To the editor:
Zika fever is an Aedes-borne disease caused by a flavivirus (Zika virus [ZIKV], genus Flavivirus). During the past years, ZIKV has spread in Polynesia, South America, and the Caribbean.1 Most ZIKV infections are asymptomatic or result in mild febrile disease with rash and conjunctivitis. Attention was recently drawn to nonvectored ZIKV transmission,2 including sexual,3,4 probable blood-borne,5 and mother-to-fetus transmission, and to severe forms such as Guillain-Barré syndrome,6,7 acute neurological infections,8,9 and fetal abnormalities10 (including microcephaly).
Many aspects of Zika fever natural history remain unknown (eg, the proportion of asymptomatic cases and the duration of viremia). Estimating the prevalence of Zika infections is difficult because a large proportion of infected individuals do not seek medical attention, and seroprevalence studies are hampered by antigenic cross-reactivity with other flaviviruses (eg, dengue virus).11 Here, we present a study of ZIKV infection in blood donors from Martinique island (French West Indies, Caribbean region), with novel epidemiological, biological, and clinical information that refines the picture of Zika fever in adults.
After the ZIKV outbreak in 2013 to 2014 in French Polynesia and the evidence that a significant proportion (3%) of donors were viremic,12 ZIKV has been recognized in Martinique since the end of 2015, leading to implementation by the French Blood Bank of a systematic individual nucleic acid testing (NAT) of blood donations. Virus detection was performed on plasma samples, using a semiautomated platform consisting of a Microlab-STARlet (Hamilton, Bonaduz, Switzerland) and the NucleoSpin 96 Virus Extraction Kit (Macherey-Nagel, Duren, Germany) for nucleic acids extraction, and a CFX96 thermocycler (Bio-Rad Laboratories, California) and the RealStar Zika Virus RT-PCR Kit_1.1 (Altona Diagnostics, Hamburg, Germany) for reverse transcriptase polymerase chain reaction testing.
Between January 19 and June 10, 2016, 4129 consecutive blood donations were tested (mean age, 41.9 years; sex ratio [M/F], 0.88). Positive NAT detection occurred in 76 blood donations (1.84%), with the most intense detection rate (3%) during weeks 17-20 (mean age, 41.8 years; sex ratio, 1.2). Postdonation inquiry consisted of a telephone call at day 7 postdonation to identify symptoms compatible with ZIKV infection. When the donor declared no sign, a new call was organized 14 days after donation. This information was obtained from 75 viremic donors: 34 (45.3%) remained asymptomatic, and 41 (54.7%) reported symptoms (1-6 days postdonation; Figure 1A) such as fever, conjunctivitis, myalgia, arthralgia, and rash (Figure 1B). There was a trend for higher values of molecular viral load in symptomatic vs asymptomatic donors (mean values, 5.36 vs 4.93 log10 RNA genomic equivalents per milliliter; independent Wilcoxon test, P value = .0013; Figure 1C). This difference does not imply that symptomatic donors reached higher viremia than asymptomatic donors: sampling could occur in the former only during the early steps of viremia, whereas in the latter, it could also occur during the decreasing phase of viremia. The range of viral loads (2.09-6.50 log10 RNA copies/mL) was comparable to that previously described in French Polynesian asymptomatic blood donors.13 Men declared symptoms less frequently than women (45.2% vs 66.7%; χ2 test P value = .06), but viral load and time to declaration of symptoms were not significantly different in men and women. No significant association of viremia with ABO, Rhesus, and Kell blood groups was detected.
Clinical symptoms and Zika viral loads in blood donors. The distribution of symptomatic donors according to time of inaugural symptoms (A) and the frequency of reported symptoms (B) are presented. Zika viral loads (log10 copies/mL) are provided in both asymptomatic and symptomatic blood donors (C). Zika viral load was determined by real-time reverse transcription polymerase chain reaction in 41 donors reporting clinical signs, and 34 donors who did not. The diagram reports for each series the quantitative distribution of viral load, together with the median value ± SD.
Clinical symptoms and Zika viral loads in blood donors. The distribution of symptomatic donors according to time of inaugural symptoms (A) and the frequency of reported symptoms (B) are presented. Zika viral loads (log10 copies/mL) are provided in both asymptomatic and symptomatic blood donors (C). Zika viral load was determined by real-time reverse transcription polymerase chain reaction in 41 donors reporting clinical signs, and 34 donors who did not. The diagram reports for each series the quantitative distribution of viral load, together with the median value ± SD.
We performed seroprevalence analyses at 2 time points: in 418 donors sampled in early March (March 9-23), and in 176 donors sampled in early June (June 6-13). Samples were tested using an anti-Zika virus NS1 immunoglobulin G enzyme-linked immunosorbent assay (ELISA) kit (anti-Zika Virus ELISA immunoglobulin G; Euroimmun, Germany), and positives were further processed by microneutralization in a 96-well format, using Vero cells, 50 pfu of the MRS_OPY_Martinique_PaRi_2015 strain,14 and a threshold titer of 40, as recommended by the French National Reference Centre for Arboviruses. The seroprevalence after seroneutralization was 13.5% in early March and 42.2% in early June. An ELISA ratio greater than 4 was associated with a positive seroneutralization in more than 95% of cases. Sera with an ELISA ratio greater than 5 were associated with seroneutralization in 100% of cases.
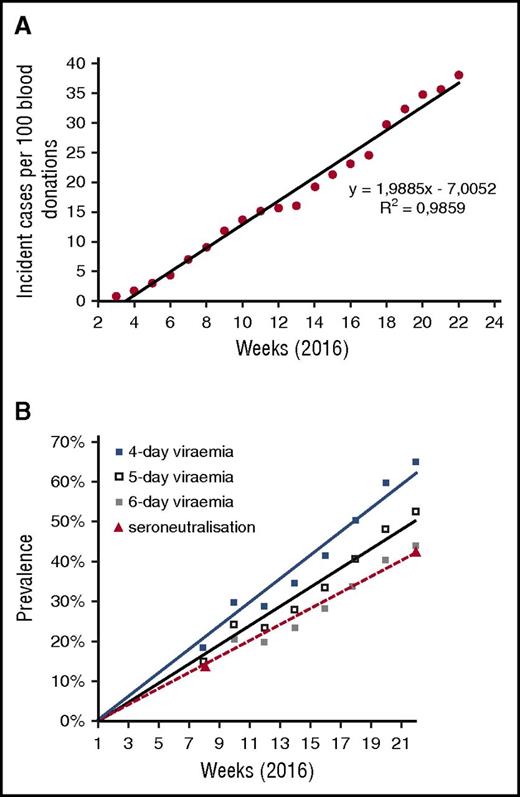
During the same period, the cumulative curve of incident cases detected by NAT in blood donors was almost linear (Figure 2A). This allowed performance of a simulation analysis and determination, on the basis of the incident cases detected among blood donors, of the number of donors infected by ZIKV at different points. Starting from the hypothesis of a random distribution of the first day of detectable viremia in infected donors during all days of the period studied, and the day of donation during the days open to collection during the same period, we estimated the number of cases for which donation would occur during the phase of asymptomatic viremia detectable by NAT. We deduced the expected prevalence of ZIKV infection in donors for theoretical durations of asymptomatic viremia in plasma of 4 to 6 days (Figure 2B), by reference to dengue15 and chikungunya fever.16 A faithful correlation with actual seroprevalence results was observed for a duration of asymptomatic viremia of 6 days (prevalence estimated at 12.5% vs 13.5% by seroneutralization in early March, and at 43.5% vs 42.2% in early June). This is consistent with the observation that the longest period reported between blood donation and symptoms (presymptomatic viremia) was 6 days (Figure 1A). Because detection methods using whole blood samples have allowed detecting virus RNA up to 68 days after the onset of symptoms,17 it will be important to determine whether such samples contain infectious virus, and to reassess ZIKV transfusion risk accordingly.
Epidemiological dynamics of Zika outbreak in Martinique.Figure 2A reports the cumulative incidence of Zika virus NAT detection in blood donors (normalized for 100 weekly blood donations). Figure 2B shows the simulated prevalence of Zika virus infections in blood donors at different points, based on incident cases and on different hypotheses for the duration of asymptomatic viremia (4-6 days). It also presents the actual seroprevalence data obtained by seroneutralization at weeks 8 and 22.
Epidemiological dynamics of Zika outbreak in Martinique.Figure 2A reports the cumulative incidence of Zika virus NAT detection in blood donors (normalized for 100 weekly blood donations). Figure 2B shows the simulated prevalence of Zika virus infections in blood donors at different points, based on incident cases and on different hypotheses for the duration of asymptomatic viremia (4-6 days). It also presents the actual seroprevalence data obtained by seroneutralization at weeks 8 and 22.
The estimated number of clinically suspect cases in Martinique (French Public Health Institute) was ∼7600, or ∼2% of the population, in early March (week 8), and ∼28 900, or ∼7.6% of the population, in early June (week 22).18 With reference to seroprevalence results, this suggests that the proportion of cases that did not seek medical attention was 80% to 85% and did not change significantly along the study period. Our analyses suggest, therefore, that ZIKV has spread in the general population silently, with a minor effect of the acute disease on medical structures (eg, 10-35 weekly consultations for Zika syndromes in emergency departments, and 10-20 in obstetric departments). The most significant medical effect consisted of 19 Zika-related GBS confirmed cases and Zika infection in 342 pregnant women at the end of our study (June 2016).19 Therefore, the situation is significantly different from that previously observed in Martinique during the 2014 Chikungunya outbreak, characterized by a high proportion of cases seeking medical attention.20
We conclude that ZIKV individual NAT screening in Martinique during 5 months of circulation of the virus allowed the detection of approximately 2% of contaminated blood donations. The proportion of truly asymptomatic cases of Zika disease among Martiniquan blood donors infected by ZIKV was approximately 45%, and the proportion of cases that did not require medical attention was even higher (80%-85%). The duration of plasma asymptomatic and presymptomatic viremia was estimated to be close to 6 days. We suggest that, once the duration of asymptomatic viremia has been estimated (eg,, from early analysis of seroprevalence and PCR detection datasets), NAT incidence studies in blood donors could improve prevalence estimates in the general population in the case of a disease with frequent mild or asymptomatic clinical presentations such as Zika fever. The French Blood Bank experience of individual ZIKV NAT screening and epidemiological studies performed from French blood donor population may be of interest at the time the US Food and Drug Administration is recommending that blood banks screen all blood donations for ZIKV or use an approved pathogen reduction technology.
Authorship
Acknowledgments: This work was supported by the Etablissement Français du Sang, INSERM, and Aix-Marseille University. P.G., A.C., R.N.C., B.P., I.L.-G., and X.d.L. are members of the ZIKAlliance project, funded by the European Commission.
Contribution: P.R. and L.P. collected samples and data from blood donors. A.C. collected and interpreted data from general population. P.G. and P.T. established the RT-PCR platform and performed NAT screening. B.P. and I.L-G. designed and performed serological detection. P.G., P.T., R.N.C., and X.d.L. designed the study, interpreted the data, and wrote and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Pierre Gallian, Etablissement Français du Sang Alpes-Méditerranée, 149 Bd Baille, 13005 Marseille, France; e-mail; pierre.gallian@efs.sante.fr.