Key Points
A pooled analysis of 2 daratumumab trials showed no new safety signals, an overall response rate of 31%, and deep and durable responses.
Median overall survival was 20.1 months; benefit was also shown in patients who achieved minimal response/stable disease.
Abstract
The efficacy and favorable safety profile of daratumumab monotherapy in multiple myeloma (MM) was previously reported. Here, we present an updated pooled analysis of 148 patients treated with daratumumab 16 mg/kg. Data were combined from part 2 of a first-in-human phase 1/2 study of patients who relapsed after or were refractory to ≥2 prior therapies and a phase 2 study of patients previously treated with ≥3 prior lines of therapy (including a proteasome inhibitor [PI] and an immunomodulatory drug [IMiD]) or were double refractory. Among the pooled population, patients received a median of 5 prior lines of therapy (range, 2 to 14 prior lines of therapy), and 86.5% were double refractory to a PI and an IMiD. Overall response rate was 31.1%, including 13 very good partial responses, 4 complete responses, and 3 stringent complete responses. The median duration of response was 7.6 months (95% confidence interval [CI], 5.6 to not evaluable [NE]). The median progression-free survival (PFS) and overall survival (OS) were 4.0 months (95% CI, 2.8-5.6 months) and 20.1 months (95% CI, 16.6 months to NE), respectively. When stratified by responders vs stable disease/minimal response vs progressive disease/NE, median PFS was 15.0 months (95% CI, 7.4 months to NE) vs 3.0 months (95% CI, 2.8-3.7 months) vs 0.9 months (95% CI, 0.9-1.0 months), respectively, and median OS was NE (95% CI, NE to NE) vs 18.5 months (95% CI, 15.1-22.4 months) vs 3.7 months (95% CI, 1.7-7.6 months), respectively. No new safety signals were identified. In this pooled data set, daratumumab 16 mg/kg monotherapy demonstrated rapid, deep, and durable responses, with a clinical benefit that extended to patients with stable disease or better.
Introduction
Over the last decade, the introduction of proteasome inhibitors (PIs) and immunomodulatory drugs (IMiDs) has significantly prolonged survival of patients with multiple myeloma (MM).1,2 Despite the availability of these classes of drugs for the treatment of MM, a recent analysis of patients with relapsed and refractory MM (RRMM) who were double refractory to a PI and an IMiD or had relapsed after ≥3 prior lines of therapy, including the novel agents pomalidomide (third-generation IMiD) and carfilzomib (second-generation PI), showed a median overall survival (OS) of 8 months.3
Daratumumab is a first-in-class, human immunoglobulin G1 kappa (IgG1κ) monoclonal antibody that binds malignant cells expressing CD38 with high affinity and induces tumor cell death through several immune-mediated mechanisms of action, including complement-dependent cytotoxicity, antibody-dependent cellular phagocytosis, antibody-dependent cell-mediated cytotoxicity, induction of apoptosis, and modulation of CD38 enzyme activities.4-6 Although normal lymphoid, myeloid, and some nonhematopoietic cells or tissues express low levels of CD38,7 myeloma cells overexpress the protein,8,9 thus providing a clinical rationale for the protein as a therapeutic target in MM. In addition to these immune-mediated killing mechanisms, daratumumab may also have an immunomodulatory role via T-cell activation and expansion as well as mitigation of immunosuppression in patients with MM.10
The efficacy and safety profiles of daratumumab monotherapy in heavily pretreated patients with RRMM were examined in the open-label, phase 1/2 GEN501 study and the phase 2 SIRIUS study.11,12 In these studies, overall response rates (ORRs) were 36% and 29% in patients treated with daratumumab 16 mg/kg after a median follow-up of 16.9 months (range, 0.4-24.9 months) and 9.3 months (range, 0.5-14.4 months), respectively, and many of these responses deepened over time to include complete responses (CRs) and stringent complete responses (sCRs). Daratumumab also demonstrated a manageable safety profile marked by a low incidence of grade ≥3 infusion-related reactions (IRRs) and few treatment discontinuations as a result of adverse events (AEs). On the basis of these findings, the US Food and Drug Administration granted accelerated approval of daratumumab monotherapy (16 mg/kg) for the treatment of patients with MM who have received ≥3 prior treatments that included a PI and an IMiD or who were double refractory to a PI and an IMiD.13 The aim of this study is to provide a pooled, updated analysis of these 2 studies (GEN501 part 2 and SIRIUS) of patients treated with daratumumab 16 mg/kg.
Patients and methods
Study design, patients, and treatment
Details regarding the eligibility criteria, study designs, and primary results of the GEN50111 and SIRIUS12 studies have been published elsewhere. In both studies, patients with MM who were age 18 years or older and had an Eastern Cooperative Oncology Group performance status of ≤2 were included.
GEN501 (NCT00574288) was an open-label, multicenter, phase 1/2, dose-escalation and dose-expansion study that included patients with MM who relapsed from or were refractory to ≥2 prior lines of therapy that included a PI and an IMiD.11 Patients who had an absolute neutrophil count ≥1000/μL, platelet count ≥75 × 109/L, hemoglobin ≥7.5 g/dL, alanine aminotransferase ≤3.5 times the upper limit of normal, and bilirubin levels ≤2.5 times the upper limit of normal were eligible. Patients with serum creatinine levels >2 times the upper limit of normal were excluded from the study. In the dose-escalation phase of the study (part 1), patients received daratumumab doses of 0.005 to 24 mg/kg and, after evaluation of safety and responses at these doses, a dose-expansion phase of the study (part 2) was conducted to investigate daratumumab doses of 8 and 16 mg/kg (supplemental Figure 1, available on the Blood Web site). Daratumumab 16 mg/kg was administered once per week for the first 7 doses after the initial dose, which had a 3-week washout period to collect pharmacokinetic data, twice monthly for the subsequent 8 doses, and monthly thereafter. The primary end point in GEN501 was safety, and efficacy was a secondary end point.
SIRIUS (NCT01985126) was an open-label, multicenter, phase 2 study that included patients with MM who were previously treated with ≥3 prior lines of therapy (including a PI and an IMiD) or who were double refractory.12 Patients with absolute neutrophil count >1000/μL, hemoglobin >7.5 g/dL, platelet count ≥50 × 109/L, creatinine clearance >20 mL/min, and alanine aminotransferase <2.5 times the upper limit of normal were eligible. Patients were randomly assigned 1:1 to daratumumab 8 mg/kg or 16 mg/kg treatment groups in part 1 and, after interim analysis that assessed the responses of patients in each treatment group, additional patients were enrolled into the 16 mg/kg cohort (supplemental Figure 1). Patients in the SIRIUS study received daratumumab 16 mg/kg once per week for 8 weeks, once every 2 weeks for 16 weeks, and then once every 4 weeks thereafter. The primary end point for this study was ORR (the sum of patients who achieved partial responses [PRs], very good partial responses [VGPRs], CRs, and sCRs). Responses were confirmed on the basis of 2 consecutive measurements and were assessed by an independent review committee. AEs were also monitored as part of the safety assessment.
In both studies, if patients developed progressive disease (PD) and subsequently discontinued daratumumab treatment, their subsequent therapies and best clinical responses to those therapies were captured, when possible. Both studies obtained approval from relevant institutional review boards or ethics committees, and all patients provided written informed consent.11,12 All authors had full access to the data.
Statistical analyses
In both the GEN501 part 2 and SIRIUS studies, responses were evaluated by using the International Myeloma Working Group response criteria. The Kaplan-Meier method was used to analyze all time-to-event end points, including duration of response, progression-free survival (PFS), and OS. No formal statistical hypotheses were formulated or tested. For the combined analysis, data from all patients who received daratumumab 16 mg/kg in GEN501 part 2 and SIRIUS were included, and similar statistical parameters were applied. An exploratory analysis was conducted to assess PFS and OS in patients stratified by their response status (responders [PR + VGPR + CR + sCR] vs minimal response [MR]/stable disease [SD] vs PD/not evaluable [NE]).
Results
Patients and treatment
Enrollment in the GEN501 study commenced on March 27, 2008, and in the SIRIUS study on September 30, 2013. Data were previously reported from the cutoff date of January 9, 2015.11,12 At the cutoff date of December 31, 2015, a total of 148 patients who received daratumumab 16 mg/kg in the GEN501 part 2 (n = 42) and SIRIUS (n = 106) studies were included in the combined analysis. The median duration of follow-up was 20.7 months (range, 0.5-27.1 months) on the basis of a Kaplan-Meier analysis. Baseline characteristics and refractory status for patients within the individual studies and the combined data set are summarized in Table 1. Overall, patients were heavily pretreated, with a median of 5 prior therapies (range, 2-14 prior therapies); 76.4% of patients received >3 prior lines of therapy. Many patients (86.5%) were double refractory to both a PI and an IMiD, 39.2% of patients were refractory to carfilzomib, and 55.4% of patients were refractory to pomalidomide. The 2 study populations were well balanced. The higher percentages of patients previously treated with carfilzomib and pomalidomide in the SIRIUS study vs the GEN501 study are indicative of the broader availability of these agents. The fewer median lines of prior therapy in the GEN501 study compared with the SIRIUS study (4 vs 5) reflect the inclusion criteria for these studies.
Baseline characteristics and refractory status among patients receiving daratumumab 16 mg/kg
| Characteristic . | GEN501 (n = 42) . | SIRIUS (n = 106) . | Combined studies (N = 148) . | |||
|---|---|---|---|---|---|---|
| No. . | % . | No. . | % . | No. . | % . | |
| Age, y | ||||||
| Median (range) | 64.0 (44-76) | 63.5 (31-84) | 64.0 (31-84) | |||
| 65 to <75 | 16 | 38 | 36 | 34 | 52 | 35 |
| ≥75 | 4 | 10 | 12 | 11 | 16 | 11 |
| Sex | ||||||
| Female | 15 | 36 | 54 | 51 | 69 | 47 |
| Male | 27 | 64 | 52 | 49 | 79 | 53 |
| ECOG performance score | ||||||
| 0 | 12 | 29 | 29 | 27 | 41 | 28 |
| 1 | 28 | 67 | 69 | 65 | 97 | 66 |
| 2 | 2 | 5 | 8 | 8 | 10 | 7 |
| No. of extramedullary plasmacytomas | ||||||
| 0 | 38 | 90 | 92 | 87 | 130 | 88 |
| ≥1 | 4 | 10 | 14 | 13 | 18 | 12 |
| Cytogenetic profile* | ||||||
| t(4;14) | 9 | 10 | ||||
| del17p | 16 | 17 | ||||
| del13q | 30 | 32 | ||||
| amp1q21 | 23 | 24 | ||||
| Other | 43 | 45 | ||||
| Median time since diagnosis, y (range) | 5.8 (0.8-23.7) | 4.8 (1.1-23.8) | 5.1 (0.8-23.8) | |||
| Renal function at baseline (creatinine clearance), mL/min | ||||||
| ≥60 | 29 | 69 | 60 | 57 | 89 | 60 |
| ≥30 to <60 | 12 | 29 | 42 | 40 | 54 | 37 |
| <30 | 1 | 2 | 4 | 4 | 5 | 3 |
| Bone marrow percent plasma cells at baseline, N | 42 | 104 | 146 | |||
| ≤30 | 37 | 88 | 48 | 46 | 85 | 58 |
| >30 to ≤60 | 5 | 12 | 21 | 20 | 26 | 18 |
| >60 | 0 | 0 | 35 | 34 | 35 | 24 |
| No. of prior lines of therapy | ||||||
| Median (range) | 4 (2-12) | 5 (2-14) | 5 (2-14) | |||
| >3 | 26 | 62 | 87 | 82 | 113 | 76 |
| Prior ASCT | 31 | 74 | 85 | 80 | 116 | 78 |
| Prior PI | 42 | 100 | 106 | 100 | 148 | 100 |
| Bortezomib | 42 | 100 | 105 | 99 | 147 | 99 |
| Carfilzomib | 8 | 19 | 53 | 50 | 61 | 41 |
| Prior IMiD | 40 | 95 | 106 | 100 | 146 | 99 |
| Lenalidomide | 40 | 95 | 105 | 99 | 145 | 98 |
| Pomalidomide | 15 | 36 | 67 | 63 | 82 | 55 |
| Thalidomide | 19 | 45 | 47 | 44 | 66 | 45 |
| Refractory to: | ||||||
| Last line of therapy | 32 | 76 | 103 | 97 | 135 | 91 |
| Both a PI and an IMiD | 27 | 64 | 101 | 95 | 128 | 87 |
| PI only | 3 | 7 | 3 | 3 | 6 | 4 |
| IMiD only | 4 | 10 | 1 | 1 | 5 | 3 |
| PI + IMiD + alkylating agent | 21 | 50 | 79 | 75 | 100 | 68 |
| Bortezomib | 30 | 71 | 95 | 90 | 125 | 85 |
| Carfilzomib | 7 | 17 | 51 | 48 | 58 | 39 |
| Lenalidomide | 31 | 74 | 93 | 88 | 124 | 84 |
| Pomalidomide | 15 | 36 | 67 | 63 | 82 | 55 |
| Thalidomide | 12 | 29 | 29 | 27 | 41 | 28 |
| Alkylating agent only | 25 | 60 | 82 | 77 | 107 | 72 |
| Characteristic . | GEN501 (n = 42) . | SIRIUS (n = 106) . | Combined studies (N = 148) . | |||
|---|---|---|---|---|---|---|
| No. . | % . | No. . | % . | No. . | % . | |
| Age, y | ||||||
| Median (range) | 64.0 (44-76) | 63.5 (31-84) | 64.0 (31-84) | |||
| 65 to <75 | 16 | 38 | 36 | 34 | 52 | 35 |
| ≥75 | 4 | 10 | 12 | 11 | 16 | 11 |
| Sex | ||||||
| Female | 15 | 36 | 54 | 51 | 69 | 47 |
| Male | 27 | 64 | 52 | 49 | 79 | 53 |
| ECOG performance score | ||||||
| 0 | 12 | 29 | 29 | 27 | 41 | 28 |
| 1 | 28 | 67 | 69 | 65 | 97 | 66 |
| 2 | 2 | 5 | 8 | 8 | 10 | 7 |
| No. of extramedullary plasmacytomas | ||||||
| 0 | 38 | 90 | 92 | 87 | 130 | 88 |
| ≥1 | 4 | 10 | 14 | 13 | 18 | 12 |
| Cytogenetic profile* | ||||||
| t(4;14) | 9 | 10 | ||||
| del17p | 16 | 17 | ||||
| del13q | 30 | 32 | ||||
| amp1q21 | 23 | 24 | ||||
| Other | 43 | 45 | ||||
| Median time since diagnosis, y (range) | 5.8 (0.8-23.7) | 4.8 (1.1-23.8) | 5.1 (0.8-23.8) | |||
| Renal function at baseline (creatinine clearance), mL/min | ||||||
| ≥60 | 29 | 69 | 60 | 57 | 89 | 60 |
| ≥30 to <60 | 12 | 29 | 42 | 40 | 54 | 37 |
| <30 | 1 | 2 | 4 | 4 | 5 | 3 |
| Bone marrow percent plasma cells at baseline, N | 42 | 104 | 146 | |||
| ≤30 | 37 | 88 | 48 | 46 | 85 | 58 |
| >30 to ≤60 | 5 | 12 | 21 | 20 | 26 | 18 |
| >60 | 0 | 0 | 35 | 34 | 35 | 24 |
| No. of prior lines of therapy | ||||||
| Median (range) | 4 (2-12) | 5 (2-14) | 5 (2-14) | |||
| >3 | 26 | 62 | 87 | 82 | 113 | 76 |
| Prior ASCT | 31 | 74 | 85 | 80 | 116 | 78 |
| Prior PI | 42 | 100 | 106 | 100 | 148 | 100 |
| Bortezomib | 42 | 100 | 105 | 99 | 147 | 99 |
| Carfilzomib | 8 | 19 | 53 | 50 | 61 | 41 |
| Prior IMiD | 40 | 95 | 106 | 100 | 146 | 99 |
| Lenalidomide | 40 | 95 | 105 | 99 | 145 | 98 |
| Pomalidomide | 15 | 36 | 67 | 63 | 82 | 55 |
| Thalidomide | 19 | 45 | 47 | 44 | 66 | 45 |
| Refractory to: | ||||||
| Last line of therapy | 32 | 76 | 103 | 97 | 135 | 91 |
| Both a PI and an IMiD | 27 | 64 | 101 | 95 | 128 | 87 |
| PI only | 3 | 7 | 3 | 3 | 6 | 4 |
| IMiD only | 4 | 10 | 1 | 1 | 5 | 3 |
| PI + IMiD + alkylating agent | 21 | 50 | 79 | 75 | 100 | 68 |
| Bortezomib | 30 | 71 | 95 | 90 | 125 | 85 |
| Carfilzomib | 7 | 17 | 51 | 48 | 58 | 39 |
| Lenalidomide | 31 | 74 | 93 | 88 | 124 | 84 |
| Pomalidomide | 15 | 36 | 67 | 63 | 82 | 55 |
| Thalidomide | 12 | 29 | 29 | 27 | 41 | 28 |
| Alkylating agent only | 25 | 60 | 82 | 77 | 107 | 72 |
ASCT, autologous stem cell transplantation; ECOG, Eastern Cooperative Oncology Group.
Cytogenetic data were not assessed in GEN501.
The median treatment duration was 3.4 months (range, 0.03-26.0 months), and the median number of infusions was 12 (range, 1-40 infusions). The majority of patients discontinued from the study as a result of PD (83.1%); only 6 patients (4.1%) in the combined data set discontinued from the study as a result of AEs (supplemental Table 1). Three deaths were recorded as being a result of AEs. These deaths consisted of 1 case each of viral H1N1 infection, pneumonia, and aspiration pneumonia, and they were determined to be not related to study treatment.
Efficacy
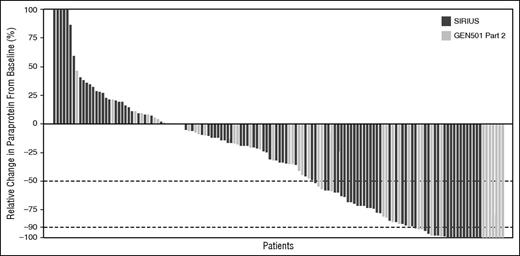
In the combined data set, 59 (39.9%) and 28 (18.9%) of 148 patients treated with daratumumab 16 mg/kg demonstrated ≥50% and ≥90% reductions in paraprotein from baseline, respectively (Figure 1). The median time to response in patients with PR or better was 0.95 months (range, 0.5-5.6 months). The ORR for the combined data set was 31.1% (95% confidence interval [CI], 23.7%-39.2%). Responses included 3 patients with sCR (2.0%; 95% CI, 0.4%-5.8%), 4 with CR (2.7%; 95% CI, 0.7%-6.8%), 13 with VGPR (8.8%; 95% CI, 4.8%-14.6%), and 26 with PR (17.6%; 95% CI, 11.8%-24.7%) (Table 2). The clinical benefit rate (ORR + MR) was 37.2% (95% CI, 29.4%-45.5%), and 83.1% of patients achieved SD or better. The median duration of response was 7.6 months (95% CI, 5.6 months to NE). Responses deepened with continued daratumumab treatment in 14 patients across the 2 studies. Of 10 patients with an initial PR, 7 went to on to achieve VGPR with further treatment. In addition, 3 patients with an initial PR achieved deeper responses of CR (1 patient) and sCR (2 patients). Responses in 4 patients with an initial VGPR continued to deepen to CR (3 patients) and sCR (1 patient). The timing of the first and best responses are shown in supplemental Figure 2. Within the individual studies, the ORR was 35.7% (95% CI, 21.6%-52.0%) in the GEN501 study part 2 and 29.2% (95% CI, 20.8%-38.9%) in the SIRIUS study.
Maximum change in paraprotein from baseline. In addition to reduction in paraprotein, International Myeloma Working Group criteria require (1) results from 2 consecutive tests demonstrating the necessary percent reduction in paraprotein, (2) reduction in paraprotein in both serum and urine, if measurable disease was determined by both serum and urine paraprotein, and (3) a ≥50% reduction in the size of soft tissue plasmacytomas, if these were present at baseline. Thus, ≥50% and ≥90% reduction in paraprotein do not correlate directly with PR and VGPR, respectively.
Maximum change in paraprotein from baseline. In addition to reduction in paraprotein, International Myeloma Working Group criteria require (1) results from 2 consecutive tests demonstrating the necessary percent reduction in paraprotein, (2) reduction in paraprotein in both serum and urine, if measurable disease was determined by both serum and urine paraprotein, and (3) a ≥50% reduction in the size of soft tissue plasmacytomas, if these were present at baseline. Thus, ≥50% and ≥90% reduction in paraprotein do not correlate directly with PR and VGPR, respectively.
Summary of responses in the combined data set of patients receiving daratumumab 16 mg/kg
| Response . | Patients receiving daratumumab 16 mg/kg (N = 148) . | ||
|---|---|---|---|
| No. . | % . | 95% CI . | |
| ORR | 46 | 31.1 | 23.7-39.2 |
| Clinical benefit (ORR + MR) | 55 | 37.2 | 29.4-45.5 |
| VGPR or better | 20 | 13.5 | 8.5-20.1 |
| CR or better | 7 | 4.7 | 1.9-9.5 |
| sCR | 3 | 2.0 | 0.4-5.8 |
| CR | 4 | 2.7 | 0.7-6.8 |
| VGPR | 13 | 8.8 | 4.8-14.6 |
| PR | 26 | 17.6 | 11.8-24.7 |
| MR | 9 | 6.1 | 2.8-11.2 |
| SD | 68 | 45.9 | 37.7-54.3 |
| PD | 18 | 12.2 | 7.4-18.5 |
| NE | 7 | 4.7 | 1.9-9.5 |
| Response . | Patients receiving daratumumab 16 mg/kg (N = 148) . | ||
|---|---|---|---|
| No. . | % . | 95% CI . | |
| ORR | 46 | 31.1 | 23.7-39.2 |
| Clinical benefit (ORR + MR) | 55 | 37.2 | 29.4-45.5 |
| VGPR or better | 20 | 13.5 | 8.5-20.1 |
| CR or better | 7 | 4.7 | 1.9-9.5 |
| sCR | 3 | 2.0 | 0.4-5.8 |
| CR | 4 | 2.7 | 0.7-6.8 |
| VGPR | 13 | 8.8 | 4.8-14.6 |
| PR | 26 | 17.6 | 11.8-24.7 |
| MR | 9 | 6.1 | 2.8-11.2 |
| SD | 68 | 45.9 | 37.7-54.3 |
| PD | 18 | 12.2 | 7.4-18.5 |
| NE | 7 | 4.7 | 1.9-9.5 |
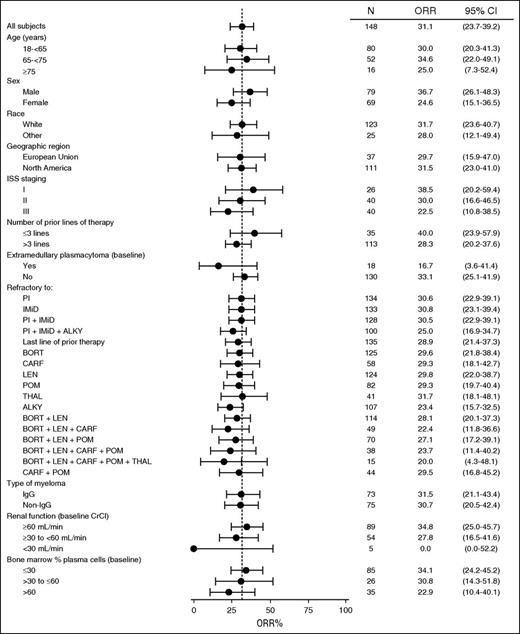
Subgroup analyses revealed that responses were observed across all subgroups regardless of the number or type of prior lines of therapy, refractory status, renal function, or baseline percentage of plasma cells in the bone marrow (Figure 2). The ORRs in patients with heavy chain (31.8% [107 patients]) and light chain (29.3% [41 patients]) disease were consistent with those in the total population. Cytogenetic data were not collected for GEN501 part 2. ORRs for high-, standard-, and low-risk patients in SIRIUS were reported previously.12 Importantly, the ORR in patients with impaired renal function (ie, creatinine clearance of >30 to ≤60 mL/min) was consistent with that observed in the overall population.
ORR in patient subgroups in the combined daratumumab 16 mg/kg group. Subgroup analysis of the overall best response in the 148 patients treated with daratumumab at a dose of 16 mg /kg. The dashed vertical line indicates 31.1%, which was the ORR in the total patient cohort. Exact 95% CIs are provided. International Staging System data are not available in GEN501 part 2. ALKY, alkylating agents, including autologous stem cell transplant; BORT, bortezomib; CARF, carfilzomib; CrCl, creatinine clearance; LEN, lenalidomide; POM, pomalidomide; THAL, thalidomide.
ORR in patient subgroups in the combined daratumumab 16 mg/kg group. Subgroup analysis of the overall best response in the 148 patients treated with daratumumab at a dose of 16 mg /kg. The dashed vertical line indicates 31.1%, which was the ORR in the total patient cohort. Exact 95% CIs are provided. International Staging System data are not available in GEN501 part 2. ALKY, alkylating agents, including autologous stem cell transplant; BORT, bortezomib; CARF, carfilzomib; CrCl, creatinine clearance; LEN, lenalidomide; POM, pomalidomide; THAL, thalidomide.
After a median follow-up of 20.7 months (range, 0.5-27.1 months), the median PFS for the combined data set in the overall population was 4.0 months (95% CI, 2.8-5.6 months; Figure 3A). The overall, 12-month PFS rate was 21.6% (95% CI, 14.4%-29.8%). Median PFS within the GEN501 study part 2 was 6.2 months (95% CI, 4.2-11.6 months) and, within SIRIUS, it was 3.7 months (95% CI, 2.8-4.6 months; supplemental Figure 3). As expected in the combined data set, median PFS was longer for patients who achieved a PR or better compared with those who achieved MR/SD or PD/NE (15.0 months [95% CI, 7.4 months to NE] vs 3.0 months [95% CI, 2.8-3.7 months] vs 0.9 months [95% CI, 0.9-1.0 months]; Figure 3B). The 6-month PFS rates were 77.9% (95% CI, 62.7%-87.4%), 26.1% (95% CI, 15.5%-37.9%), and 0.0% (95% CI, NE to NE) in patients with PR or better, MR/SD, and PD/NE, respectively.
PFS in the combined daratumumab 16 mg/kg group. At a median follow-up of 20.7 months, the median PFS of patients in (A) the combined data set and (B) stratified by response category are shown.
PFS in the combined daratumumab 16 mg/kg group. At a median follow-up of 20.7 months, the median PFS of patients in (A) the combined data set and (B) stratified by response category are shown.
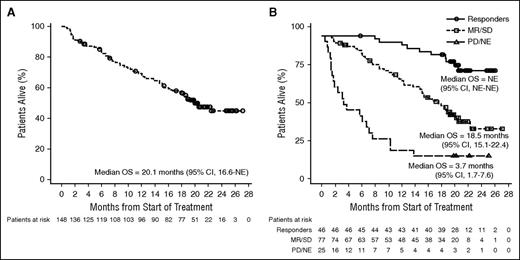
The median OS of the combined study population was 20.1 months (95% CI, 16.6 months to NE) (Figure 4A). In the GEN501 study part 2, the median OS was NE (95% CI, 18.7 months to NE), and in SIRIUS, it was 18.6 months (95% CI, 13.7 months to NE; supplemental Figure 4). For the combined analysis, the 18-month and 24-month OS rates were 56.5% (95% CI, 47.9%-64.2%) and 45.0% (95% CI, 35.5%-54.1%), respectively. Clear differences in survival outcomes were observed when patients were stratified by response type. The median OS for patients who achieved a PR or better was NE (95% CI, NE to NE; Figure 4B). At a median follow-up of 20.7 months, 36 of 46 patients who responded to daratumumab were alive. Eighteen-month and 24-month OS rates were 86.7% (95% CI, 72.7%-93.8%) and 75.6% (95% CI, 59.9%-86.2%), respectively, in these patients. Remarkably, a clear OS benefit was extended to those patients who achieved MR/SD who had a median OS of 18.5 months (95% CI, 15.1-22.4 months) and 18- and 24-month OS rates of 51.1% (95% CI, 38.9%-62.0%) and 34.9% (95% CI, 21.3%-48.8%), respectively (Figure 4B). Median OS in patients with PD or NE was 3.7 months (95% CI, 1.7-7.6 months; Figure 4B), and the 18- and 24-month OS rates were 16.0% (95% CI, 5.0%-32.5%) for both time periods.
OS in the combined daratumumab 16 mg/kg group. The median OS of patients in (A) the combined data set and (B) stratified by response category are shown.
OS in the combined daratumumab 16 mg/kg group. The median OS of patients in (A) the combined data set and (B) stratified by response category are shown.
During these studies, treatment with daratumumab ended with disease relapse or progression. Of the 107 patients for whom data on subsequent therapies were available, the most common therapies included dexamethasone (58%), pomalidomide (34%), cyclophosphamide (32%), carfilzomib (28%), bortezomib (24%), and lenalidomide (16%). Forty-two patients (39.2%) responded to their first subsequent therapy with a PR or better, 40 (37.4%) had MR/SD, 16 (15.0%) had PD/NE, and the response to first subsequent therapy was unknown in 9 patients (8.4%) (supplemental Table 2). In the pooled population, 125 patients were refractory to bortezomib before being treated with daratumumab. Refractoriness was defined as evidence of PD per International Myeloma Working Group criteria during or within 60 days (measured from the end date of the last MM therapy) after completing treatment with the last antimyeloma drug regimen used just before study entry. Of 31 patients who were re-treated with bortezomib-based regimens, 9 (29%) responded to re-treatment on the basis of investigator assessment.
Safety
The incidences of overall treatment-emergent AEs (TEAEs) and grade ≥3 TEAEs for the combined data set are summarized in Table 3. The most common (≥20%) TEAEs included fatigue, nausea, anemia, back pain, cough, upper respiratory tract infection, thrombocytopenia, and neutropenia. IRRs were observed in 48% of patients, and those that occurred in ≥5% of patients consisted mainly of respiratory conditions such as nasal congestion, cough, allergic rhinitis, throat irritation, and dyspnea (supplemental Table 3). Nonrespiratory IRRs that occurred in ≥5% of patients were chills and nausea. Only 4 patients (2.7%) had grade ≥3 IRRs (bronchospasm [n = 2]; dyspnea, hypoxia, and hypertension [n = 1 each]). When IRRs occurred, most (95.8%) were observed during the first infusion, and the incidence of IRRs decreased during the second (7.0%) and subsequent (7.0%) infusions (some patients experienced >1 IRR). IRRs were safely managed with pre- and postinfusion medications, which consisted of antihistamines, corticosteroids, and paracetamol/acetaminophen. Supportive care treatment with granulocyte colony-stimulating factor was required by 12 patients (8.1%). There were 199 transfusions in 46 patients (31.1%) during the study. Of those transfusions, red blood cell (concentrated or packed cells and erythrocytes) and platelet transfusions were received by 44 patients (29.7%) and 14 patients (9.5%) in the combined data set, respectively. It has previously been reported that daratumumab binds to CD38 expressed on the surface of red blood cells.14,15 Although this additional activity may interfere with blood typing and cross-matching, no AEs related to hemolysis were reported.
Incidence and severity of most common (≥20%) TEAEs (N = 148)
| TEAE . | All grades . | Grade 3 . | Grade 4 . | |||
|---|---|---|---|---|---|---|
| No. . | % . | No. . | % . | No. . | % . | |
| Fatigue | 62 | 41.9 | 3 | 2.0 | 0 | |
| Nausea | 44 | 29.7 | 0 | 0 | ||
| Anemia | 42 | 28.4 | 26 | 17.6 | 0 | |
| Back pain | 40 | 27.0 | 4 | 2.7 | 0 | |
| Cough | 38 | 25.7 | 0 | 0 | ||
| Thrombocytopenia | 32 | 21.6 | 13 | 8.8 | 8 | 5.4 |
| Upper respiratory tract infection | 32 | 21.6 | 1 | 0.7 | 0 | |
| Neutropenia | 31 | 20.9 | 11 | 7.4 | 4 | 2.7 |
| TEAE . | All grades . | Grade 3 . | Grade 4 . | |||
|---|---|---|---|---|---|---|
| No. . | % . | No. . | % . | No. . | % . | |
| Fatigue | 62 | 41.9 | 3 | 2.0 | 0 | |
| Nausea | 44 | 29.7 | 0 | 0 | ||
| Anemia | 42 | 28.4 | 26 | 17.6 | 0 | |
| Back pain | 40 | 27.0 | 4 | 2.7 | 0 | |
| Cough | 38 | 25.7 | 0 | 0 | ||
| Thrombocytopenia | 32 | 21.6 | 13 | 8.8 | 8 | 5.4 |
| Upper respiratory tract infection | 32 | 21.6 | 1 | 0.7 | 0 | |
| Neutropenia | 31 | 20.9 | 11 | 7.4 | 4 | 2.7 |
All patients had their blood types assessed before their first daratumumab infusion. They were also encouraged to carry a card indicating their blood type for the duration of the study. In the event of a positive Coombs test, individual blood banks used a variety of mitigation methods to safely provide blood products. Blood banks were also made aware that this effect could persist for up to 6 months after cessation of daratumumab treatment.
Discussion
In the combined data set, consistent with the findings of the individual studies,11,12 daratumumab monotherapy at a dose of 16 mg/kg demonstrated notable activity in heavily pretreated patients with highly refractory MM. Responses achieved by daratumumab-treated patients were rapid (median time to response, 1 month), deep (14% with VGPR or better), and durable (median duration of response, 7.6 months). The median OS was 20.1 months but, importantly, the clinical benefit of daratumumab was not restricted to those with a response of PR or better, because patients who achieved MR/SD experienced an OS benefit of more than 12 months compared with the PD/NE subgroup.
Daratumumab is administered as monotherapy, although peri-infusion methylprednisolone is used to mitigate the effects of IRRs. Because of the reduced frequency of dosing later in the daratumumab dosing schedule, these subtherapeutic corticosteroid doses are reduced further over time.12 Thus, when daratumumab is considered as monotherapy, its activity is noteworthy when placed in the context of other recently approved agents for refractory MM. Compared with the ORR of 31% observed in the highly refractory MM population in this study, lower ORRs were observed with single-agent pomalidomide (18%),16,17 carfilzomib (24%),18 and lenalidomide (26%)19 in less heavily pretreated patient populations. Two other recently approved drugs for refractory MM, panobinostat and elotuzumab, were previously shown to have little and no single-agent activity in patients with RRMM, respectively.20,21
Although many of the patients in the combined data set were refractory to newer agents such as carfilzomib and pomalidomide, subgroup analyses revealed that responses were maintained in these patients and that meaningful responses were observed in patients with triple-, quadruple-, and even quintuple-refractory MM. The observation that prolonged responses were attained and remarkably were deepened further in some patients treated with daratumumab monotherapy suggests that targeting CD38 may address an unmet clinical need in situations in which other available treatment options have been exhausted.3
Other recent late-/last-line myeloma studies in refractory disease have reported median OS values ranging from 10.2 to 15.4 months compared with 20.1 months in this study.17,18,22,23 Thus, although these studies of daratumumab monotherapy enrolled a generally more refractory population, the median OS appeared to be longer. However, the median PFS seen in this combined data set (4.0 months) was comparable to the median PFS of studies of the other agents (ranging from 3.7 to 4.6 months).17,18,22,23 The underlying mechanisms that drove the extended OS in patients treated with daratumumab remain to be elucidated, but it can be hypothesized that a number of factors may be promoting this outcome. First, patients achieved deep responses with daratumumab treatment, including CRs and sCRs, which arose over time after initial PRs and VGPRs. Second, a survival benefit was observed, even in those patients who achieved only MR or SD; these patients continued to receive daratumumab because disease progression was the only disease-related criterion for discontinuation of study treatment. Prolonged exposure to daratumumab, even in the absence of deep clinical responses, may have provided the opportunity for other aspects of the drug’s mechanisms of action to exert their effects. In this group of patients, the role of daratumumab in immune-mediated4-6 as well as in immunomodulatory mechanisms may, in part, contribute to the survival benefit of these patients.10 It has recently been proposed that additional immunomodulatory effects mediated by T-cell activation and expansion, which are driven by elimination of CD38-expressing regulatory T and B cells and myeloid-derived suppressor cells, may promote deeper and prolonged responses,10 which is in line with observations of immune-checkpoint inhibitors that were recently approved for treatment of other advanced cancers.24-26
Mechanisms of action of daratumumab may also enable patients to mount new responses to drugs to which they were previously exposed. The preliminary observation that clinical responses were observed in a subset of patients re-treated with bortezomib-containing regimens in this study population (which was highly refractory to bortezomib) is encouraging. This hypothesis that daratumumab may enhance a patient’s response to subsequent treatments, including those to which they were previously exposed, is under active investigation. Another possibility is that, because of the tolerability of daratumumab, patients may be afforded an opportunity to recover from the stress of prior therapies, which allows them to receive subsequent therapies at higher doses or more aggressive dosing schedules. Further studies will be required to conclusively determine the reasons for the extended difference between observed median PFS and median OS with daratumumab treatment compared with other monotherapies.
No new safety signals were identified for daratumumab monotherapy in this updated analysis of the combined data set of the GEN501 part 2 and SIRIUS studies. Consistent with the findings from the individual studies,11,12 most IRRs had a low grade (grade ≤2), occurred during the first infusion, and were manageable; no IRRs led to treatment discontinuations. Patients safely received blood transfusions, and few required support with granulocyte colony-stimulating factor or other growth factors. The favorable safety profile and activity of daratumumab monotherapy suggests that it is a promising partner for potential combination therapy regimens, and ongoing phase 3 studies include daratumumab in combination with lenalidomide or bortezomib-based regimens in RRMM (NCT02076009 and NCT02136134) and in newly diagnosed MM (NCT02252172, NCT02195479, and NCT02541383). An additional phase 1 study is also investigating the subcutaneous delivery of daratumumab in combination with recombinant human hyaluronidase in patients with RRMM (NCT02519452).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Swarna Ramanjulu and Brian Berkey for additional data analyses.
This study was sponsored by Janssen Research & Development, LLC. Medical writing and editorial support were provided by Erica Chevalier-Larsen of MedErgy and were funded by Janssen Global Services, LLC.
Authorship
Contribution: S.Z.U., B.M.W., T.P., N.J.B., A.B., S.L., H.M.L., P.M.V., P.G.R., A.C., and H.N. collected the data; A.K.S., A.A., H.F., C.M.U., J.W., I.K., and T.A. compiled the data for summation and analysis and confirmed the accuracy of the data; S.Z.U. and H.N. wrote the first draft of the manuscript; and all authors had full access to the data, contributed to the study design and statistical analysis plan, reviewed and revised the manuscript, approved the final version, and agreed to submit the manuscript for publication.
Conflict-of-interest disclosure: S.Z.U. consulted for Celgene, Millennium Takeda, Onyx, and Sanofi; received speaker’s fees from Celgene, Millennium Takeda, and Onyx; and received research funding from Array BioPharma, Celgene, Janssen Oncology, Onyx, Pharmacyclics, and Sanofi. B.M.W. consulted for Janssen Research & Development and Millennium Pharmaceuticals and received research funding from Janssen Research & Development and Prothena. N.J.B. received honoraria from Amgen, Celgene, and Johnson & Johnson; speaker’s fees from Celgene and Johnson & Johnson; and research funding from Celgene and Johnson & Johnson. S.L. consulted for Bristol-Myers Squibb, Celgene, Janssen Research & Development, Millennium Pharmaceuticals, Novartis, and Onyx and received research funding from Janssen Research & Development. H.M.L. served on advisory boards for Amgen, Genmab, and Janssen Research & Development and received research funding from Genmab and Janssen Research & Development. P.M.V. received honoraria from Millennium Takeda and Novartis; consulted for Array BioPharma, Bristol-Myers Squibb, Celgene, GlaxoSmithKline, and Oncopeptides; and received research funding from Celgene, GlaxoSmithKline, and Oncopeptides. P.G.R. served on an advisory committee for Genmab. A.C. consulted for Array BioPharma, Celgene, Millennium Takeda, Novartis, and Onyx and received research funding from Array BioPharma, Celgene, Janssen Pharmaceuticals, Millennium Takeda, Novartis, Onyx, and Pharmacyclics. T.P. consulted for Genmab, Janssen Research & Development, Takeda, and Celgene and received research funding from Janssen Research & Development. A.K.S., A.A., H.F., C.M.U., J.W., I.K., and T.A. are employees of Janssen Research & Development, LLC. The remaining authors declare no competing financial interests.
Correspondence: Saad Z. Usmani, Levine Cancer Institute, 1021 Morehead Medical Dr, Charlotte, NC 28204; e-mail: saad.usmani@carolinashealthcare.org.