To the editor:
Elevated levels of fetal hemoglobin (HbF; α2γ2) lessen the severity of symptoms and increase the life span of patients with sickle cell disease (SCD).1,2 Hydroxyurea, the only approved drug for the treatment of SCD, is ineffective in a large proportion of patients, and therefore a genuine need for new and more effective treatments exists.
Simian primates are widely acknowledged as the best animal model to test the ability of new drugs to increase γ-globin expression because results in the baboon are predictive of effects in humans due to conservation of the structure and developmental stage-specific regulation of the β-like globin genes in simian primates.3-5 The usefulness of the baboon model was demonstrated by experiments showing that the DNA methyltransferase (DNMT) inhibitor 5-azacytidine increased HbF to high levels in baboons rendered anemic by phlebotomy,6 and these studies were rapidly translated in two clinical studies in patients with SCD and β-thalassemia.7,8 Additional trials showed that decitabine, the deoxy analog, increased HbF in patients with SCD.9-11 An orally administered combination of tetrahydrouridine and decitabine, developed in baboons,12 is currently in clinical trials.13
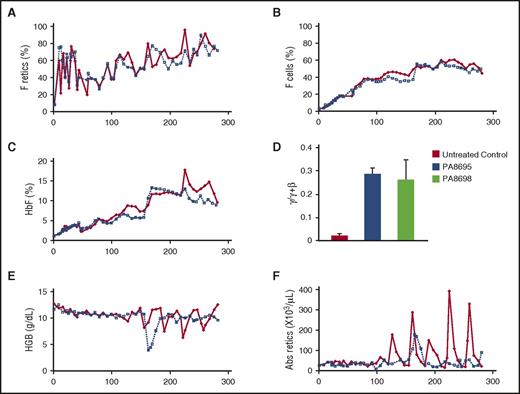
DNMT1 and the lysine-specific demethylase 1 (LSD1) are components of multiprotein corepressor complexes that repress γ-globin gene expression in adult erythroid cells.14,15 Experiments in β-YAC transgenic mice have shown that LSD1 is also an effective target for HbF-inducing therapies,16 and treatment of SCD mice with the LSD1 inhibitor RN-1 increased γ-globin mRNA, F cells, and F retics, although levels achieved were low because the human γ-globin gene is not efficiently reactivated in this mouse model.17,18 In phlebotomized baboons, RN-1 stimulated high levels of γ-globin synthesis and increased HbF.19 Doses of RN-1 that produced high levels of HbF in anemic baboons were invariably associated with neutropenia, but when normal, nonanemic baboons were treated, adverse hematological effects were minimized while increases in γ-globin synthesis, HbF, and F cells were still observed. To evaluate the safety and effectiveness of RN-1 over a prolonged period, we treated two juvenile (4- to 5-year- old) female baboons (PA8695, PA8698) with RN-1 (0.25 mg/kg per day; subcutaneous; 5 d/wk) for 264 and 278 days, respectively. All procedures were approved by the animal care committee of the University of Illinois at Chicago. Blood samples were drawn weekly for complete blood count (CBC) analysis and determination of HbF, F cells, and F retic levels. Both animals exhibited weight gain during the course of the study (PA8695, 14.4%; PA8698, 20%). Low bilirubin levels were the only abnormality observed in liver function and blood chemistry analysis performed on day 77 and day 207 (PA8695, day 77 = 0.19 mg/dL, day 207 = 0.12 mg/dL; PA8698, day 77 = 0.25 mg/dL [N], day 207 = 0.15 mg/dL). By the second week (day 9), an eight- to 10-fold increase in F retics was observed. Elevated levels of F retics were consistently maintained throughout the treatment phase at levels seven- to eightfold higher than those at pretreatment (PA8695 = 53.8% ± 16.5% [mean ± standard deviation (SD)]; median [M] = 56.4%; PA8698 = 55.8% ± 13.4% [mean ± SD]; M = 55.8%; Figure 1A). F cell levels increased until approximately day 170 in both animals, and following that, elevated F cell levels were maintained that were 18-25 times greater than those at pretreatment levels (PA8695 = 54.8% ± 2.6% [mean ± SD]; M = 54.1%; PA8698 = 52.7% ± 2.3% [mean ± SD]; M = 52.6%; Figure 1B). HbF levels also increased until approximately day 170 in both animals and were then maintained at levels 10-12 times greater than at pretreatment levels for the duration of the study (PA8695 = 12.5% ± 12.0%; M = 12.0%; PA8698 = 11.9% ± 1.3%; M = 12.3%; Figure 1C). Measurement of globin chain synthesis in peripheral blood reticulocytes on day 162, day 190, and day 267 showed that γ-globin chain synthesis was elevated (PA8695, 0.29% ± 0.03%, γ/γ + β; PA8698 0.26% ± 0.08%, γ/γ + β) in comparison with untreated controls (Figure 1D).
Long-term RN-1 treatment increases F retics, F cells, and HbF. (A) F retics, (B) F cells, (C) HbF, (D) globin chain synthesis in peripheral blood reticulocytes, (E) hemoglobin (HGB) levels, and (F) absolute (Abs) reticulocyte counts during days of treatment: PA8695 (solid red symbols, solid red line); PA8698 (open symbols, dotted line).
Long-term RN-1 treatment increases F retics, F cells, and HbF. (A) F retics, (B) F cells, (C) HbF, (D) globin chain synthesis in peripheral blood reticulocytes, (E) hemoglobin (HGB) levels, and (F) absolute (Abs) reticulocyte counts during days of treatment: PA8695 (solid red symbols, solid red line); PA8698 (open symbols, dotted line).
Total hemoglobin (Figure 1E), red blood cell number, and hematocrit levels exhibited small overall decreases during the course of treatment in comparison with pretreatment values in each animal but remained within the normal range. These small effects may have been due to perturbation of erythroid differentiation. Flow cytometry analysis of bone marrow aspirates showed a 2.5-fold increase in CD105+ CD117+gly+ proerythroblasts in RN-1-treated animals in comparison with normal untreated controls. RNA sequencing analysis of this subpopulation showed increased expression of GFI1B (Q value = 0.0006) and GATA-2 (Q value = 0.01), genes associated with expansion and inhibition of primitive erythroblasts in the RN-1-treated baboons.
PA8698 suffered acute blood loss due to a laceration of the perineal swelling during menstruation between day 155 and day 162. In PA8695, heavy bleeding associated with menstruation was observed beginning on day 155. Rapid recovery was observed in both animals following these episodes of blood loss, whereas increased reticulocyte levels during these recovery periods (Figure 1F) contributed to increased levels of F retics, F cells, and HbF. For example, at the time of the perineal laceration in PA8698, HbF levels rose from 5.7% (day 155) to 13.2% (day 166), whereas Hb levels decreased >60% and reticulocytes increased >threefold (Figure 2A). F retics were elevated prior to the bleeding episode and increased approximately 50% during recovery. Increases in mean corpuscular volume were observed in PA8695, which coincided with recovery from periods of increased menstrual bleeding (Figure 2B), whereas mean corpuscular hemoglobin concentration levels were maintained within the normal range.20
Effects of RN-1 treatment on additional hematologic parameters. (A) Changes in hemoglobin (solid red squares, dashed red line), fetal hemoglobin (blue circles, dotted blue line), absolute reticulocytes (purple triangles, solid purple line), and F retics (open green squares, solid green line) between days 148 and 190, coinciding with an incident of acute bleeding due to accidental laceration of the perineal swelling in PA8698. (B) Mean corpuscular volume (MCV), (C) neutrophils, (D) platelets (PLT), and (E) monocytes during days of treatment: PA8695 (solid red symbols, solid red line); PA8698 (open blue symbols, dashed blue line). (F) CD62 expression (mean fluorescence intensity [MFI]) on surface of platelets following addition of thrombin: untreated controls (red bar); PA8695 during treatment phase (blue bar); PA8698 during treatment phase (green bar); PA8695 1 week following cessation of RN-1 treatment (purple bar); PA8698 1 week following cessation of RN-1 treatment (orange bar). ANC, absolute neutrophil count.
Effects of RN-1 treatment on additional hematologic parameters. (A) Changes in hemoglobin (solid red squares, dashed red line), fetal hemoglobin (blue circles, dotted blue line), absolute reticulocytes (purple triangles, solid purple line), and F retics (open green squares, solid green line) between days 148 and 190, coinciding with an incident of acute bleeding due to accidental laceration of the perineal swelling in PA8698. (B) Mean corpuscular volume (MCV), (C) neutrophils, (D) platelets (PLT), and (E) monocytes during days of treatment: PA8695 (solid red symbols, solid red line); PA8698 (open blue symbols, dashed blue line). (F) CD62 expression (mean fluorescence intensity [MFI]) on surface of platelets following addition of thrombin: untreated controls (red bar); PA8695 during treatment phase (blue bar); PA8698 during treatment phase (green bar); PA8695 1 week following cessation of RN-1 treatment (purple bar); PA8698 1 week following cessation of RN-1 treatment (orange bar). ANC, absolute neutrophil count.
Absolute neutrophil counts (Figure 2C) overall showed no overall decline in comparison with pretreatment values (PA8695; pretreatment = 2110 per μL; posttreatment = 3237 ± 1460 per μL; M = 2855 per μL; PA8698 pretreatment = 2690 per μL; posttreatment = 3031 ± 1784 per μL; M = 2470 per μL), although short variations in levels were observed. In PA8695 the absolute neutrophil count declined below 1500 per μL on 1 occasion (1490), and in PA8698, 5 measurements below 1500 were observed (1250, 1410, 1420, 1330, 1000). Platelet levels decreased approximately 40% in each animal but were nevertheless maintained within the normal range (PA8695: pretreatment = 351 × 103 per μL, posttreatment = 219 ± 60 × 103 per μL, M = 235 × 103 per μL; PA8698: pretreatment = 224 × 103 per μL, posttreatment = 130 ± 82 × 103 per μL, M = 146 × 103 per μL; Figure 2D). Monocytes increased two- to threefold in each animal (PA8695; pretreatment = 100 per μL; posttreatment = 252 ± 96 per μL; M = 258 per μL; PA8698: pretreatment = 161 per μL; posttreatment = 423 ± 334 per μL; M = 336 per μL). A monocyte count of >400 per μL was observed in 4 of 39 CBC analysis for PA8695 and 12 of 40 CBC analyses for PA8698 (Figure 2E).
The heavy loss of blood following perineal laceration (PA8698) and menstrual bleeding (PA8695) prompted us to investigate effects of RN-1 on blood coagulation pathways. No significant differences in prothrombin time or activated partial thromboplastin time were observed between control and RN-1-treated baboons. In vitro platelet activation assays to assess platelet function by flow cytometric analysis of CD62 expression on the surface of platelets following addition of thrombin21 showed that the fraction of platelets expressing CD62 was reduced approximately 14% (P < .02), and the level of CD62 expression was reduced 46% (P < .0001) in RN-1-treated baboons in comparison with controls (Figure 2F). These effects were predicted by previous RNA interference knockdown studies in mice.22,23 A phase II study of the platelet inhibitor prasugrel in children with sickle cell disease was designed to identify drug doses that inhibit platelet function between 30% and 50%, a level thought to balance safety and efficacy,24 similar to the level of inhibition observed here, although the dual effects of RN-1 on platelet function and platelet counts could pose an additional risk for bleeding that will require further monitoring.
Our results show that administration of RN-1 to normal baboons over a prolonged period increases HbF, F cells, and F retics and is generally well tolerated, supporting further development of LSD1 inhibitors as therapeutic agents for SCD. Because LSD1 also has an important functional role in neural stem cell maintenance and proliferation, effects of LSD1 inhibitors on the brain and nervous system should be carefully evaluated.25
Authorship
Acknowledgment: This work was supported by National Institutes of Health, National Heart, Lung, and Blood Institute grant U01 HL117658.
Contribution: V.I. and K.V. performed research and collected, analyzed, and interpreted the data; A.R. and R.M. analyzed data and reviewed the manuscript; S.C. and J.D.E. reviewed the manuscript; J.D. analyzed data and reviewed the manuscript; D.L. designed and performed the research, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Donald Lavelle, Jesse Brown Veterans Affairs Medical Center, Room 6215, 820 S Damen Ave, Chicago, IL 60612; e-mail: dlavelle@uic.edu.
References
Author notes
V.I. and K.V. contributed equally to this work.

![Figure 2. Effects of RN-1 treatment on additional hematologic parameters. (A) Changes in hemoglobin (solid red squares, dashed red line), fetal hemoglobin (blue circles, dotted blue line), absolute reticulocytes (purple triangles, solid purple line), and F retics (open green squares, solid green line) between days 148 and 190, coinciding with an incident of acute bleeding due to accidental laceration of the perineal swelling in PA8698. (B) Mean corpuscular volume (MCV), (C) neutrophils, (D) platelets (PLT), and (E) monocytes during days of treatment: PA8695 (solid red symbols, solid red line); PA8698 (open blue symbols, dashed blue line). (F) CD62 expression (mean fluorescence intensity [MFI]) on surface of platelets following addition of thrombin: untreated controls (red bar); PA8695 during treatment phase (blue bar); PA8698 during treatment phase (green bar); PA8695 1 week following cessation of RN-1 treatment (purple bar); PA8698 1 week following cessation of RN-1 treatment (orange bar). ANC, absolute neutrophil count.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/129/2/10.1182_blood-2016-10-746727/4/m_blood746727f2.jpeg?Expires=1763674271&Signature=RdQpXK9EjoXTp440FY8eG3DarcD8GFkFatxOS8grX1cIdKyiYTpkCP0CeF36PXZkqSO4ZUcZc8t60mswPtGwLC9JtcXBIqrHmoMUNHOaaU-pgu4SpmKaL391fMIV8KZvrRSFc6ZaED29CC2RYo0puB72MJsU9ftsWHlS1PQ2l7Fu1mHrwrQlDSuTNnwXi4uFb-rik354YiNY3vxVeuMWrfhY8pxrSKK~Fiv~9LO2x1MOy3U~hVw~lf-nsRJPIxCEoN6VWM0JGP1~AUFrE1hvAJv1~OiL1scrhYllM4pbAYwCPNgEzdMPV1r9HAN~x9Yq1qJe3jWf~Rbgyzht2N4Z3Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)