; e-mail: dpl@queensu.ca.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal