To the editor:
The recently approved anti-CD38 monoclonal antibody daratumumab (DARA) provides a unique therapeutic strategy that more selectively targets plasma cells in patients with plasma cell myeloma.1-6 While DARA recognizes and removes malignant plasma cells, red blood cells (RBCs) also express CD38.7 However, many patients on DARA unexpectedly display a negative direct antiglobulin test (DAT) result, despite producing pan-reactive indirect antibody test (IAT) results observed on antibody screens.8-11 Pan-reactive IAT results caused by DARA complicate pretransfusion testing,8-11 and the discrepancy between positive IAT results and negative DAT results observed in patients on DARA remains a common, yet incompletely understood, outcome of DARA treatment. Furthermore, while DARA eliminates plasma cell myeloma through a variety of mechanisms,1-6 this antibody fails to induce detectable RBC hemolysis.8,9,12,13 The negative DAT result following DARA treatment coupled with the apparent resistance of RBCs to DARA-induced hemolysis led us to hypothesize that DARA may induce removal of CD38 from the RBC surface, resulting in a negative DAT result that may protect RBCs from DARA-induced removal.
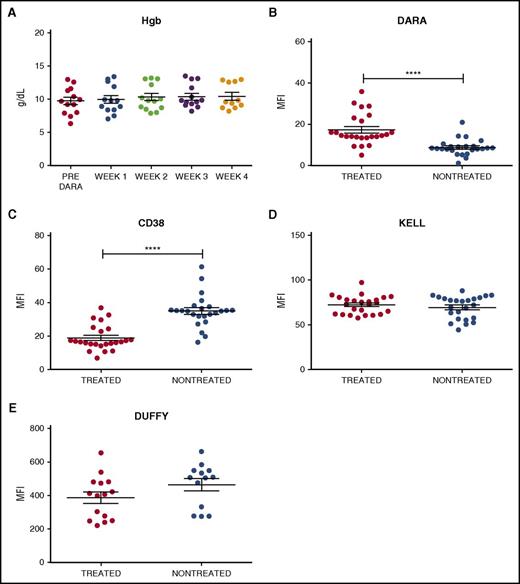
To examine the impact of DARA on RBC CD38 levels, 24 samples were obtained from 13 DARA-treated patients enrolled in a phase 2 efficacy and safety trial (www.clinicaltrials.gov identifier #NCT01985126). Samples from 24 nontreated controls were obtained from the clinical laboratory. Similar to previous reports,8,9 the 24 samples from DARA-treated patients demonstrated anti-RBC antibodies in the serum (antibody screen reactivity: weak positive to 2+; supplemental Table 1, available on the Blood Web site). In contrast, none of the samples from DARA-treated patients demonstrated a positive DAT, indicating that standard clinical tests could not detect binding of serum DARA to the patients’ own RBCs (supplemental Table 2). Consistent with these and previous results,8,9,12,13 DARA also failed to cause significant anemia, suggesting minimal (if any) DARA-mediated hemolysis (Figure 1A). To more specifically investigate the interaction between DARA and RBCs, we examined antibody reactivity on the RBC surface using a more sensitive flow cytometry–based DAT in which antibody engagement of RBCs is assessed using fluorescently labeled anti–human immunoglobulin G.14,15 RBCs isolated from DARA-treated patients demonstrated weak antibody positivity when compared with RBCs isolated from nontreated patients (Figure 1B). To determine whether low antibody binding to RBCs from DARA-treated individuals reflected incomplete DARA engagement of CD38, we next incubated RBCs from DARA-treated or nontreated patients with saturating levels of DARA. RBCs from nontreated patients displayed higher DARA engagement than RBCs from DARA-treated individuals (Figure 1C), strongly suggesting that the reduced DAT level following DARA treatment did not reflect incomplete CD38 binding but instead possibly reflected an actual loss of the CD38 target antigen. In contrast to CD38, DARA treatment failed to alter the levels of Kell or Duffy RBC antigens (Figure 1D-E), indicating that the effects of DARA were CD38 specific.
Antibody and CD38 antigen levels are decreased on RBCs isolated from DARA treated individuals. (A) Hemoglobin (Hgb) levels of DARA-treated patients before DARA exposure and at weeks 1, 2, 3, and 4 of treatment (week 4 n = 11, as hemoglobin data were not available for 2 patients). (B-E) Flow cytometric detection of in vivo DARA binding (B), CD38 antigen (C), Kell antigen (D), and Duffy antigen (E) on RBCs isolated from DARA-treated and nontreated patients. Significance was determined by Student t test (****P ≤ .0001). MFI, mean fluorescence intensity.
Antibody and CD38 antigen levels are decreased on RBCs isolated from DARA treated individuals. (A) Hemoglobin (Hgb) levels of DARA-treated patients before DARA exposure and at weeks 1, 2, 3, and 4 of treatment (week 4 n = 11, as hemoglobin data were not available for 2 patients). (B-E) Flow cytometric detection of in vivo DARA binding (B), CD38 antigen (C), Kell antigen (D), and Duffy antigen (E) on RBCs isolated from DARA-treated and nontreated patients. Significance was determined by Student t test (****P ≤ .0001). MFI, mean fluorescence intensity.
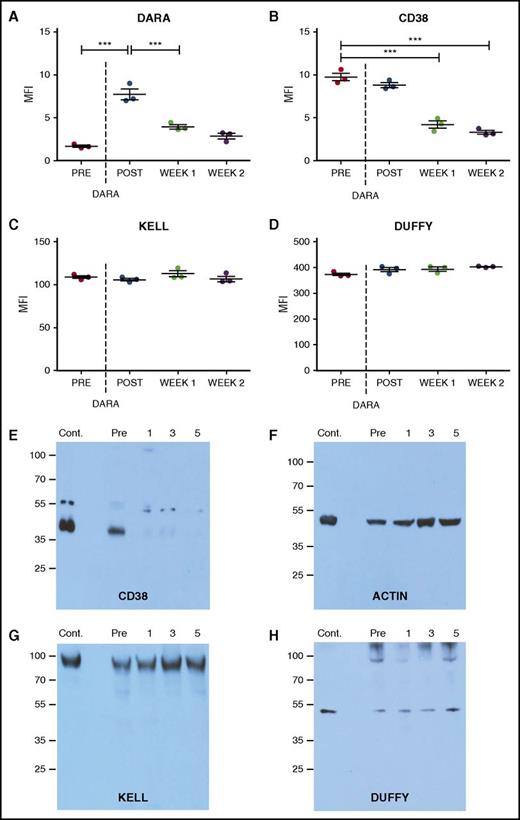
While this cross-sectional analysis of the impact of DARA on CD38 levels suggests that DARA treatment may alter CD38 detection, it remained possible that the reduced DAT observed following DARA treatment might simply reflect an inability of DARA to fully engage RBCs in vivo. To test this, we examined the impact of DARA treatment on RBC CD38 levels before and after infusion. Antibody (DAT) reactivity on the RBC surface immediately following DARA infusion was robust and occurred at a level similar to that observed following DARA incubation with RBCs from nontreated individuals in vitro (Figure 2A). Additional DARA incubation failed to increase antibody engagement, strongly suggesting that DARA infusion led to complete saturation of CD38-binding sites on RBCs in vivo. To determine whether DARA engagement persists over time, we examined RBCs 1 week following DARA infusion (Figure 2A). Unlike the DAT level observed immediately following DARA exposure, DAT levels dropped considerably 1 week later, largely matching the levels observed in our cross-sectional analysis of patients on active DARA therapy (Figure 1). Incubation of RBCs with saturating levels of DARA failed to increase antibody reactivity, consistent with the excess DARA detected in serum samples when conducting IATs (supplemental Table 1), strongly suggesting that decreases in DATs reflected loss of CD38. DARA-induced decreases in detectable CD38 could be observed within 6 hours of initiating treatment and also exhibited reversibility, as CD38 was again detectable 6 months after discontinuing treatment and corresponded with a loss of serum DARA detectable by IATs (supplemental Figures 1 and 2; supplemental Table 3). Similar to our cross-sectional analysis, DARA-induced changes over time also appeared to be CD38 specific (Figure 2B), as no alterations in Kell or Duffy antigens were detected (Figure 2C-D).
CD38 antigen and bound antibody specifically decline following DARA treatment. (A-D) Bound DARA (ie, flow cytometry–based DAT) (A), CD38 antigen (B), Kell antigen (C), and Duffy antigen (D) before and after initial DARA administration (post, same day as administration; week 1, 1 week after initial administration; week 2, 2 weeks after initial administration). (E-H) Western blot analysis of the CD38 antigen (E), β-actin (F), Kell antigen (G), and Duffy antigen (H) before and after DARA administration (1, 1 week following initial administration; 3, 3 weeks following initial administration; 5, 5 weeks following initial administration). Each time point consists of 3 repeat runs of the same sample. Dotted line represents first DARA administration. Significance was determined in panels A-D by 1-way analysis of variance with Tukey post-test (***P ≤ .001).
CD38 antigen and bound antibody specifically decline following DARA treatment. (A-D) Bound DARA (ie, flow cytometry–based DAT) (A), CD38 antigen (B), Kell antigen (C), and Duffy antigen (D) before and after initial DARA administration (post, same day as administration; week 1, 1 week after initial administration; week 2, 2 weeks after initial administration). (E-H) Western blot analysis of the CD38 antigen (E), β-actin (F), Kell antigen (G), and Duffy antigen (H) before and after DARA administration (1, 1 week following initial administration; 3, 3 weeks following initial administration; 5, 5 weeks following initial administration). Each time point consists of 3 repeat runs of the same sample. Dotted line represents first DARA administration. Significance was determined in panels A-D by 1-way analysis of variance with Tukey post-test (***P ≤ .001).
Detection of CD38 on RBCs following DARA treatment could be obscured by the masking of CD38 by degraded DARA fragments and/or complement split products that prevent typical anti–immunoglobulin G antibodies from recognizing bound DARA or the CD38 antigen. As a result, we next determined whether the reduced DATs observed following DARA treatment actually reflected masking of CD38 by DARA fragments and/or complement split products. To accomplish this, we first examined whether DARA induced complement fixation on the RBC surface. No complement was detected on RBCs from DARA-treated individuals (supplemental Figure 3), signifying that complement-mediated masking of CD38 was not likely responsible for decreased CD38 detection. We then directly examined CD38 levels using western blot analysis. While CD38 protein was clearly present on RBCs before DARA infusion, it became undetectable 1 week after treatment (Figure 2E). DARA-induced changes in CD38 were once again antigen specific, as DARA treatment failed to induce similar changes in Kell or Duffy antigens (Figure 2F-G). Taken together, these results demonstrate that DARA possesses the ability to uniformly remove CD38 from the RBC surface without inducing detectable hemolysis.
The ability of DARA to induce antigen loss explains conflicting results that surfaced shortly after the introduction of DARA: the ability of DARA to cause a pan-reactive antibody screen in a patient with a negative DAT result and no detectable hemolysis. While loss of CD38 on the RBC surface may impact RBC interactions with endothelial cells or alter other RBC activities,16 DARA-induced removal of CD38 appears to prevent further DARA engagement, possibly protecting RBCs from continual removal in the face of ongoing DARA treatment. DARA-induced CD38 removal is consistent with alterations in target antigens observed in murine models of incompatible transfusion and correlative findings in patients with autoimmune hemolytic anemia.17,18 Similar to DARA, in murine models, antibody engagement can induce loss of target antigen, which typically occurs through an Fcγ receptor–dependent pathway.15,19-21 In these settings, even partial loss of antigen can render RBCs resistant to antibody-mediated hemolysis,15 suggesting that a threshold effect may exist for antibody-induced clearance. As a result, even incomplete early loss of CD38 might impact the sensitivity of RBCs to possible DARA-induced removal, although the initial density of CD38 may also play a role in the relative resistance of RBCs to DARA. While the precise mechanisms and factors that regulate antibody-induced antigen loss on the RBC surface remain to be determined, understanding factors that dictate antibody-induced antigen loss versus RBC removal may provide an opportunity to selectively enhance antigen loss over RBC clearance in the setting of autoimmune hemolytic anemia and immune-mediated hemolytic transfusion reactions.15,19-22 Future studies will explore these intriguing possibilities.
The online version of this article contains a data supplement.
Authorship
Acknowledgments: This work was supported in part by the Burroughs Wellcome Trust Career Award for Medical Scientists and the National Institutes of Health (NIH) Common Fund, NIH Director's Early Independence Award DP5OD019892 (S.R.S.).
Contribution: S.R.S., H.C.S., and S.L. designed the research study and carried out and analyzed experiments together with C.G.-S., C.M.A., and L.T.; critical support was provided by A.K.N., A.M., S.R.P., M.Y., R.M.F., C.D.J., R.M.K., and J.D.R.; and H.C.S. and S.R.S. wrote the manuscript, which was additionally edited and commented on by the other authors.
Conflict-of-interest disclosure: S.L. is a consultant for Millennium, Celgene, Novartis, BMS, Onyx, and Janssen (all <$10 000 per year) and receives research support from Millennium, Celegene, BMS, and Janssen. The remaining authors declare no competing financial interests.
Correspondence: Sean R. Stowell, Center for Transfusion Medicine and Cellular Therapies, Department of Pathology and Laboratory Medicine, 105M Whitehead Building, 615 Michael St, Atlanta, GA 30322; e-mail: srstowe@emory.edu.