Key Points
Leukemia-forming activity is enriched in endoglin-expressing AML and B-ALL blasts using a mouse xenograft model.
Inhibition of endoglin function with TRC105 reduces leukemia development and progression.
Abstract
Endoglin (CD105), a receptor of the transforming growth factor-β superfamily, has been reported to identify functional long-term repopulating hematopoietic stem cells, and has been detected in certain subtypes of acute leukemias. Whether this receptor plays a functional role in leukemogenesis remains unknown. We identified endoglin expression on the majority of blasts from patients with acute myeloid leukemia (AML) and acute B-lymphoblastic leukemia (B-ALL). Using a xenograft model, we find that CD105+ blasts are endowed with superior leukemogenic activity compared with the CD105− population. We test the effect of targeting this receptor using the monoclonal antibody TRC105, and find that in AML, TRC105 prevented the engraftment of primary AML blasts and inhibited leukemia progression following disease establishment, but in B-ALL, TRC105 alone was ineffective due to the shedding of soluble CD105. However, in both B-ALL and AML, TRC105 synergized with reduced intensity myeloablation to inhibit leukemogenesis, indicating that TRC105 may represent a novel therapeutic option for B-ALL and AML.
Introduction
Endoglin, also known as CD105, is an ancillary receptor of the transforming growth factor-β (TGF-β) superfamily, and is mostly known for its abundant expression in endothelial cells and critical function in vascular development1-3 and angiogenesis.4 Endoglin microvessel density is a negative prognostic factor in several solid cancers.5-7 This receptor is a therapeutic target and TRC105, an endoglin-neutralizing antibody, is currently in phase 2 and 3 trials as an antiangiogenic agent for the treatment of solid tumors.8-10
In addition to the endothelial lineage, endoglin also plays a key role in hematopoiesis. We have reported an important function for endoglin in cell fate specification and early hematopoiesis,11-15 and a potential role beyond the embryonic stage is suggested by the expression of this receptor on the hematopoietic stem cell (HSC) isolated from every hematopoietic site, including the aorta-gonad-mesonephros,16,17 the fetal liver,18 and the bone marrow (BM),19,20 in which endoglin has been reported to identify the long-term repopulating HSC.18,19,21,22 Transcriptional profiling data of proliferating and quiescent HSCs has demonstrated endoglin to be one of the genes selectively expressed in the quiescent HSC subset.23
Based on this evidence pointing to endoglin as a potential regulator of HSC self-renewal, we hypothesized that deregulated expression of this receptor could be associated with hematopoietic malignancies, in particular acute leukemias. Corroborating this hypothesis, a study based on immunohistochemistry has documented endoglin to be highly expressed in the BM of a subset of acute myeloid leukemia (AML) patients.24 Furthermore, a gene expression profile–based study indicated that endoglin correlates with poor outcome in childhood acute lymphoblastic leukemia (ALL).25 Nonetheless, to date, the biological and clinical relevance of endoglin expression in the context of leukemogenesis has not been elucidated.
Herein, we report that endoglin is highly expressed in a subset of acute leukemias and that endoglin-expressing AML and B-lymphoblastic leukemia (B-ALL) blasts are endowed with superior ability to initiate leukemia in a xenotransplantation model. Of significance, inhibition of endoglin signaling using TRC105, alone or in combination with a mild myeloablation regimen, results in inhibition of AML and B-ALL development and progression, suggesting endoglin as a potential target for the treatment of these diseases.
Material and methods
Leukemic cell lines and primary samples
SEMK-2, Nalm-6, RS4;11, Raji, and Jurkat cell lines were maintained in RPMI 1640 medium (Gibco) supplemented with 10% fetal bovine serum (Gemini) and 1% penicillin/streptomycin. HL-60 cells, from ATCC (CCL-240), were maintained in Iscove modified Dulbecco medium (Gibco) containing 20% fetal bovine serum and 1% penicillin/streptomycin. Single-cell suspension cultures were maintained in a humidified incubator at 37°C in an environment of 5% CO2. Deidentified, diagnostic cryopreserved mononuclear cells from BM, peripheral blood (PB), or apheresis, as detailed in Table 1, and sera samples from AML and ALL patients were obtained from the Hematology Malignancy Tissue Bank at the University of Minnesota, according to procedures approved by the institutional review board of the University of Minnesota. All leukemic samples were characterized by a high percentage of blasts and linked to an extensive database with details of the diagnosis and clinical outcomes. Analysis of healthy BM was performed in samples from BM donors at the Laboratory for the Diagnosis of Onco-Hematological Disorders in Curitiba (Brazil), under a protocol approved by the institutional review board of the University of Parana, or purchased from AllCell. The cord blood sample was obtained from St. Louis Cord Blood Bank.
Clinical and immunophenotypic characteristics of leukemic patients
| Sample ID . | Age, y . | Leukemia type . | Sample type . | Cytogenetics . | % Blasts . | % Blasts . | ||
|---|---|---|---|---|---|---|---|---|
| CD34+ . | CD38+ . | CD105+ . | ||||||
| P0001 | 3 | ALL–B cell | BM | t(12;21) TEL/AML1 | 98 | 3.60 | 99.70 | 99 |
| P0012 | 4 | ALL–B cell | BM | +x,+6,+14,+17,+18,+21,+21 hyperdiploid (54) | 97 | 95 | 100.00 | 92.60 |
| P0016 | 5 | ALL–B cell | BM | Trisomy 21 | 99 | 98 | 70.80 | 98.30 |
| P0019 | 16 | ALL–B cell | BM | t(9;22) | 98 | 96.60 | 44.70 | 98.30 |
| P0028 | 1 | ALL–B cell | Apheresis | dic(9;20) | 98 | 99.60 | 99.70 | 96.60 |
| P0021 | 12 | AML | Apheresis | +6 FLT3pos | 91 | 88 | 95 | 65 |
| A0032 | 66 | AML | PB | t(1;3)del(5),−7,idem,t(1;17); CML transformed to AML; prior dx of MDS | 88.50 | 99.80 | 7.63 | 98.50 |
| A0036 | 49 | AML | Apheresis | Normal | 87 | 0.01 | 97.80 | 47.60 |
| A0050 | 86 | AML | BM | Trisomy 8 | 55.60 | 88 | 94.60 | 74.40 |
| Sample ID . | Age, y . | Leukemia type . | Sample type . | Cytogenetics . | % Blasts . | % Blasts . | ||
|---|---|---|---|---|---|---|---|---|
| CD34+ . | CD38+ . | CD105+ . | ||||||
| P0001 | 3 | ALL–B cell | BM | t(12;21) TEL/AML1 | 98 | 3.60 | 99.70 | 99 |
| P0012 | 4 | ALL–B cell | BM | +x,+6,+14,+17,+18,+21,+21 hyperdiploid (54) | 97 | 95 | 100.00 | 92.60 |
| P0016 | 5 | ALL–B cell | BM | Trisomy 21 | 99 | 98 | 70.80 | 98.30 |
| P0019 | 16 | ALL–B cell | BM | t(9;22) | 98 | 96.60 | 44.70 | 98.30 |
| P0028 | 1 | ALL–B cell | Apheresis | dic(9;20) | 98 | 99.60 | 99.70 | 96.60 |
| P0021 | 12 | AML | Apheresis | +6 FLT3pos | 91 | 88 | 95 | 65 |
| A0032 | 66 | AML | PB | t(1;3)del(5),−7,idem,t(1;17); CML transformed to AML; prior dx of MDS | 88.50 | 99.80 | 7.63 | 98.50 |
| A0036 | 49 | AML | Apheresis | Normal | 87 | 0.01 | 97.80 | 47.60 |
| A0050 | 86 | AML | BM | Trisomy 8 | 55.60 | 88 | 94.60 | 74.40 |
CML, chronic myeloid leukemia; dx, diagnosis; ID, identification; MDS, myelodysplastic syndrome.
Flow cytometry and sorting of leukemic blasts
Cells were thawed, washed twice with phosphate buffer solution containing bovine serum albumin (BSA), resuspended in the same buffer containing 0.25 μg/106 cells of Fc block (Miltenyi), and then incubated for 30 minutes with different combinations of the following anti-human antibodies: phycoerythrin (PE)-CD105 (clone SN6; eBioscience), PE-Cy7–conjugated CD45 (clone HI30; BD Biosciences), allophycocyanin-conjugated CD34 (clone 581; Pharmingen), fluorescein isothiocyanate–conjugated CD38 (clone T16; Beckman Coulter), fluorescein isothiocyanate–conjugated CD19 (clone SJ25-C1; Abcam), and allophycocyanin-conjugated CD117 (clone 104D2; Pharmingen). Subsequently, cells were washed twice with phosphate buffer solution containing BSA and analyzed on a FACSAria cell sorter (Becton Dickinson). To exclude nonviable cells, samples were stained with 7-aminoactinomycin D (eBioscience) prior to analysis. At least 100 000 events were acquired. FlowJo software was used for analysis (Tree Star Inc). For sorting, blasts were purified based on CD105 or CD45 expression.
Mice and xenograft leukemia model
All experiments were approved by the University of Minnesota Institutional Animal Care and Use Committee. Female NSG (NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ; The Jackson Laboratory) mice were 7 to 9 weeks of age at the time of injection. Sublethally irradiated (2.0-2.5 Gy) NSG mice were IV injected with 5 × 105 primary leukemic blasts (or 10 000 in case of CD105+ or CD105− blasts). Mice were monitored weekly for signs of disease (including scruffy fur, weight loss, hunched posture, and lethargy) and monitored biweekly for the presence of human CD45+ (hCD45+) cells in the PB. To eliminate erythrocytes, blood was incubated with lysing solution (VersaLyse; Beckman Coulter) prior to staining. When the PB had >60% hCD45, BM and spleen were collected for analyses, except in the case of survival experiments.
Treatment with TRC105 in a xenograft model
TRC105 is an immunoglobulin G (IgG) monoclonal antibody specific for endoglin produced by TRACON Pharmaceuticals. At day 2 or day 30 after injection of AML or ALL blasts, mice were randomly divided into 3 groups: (1) untreated, (2) TRC105 (2 mg/kg IV, every 3 days), and (3) IgG isotype control antibody (Life Technologies) in a similar manner. Studies involving chemotherapy consisted of cyclophosphamide (90 mg/kg intraperitoneally, once a week) for ALL and cytarabin (AraC; 100 mg/kg intraperitoneally, once a week) for AML, alone or in combination with TRC105. PB was analyzed monthly for the presence of hCD45. BM and spleen were analyzed at the end of the study.
Measurement of soluble endoglin
Enzyme-linked immunosorbent assay (ELISA) for human Endoglin/CD105 (R&D Systems) was performed on supernatant of leukemic cell cultures, human plasma, and serum of leukemic mice. Cultures of NALM-6, SEMK-2, and HL-60 cells were started at 2 × 105 cells/mL and supernatant was collected 4 days later.
Western blot
Cells were lysated in radioimmunoprecipitation assay buffer containing antiproteolytics. Matrix metalloproteinase-14 (MMP-14) (EP1264Y, 1/1000; Abcam) and glyceraldehyde-3-phosphate dehydrogenase (Gapdh) (1/3000; Abcam) were diluted in Primary Antibody Signal Boost Immunoreaction Enhancer (Calbiochem). Enhanced chemiluminescence peroxidase-labeled anti-mouse and anti-rabbit antibodies (GE Biosciences) were diluted at 1/10 000 with 5% BSA in 1× Tris-buffered saline–Tween 20.
Statistical analysis
Statistical analysis was determined by the comparison of means using the unpaired Student t test (for 2 groups) or 1-way analysis of variance (ANOVA) (3 groups). The paired Student t test was used to compare means of a given group before and after treatment. The log-rank (Mantel-Cox) test was used to compare survival distributions. P values <.05 were considered significant.
Results
CD105 is highly expressed in normal CD34+ precursor cells and leukemic blasts
Flow cytometry of normal human BM confirmed distinct expression of CD105 on CD34+ cells (60%-80%; supplemental Figure 1A-B, available on the Blood Web site), as described.26 Further subfractionation of BM and cord blood based on CD38 expression revealed that CD105 is present in both fractions, but abundantly on the CD34+CD38− subpopulation (supplemental Figure 1C-D).
Before investigating the expression of this receptor on malignant CD34+ cells, we assessed CD105 expression in several human leukemic cell lines, including Nalm-6, HL-60, SEMK2, RS4;11, Jurkat, and Raji. As shown in Figure 1A, abundant CD105 expression was observed in the promyelocytic HL60 and in pre-B and precursor B-cell leukemic lines (Nalm-6, SEMK2, and RS4;11). In contrast, Raji and Jurkat, human cell lines associated with more mature B cells and T lymphoblastoid cells, respectively, lacked CD105 expression (Figure 1A). These later results are in agreement with a microarray report in childhood leukemia samples, which identified CD105 as a biomarker to distinguish between B- and T-lineage ALL.27
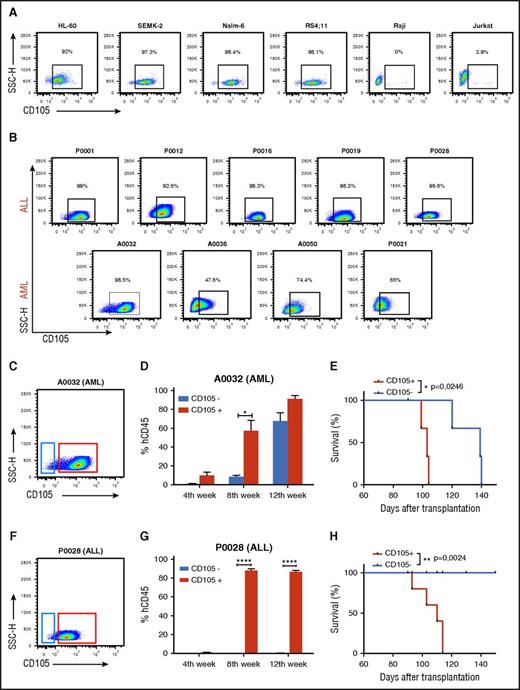
Expression and function of CD105 in acute leukemias. (A-B) Representative FACS plots show CD105/endoglin expression in several leukemic cells lines (A) and in primary ALL (n = 5) and AML (n = 4) blasts (B). Primary leukemic blasts were initially gated based on SSC and the expression of CD45, CD34, and/or CD38, CD19, or CD117, as shown in supplemental Figure 1. Percentages represent CD105 expression. (C-E) Leukemia-forming activity of AML blasts based on CD105 expression. (C) FACS plot shows gating strategy for the sorting of CD105+ (red) and CD105− (blue) subfractions from the AML blast population. (D) Presence of hCD45+ cells in the PB of NSG mice that had been injected with CD105+ (red) and CD105− (blue) AML blasts. Leukemia-forming activity was increased in the CD105+ AML blast subfraction. Bars represent average percentage of hCD45, and error bars indicate standard error of the mean (SEM) for each cohort (n = 3 per group). *P < .05 by Student t test. (E) Reduced survival rate in mice injected with CD105+ AML blasts (n = 3). *P < .05 by log-rank test. (F-H) Leukemia-forming activity of ALL blasts based on CD105 expression. (F) FACS plot shows gating strategy for the sorting of CD105+ (red) and CD105− (blue) subfractions from the ALL blast population. (G) Graphic shows expression levels of hCD45+ cells in the PB of NSG mice that had been injected with CD105+ (red) and CD105− (blue) ALL blasts. Leukemia-forming activity was restricted to the CD105+ ALL blast subfraction. Error bars indicate SEM for each experimental group (n = 5). ****P < .0001 by Student t test. (H) Reduced survival rate in mice injected with the CD105+ ALL blasts (n = 5 per group). ** P < .01 by log-rank test. SSC-H, SSC height.
Expression and function of CD105 in acute leukemias. (A-B) Representative FACS plots show CD105/endoglin expression in several leukemic cells lines (A) and in primary ALL (n = 5) and AML (n = 4) blasts (B). Primary leukemic blasts were initially gated based on SSC and the expression of CD45, CD34, and/or CD38, CD19, or CD117, as shown in supplemental Figure 1. Percentages represent CD105 expression. (C-E) Leukemia-forming activity of AML blasts based on CD105 expression. (C) FACS plot shows gating strategy for the sorting of CD105+ (red) and CD105− (blue) subfractions from the AML blast population. (D) Presence of hCD45+ cells in the PB of NSG mice that had been injected with CD105+ (red) and CD105− (blue) AML blasts. Leukemia-forming activity was increased in the CD105+ AML blast subfraction. Bars represent average percentage of hCD45, and error bars indicate standard error of the mean (SEM) for each cohort (n = 3 per group). *P < .05 by Student t test. (E) Reduced survival rate in mice injected with CD105+ AML blasts (n = 3). *P < .05 by log-rank test. (F-H) Leukemia-forming activity of ALL blasts based on CD105 expression. (F) FACS plot shows gating strategy for the sorting of CD105+ (red) and CD105− (blue) subfractions from the ALL blast population. (G) Graphic shows expression levels of hCD45+ cells in the PB of NSG mice that had been injected with CD105+ (red) and CD105− (blue) ALL blasts. Leukemia-forming activity was restricted to the CD105+ ALL blast subfraction. Error bars indicate SEM for each experimental group (n = 5). ****P < .0001 by Student t test. (H) Reduced survival rate in mice injected with the CD105+ ALL blasts (n = 5 per group). ** P < .01 by log-rank test. SSC-H, SSC height.
We next determined whether CD105 would be distinctively expressed in samples from patients with AML and precursor B-ALL. Nine deidentified primary samples from B-ALL and AML patients were evaluated (Table 1). The blast population was gated based on side scatter (SSC) and low/intermediate expression of CD45, and then analyzed for the expression of CD34 and CD38 to confirm the immature phenotype,28-30 CD19 or CD117 (to confirm B-ALL or AML phenotype, respectively), and CD105 (supplemental Figure 2; Table 1). CD105 was highly expressed on the majority of B-ALL blasts, and varying from 47.5% to 98.5% in AML blasts (Table 1; Figure 1B), indicating that CD105 is expressed on myeloid and B-lymphoid leukemic blasts.
CD105+ blasts have superior in vivo leukemogenic activity
To determine whether CD105 is differentially expressed by cells with leukemia-initiating potential, we used fluorescence-activated cell sorting (FACS) to isolate both AML (Figure 1C) and ALL (Figure 1F) blasts based on CD105 expression. For in vivo assays, primary leukemic samples A0032 (AML) and P0028 (ALL) were selected based on their ability to develop leukemia, as demonstrated by hCD45+ blast engraftment in the PB, BM, and spleen within 1 month into the xenograft model (supplemental Figure 3). FACS reanalysis of sorted samples confirmed the purity of the CD105+ and CD105− sorted subpopulations to be >95% (supplemental Figure 4A-B), and importantly, both CD105+ and CD105− subfractions were CD34+CD117+ in AML (supplemental Figure 4C) and CD34+CD19+ in ALL (supplemental Figure 4D). Following the IV injection of equal numbers of CD105+ and CD105− cells (10 000), mice were monitored monthly for the presence of hCD45+ cells in the PB.
Mice injected with CD105+ AML blasts showed signs of disease by 4 weeks after cell infusion. At this time, FACS analyses revealed a clear subpopulation of human blasts in the PB, which increased significantly by weeks 8 and 12, when it reached about 90% (supplemental Figure 5A; Figure 1D). In mice injected with CD105− AML blasts, hCD45+ was detected by week 8, albeit at much lower levels than in mice injected with CD105+ cells (Figure 1D). Most importantly, mice injected with AML CD105− blasts demonstrated a survival advantage when compared with the CD105+ cohort (Figure 1E). Of note, FACS analysis of engrafted hCD45+ cells in both cohorts revealed homogenous expression of CD105 in the leukemic blast population at week 12 (supplemental Figure 5B), suggesting that a CD105+ contaminating subpopulation was positively selected, or that injected CD105− blasts acquired expression of this receptor in vivo. A similar trend was observed with the ALL sample. Mice injected with the CD105+ cell fraction exhibited a clear subpopulation of human blasts in the PB by 4 weeks postinfusion, which increased rapidly, reaching about 90% by week 8 (Figure 1G; supplemental Figure 5C-D). Remarkably, hCD45+ cells were not detected in mice injected with the same number of CD105− ALL blasts (Figure 1G; supplemental Figure 5C). This result correlated with the prolonged survival rate in the CD105−-injected group (Figure 1H). All mice injected with CD105+ ALL blast died ∼3 months after transplantation, whereas in the group injected with CD105− ALL blasts, no mice died or showed signs of disease until time of sacrifice, 5 months after injection, which correlated with the absence of hCD45+ cells.
Inhibition of endoglin reduces leukemogenic activity
Next, we investigated whether inhibiting endoglin function using the TRC105 antibody would affect leukemia development. Two days after infusion with AML or ALL blasts, mice were randomly divided into 3 groups: treatment with TRC105 or IgG isotype control, or untreated, as outlined in Figure 2A.
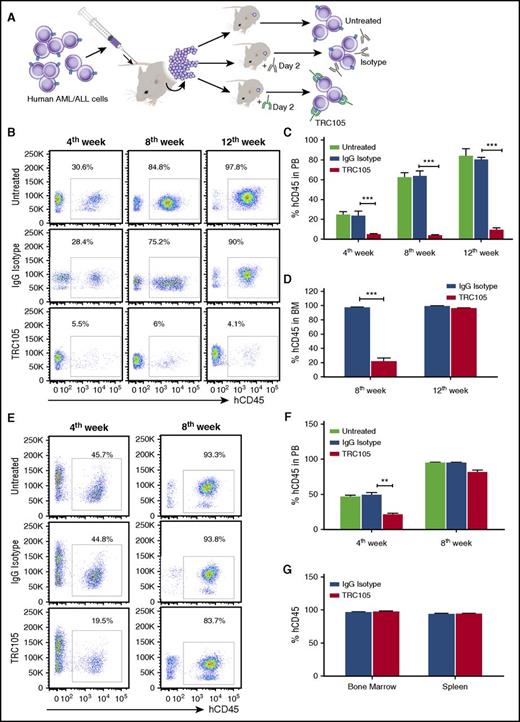
Effect of TRC105 on the ability of AML/ALL blasts to generate leukemia in a xenograft model. (A) Schematic representation of experimental design. Sublethally irradiated NSG mice were IV injected with 5 × 105 human AML or B-ALL blasts isolated from the BM of primary recipient mice that had been injected with primary human leukemic blasts (in vivo expansion). At day 2, mice were randomly divided into groups (n = 7-8 each) and injected with TRC105 or IgG isotype control. (B-D) Effect of TRC105 treatment on AML development. (B) Representative FACS plots show levels of hCD45 in the PB at 4, 8, and 12 weeks postinjection. (C-D) Percentage of hCD45+ cells in PB (C) and BM (D). Bars represent average percentage of hCD45, and error bars indicate SEM for each cohort. Leukemia development in PB was inhibited at week 4 in the TRC105-injected cohort (red), and remained low by week 12 (C). ***P < .001 by ANOVA. Presence of hCD45+ cells in the BM reveals that leukemia development is impaired in the TRC105 cohort at 8 weeks postinjection (n = 3). However, this effect is no longer observed at week 12 (n = 5) (D). ***P < .001 by Student t test. (E-G) Effect of TRC105 treatment on ALL development. (E) Representative flow cytometric plots show levels of hCD45 in the PB at 4 and 8 weeks postinjection. (F-G) Percentage of hCD45+ cells in PB (F) as well as in BM and spleen (G). Bars represent average percentage of hCD45, and error bars indicate SEM for each cohort. TRC105-treated mice exhibited significantly less leukemia in PB by week 4. However, no difference was observed by week 8 (F). **P < .01 by ANOVA. All groups showed massive leukemic cell infiltration in BM by week 8 (G).
Effect of TRC105 on the ability of AML/ALL blasts to generate leukemia in a xenograft model. (A) Schematic representation of experimental design. Sublethally irradiated NSG mice were IV injected with 5 × 105 human AML or B-ALL blasts isolated from the BM of primary recipient mice that had been injected with primary human leukemic blasts (in vivo expansion). At day 2, mice were randomly divided into groups (n = 7-8 each) and injected with TRC105 or IgG isotype control. (B-D) Effect of TRC105 treatment on AML development. (B) Representative FACS plots show levels of hCD45 in the PB at 4, 8, and 12 weeks postinjection. (C-D) Percentage of hCD45+ cells in PB (C) and BM (D). Bars represent average percentage of hCD45, and error bars indicate SEM for each cohort. Leukemia development in PB was inhibited at week 4 in the TRC105-injected cohort (red), and remained low by week 12 (C). ***P < .001 by ANOVA. Presence of hCD45+ cells in the BM reveals that leukemia development is impaired in the TRC105 cohort at 8 weeks postinjection (n = 3). However, this effect is no longer observed at week 12 (n = 5) (D). ***P < .001 by Student t test. (E-G) Effect of TRC105 treatment on ALL development. (E) Representative flow cytometric plots show levels of hCD45 in the PB at 4 and 8 weeks postinjection. (F-G) Percentage of hCD45+ cells in PB (F) as well as in BM and spleen (G). Bars represent average percentage of hCD45, and error bars indicate SEM for each cohort. TRC105-treated mice exhibited significantly less leukemia in PB by week 4. However, no difference was observed by week 8 (F). **P < .01 by ANOVA. All groups showed massive leukemic cell infiltration in BM by week 8 (G).
Heading for AML, administration of TRC105 in mice that had been injected with AML blasts suppressed the ability of these cells to give rise to leukemia in secondary recipients (Figure 2B-C). Untreated or IgG isotype antibody-injected mice displayed a clear subpopulation of human blast cells in the PB (∼25%) at week 4, which increased significantly by week 8 (∼60%) and 12 (∼90%). In contrast, TRC105-treated mice exhibited significantly lower levels of hCD45+ by week 4 (∼5%), which remained low by week 12 (Figure 2B-C; supplemental Figure 6A). Analysis of BM at week 8 revealed that the TRC105-treated cohort contained much fewer hCD45+ cells than the mice group that had been treated with IgG isotype control antibody (21% vs 98%, respectively; Figure 2D). Nevertheless, when analyzed at week 12, unlike PB and spleen (supplemental Figure 6A-B), no difference in the frequency of CD45+ blasts was observed between the 2 groups (Figure 2D), which were nearly all positive for CD105 (supplemental Figure 7A). Treatment with TRC105 prevented splenomegaly and body weight loss that accompanied leukemia development (supplemental Figure 6C-D). These findings suggest that TRC105 treatment reduces leukemogenic activity in vivo.
In the case of ALL, hCD45+ blasts were found at considerable levels by week 4 in the PB of untreated or IgG control-injected mice (∼50%), which doubled by week 8 (∼98%; Figure 2E-F; supplemental Figure 6E). On the other hand, TRC105-treated mice exhibited significantly lower levels of hCD45+ blasts by week 4 (∼20%; Figure 2E). However, these numbers increased significantly by week 8, reaching similar levels to those of untreated and isotype control groups (∼98%; Figure 2E-F), despite continuous treatment. At this time point, all groups showed massive leukemic cell infiltration in BM and spleen (Figure 2G; supplemental Figure 7B), which was accompanied by splenomegaly (supplemental Figure 6F). These results suggest that TRC105 treatment slows down the development of ALL, but not as effectively as observed in AML.
TRC105 suppresses AML progression when administered upon disease onset
To assess therapeutic relevance, we investigated whether TRC105 treatment would have antileukemogenic activity when administered after disease had been established. For this, human AML or ALL blasts (Figure 3A) were allowed to populate the PB of injected mice prior to TRC105 treatment. Four weeks postinfusion, when blasts were detected in the PB (Figure 3B,E), leukemic mice were randomly divided into 3 groups and subjected to treatment with TRC105 or IgG isotype control or no treatment. Antibodies were administered for 8 weeks in the case of AML (Figure 3B-D) and 4 weeks for ALL (Figure 3E-G). As shown in Figure 3B, when we began TRC105 treatment of AML-injected mice at week 4, the average of hCD45+ cells in the PB was about 20%. As expected, numbers of CD45+ blasts in untreated and IgG isotype injected mice increased significantly in the subsequent weeks, reaching 60% by week 8 and 90% by week 12 (Figure 3B-C; supplemental Figure 8A). In contrast, no increase, but rather reduced numbers of hCD45+ blasts, was observed in the PB of TRC105-treated mice (10% and 16% by weeks 8 and 12, respectively), confirming the effect of TRC105 in counteracting AML development and progression (Figure 3B-C). TRC105 treatment prolonged survival (Figure 3D), and prevented splenomegaly and body weight loss (supplemental Figure 8C-D). Accordingly, significantly lower leukemic cell infiltration was observed in the spleens of TRC105-treated mice relative to the IgG control group (supplemental Figure 8B). However, by 12 weeks, the BM of both groups contained equivalent high numbers of hCD45 (supplemental Figure 8B).
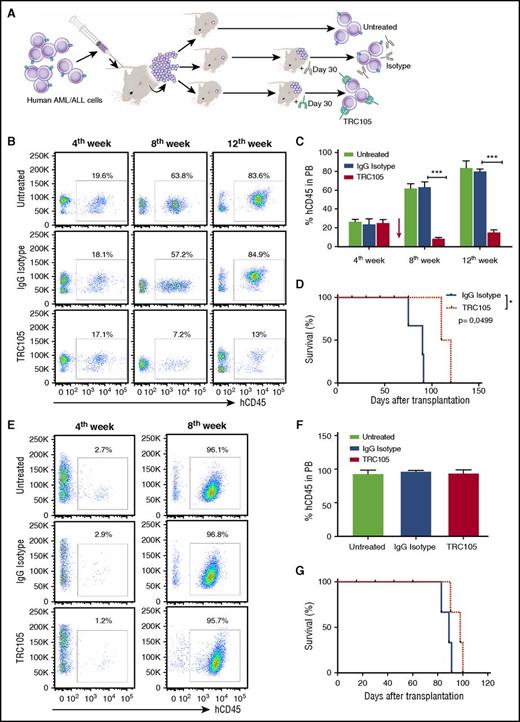
TRC105 treatment alone suppresses the in vivo progression of AML, but not of ALL. (A) Schematic representation of experimental design. Sublethally irradiated NSG mice were IV injected with human AML/ALL blast cells (5 × 105 and 4 × 104 cells, respectively) isolated from the BM of primary recipient mice that had been injected with primary human leukemic blasts. Once hCD45+ cells were detected in the PB, 4 weeks after injection, mice were randomly divided into groups (n = 7 each) and treated with TRC105 or IgG isotype control. (B-D) Effect of TRC105 treatment on AML progression. (B) Representative FACS plots show levels of hCD45 in the PB before (fourth week) and after (eighth and 12th week) treatment with TRC105 or IgG isotype control. (C) Percentage of hCD45+ cells in PB. Bars represent average percentage of hCD45, and error bars indicate SEM for each cohort (n = 7). Leukemia progression was inhibited and actually regressed at week 8 in the TRC105-injected cohort (red), and remained low by week 12 (C). ***P < .001 by ANOVA. (D) TRC105-treated mice exhibited prolonged survival compared with IgG-injected control. Each data point represents a single mouse from TRC105- (red) or IgG isotype- (gray) injected cohort. *P < .05 by log-rank test. (E-G) Effect of TRC105 treatment on ALL progression. (E) Representative FACS plots show levels of hCD45 before (fourth week) and after (eighth week) treatment with TRC105 or IgG isotype control. (F) Bars represent average percentage of hCD45 in PB, and error bars indicate SEM for each cohort (n = 7). Leukemia progressed similarly in both groups. ***P < .001 by ANOVA. (G) No significant differences were observed on survival between TRC105 (red) and IgG isotype-treated groups (gray). Each data point represents a single mouse for each cohort.
TRC105 treatment alone suppresses the in vivo progression of AML, but not of ALL. (A) Schematic representation of experimental design. Sublethally irradiated NSG mice were IV injected with human AML/ALL blast cells (5 × 105 and 4 × 104 cells, respectively) isolated from the BM of primary recipient mice that had been injected with primary human leukemic blasts. Once hCD45+ cells were detected in the PB, 4 weeks after injection, mice were randomly divided into groups (n = 7 each) and treated with TRC105 or IgG isotype control. (B-D) Effect of TRC105 treatment on AML progression. (B) Representative FACS plots show levels of hCD45 in the PB before (fourth week) and after (eighth and 12th week) treatment with TRC105 or IgG isotype control. (C) Percentage of hCD45+ cells in PB. Bars represent average percentage of hCD45, and error bars indicate SEM for each cohort (n = 7). Leukemia progression was inhibited and actually regressed at week 8 in the TRC105-injected cohort (red), and remained low by week 12 (C). ***P < .001 by ANOVA. (D) TRC105-treated mice exhibited prolonged survival compared with IgG-injected control. Each data point represents a single mouse from TRC105- (red) or IgG isotype- (gray) injected cohort. *P < .05 by log-rank test. (E-G) Effect of TRC105 treatment on ALL progression. (E) Representative FACS plots show levels of hCD45 before (fourth week) and after (eighth week) treatment with TRC105 or IgG isotype control. (F) Bars represent average percentage of hCD45 in PB, and error bars indicate SEM for each cohort (n = 7). Leukemia progressed similarly in both groups. ***P < .001 by ANOVA. (G) No significant differences were observed on survival between TRC105 (red) and IgG isotype-treated groups (gray). Each data point represents a single mouse for each cohort.
In the case of ALL, our results showed that treatment with TRC105 has no effect on leukemia progression when administered after disease has been established. Four weeks after beginning treatment, all groups displayed very high numbers of hCD45+ blasts in the PB (>95%; Figure 3E-F). Massive leukemic cell infiltration was found in the BM and spleen of both groups (supplemental Figure 8E), and accordingly, no differences in body weight or survival between TRC105- and IgG isotype-injected groups (supplemental Figure 8F and Figure 3G, respectively).
Levels of sENG correlate inversely with effectiveness of TRC105
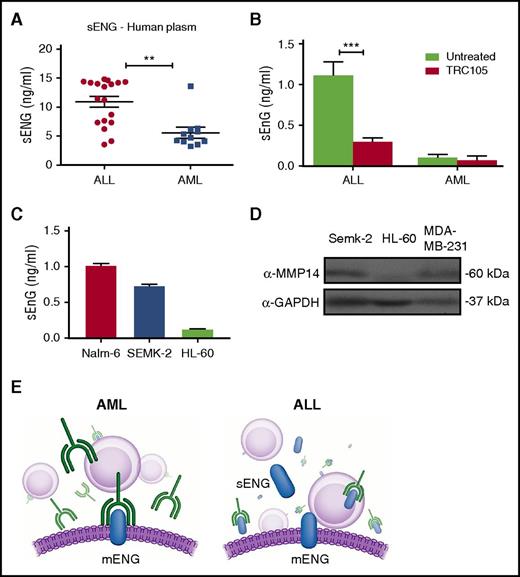
Based on these results, we hypothesized that the limited effect in the ALL model could be due to expression of soluble endoglin (sENG), which would titrate the TRC105 antibody, thus limiting its ability to bind to membrane-bound endoglin (mENG) on leukemic cells (Figure 4E). We began by determining the levels of sENG in the plasma of AML and ALL patients at the time of diagnosis and discovered that sENG was significantly higher in the plasma of ALL patients compared with AML patients (Figure 4A). Next, we measured sENG in the serum of mice engrafted with human AML or ALL blasts and treated with TRC105. Twelve weeks after transplantation, sENG could be detected in the serum of mice transplanted with ALL cells, and was significantly decreased in the group treated with TRC105 (Figure 4B). However, at the same time point, no significant detection of sENG was found in the serum of mice injected with AML cells (Figure 4B). This is in agreement with Al-Mowallad et al,31 who reported plasma levels for CD105 to be elevated in children with ALL.
Levels of sENG are higher in ALL and potentially interfere with TRC105 efficacy. (A-C) Levels of sENG in leukemic cells. Measurements were performed using standard quantitative ELISA. (A) Concentration of sENG in the plasma of AML (n = 11) and ALL (n = 18) patients. Each data point represents a single patient sample. **P < .01 by Student t test. (B) Levels of sENG in the serum of mice that had been injected with ALL or AML blasts, and subjected to TRC105 treatment, or not, for a period of 12 weeks. Error bars indicate SEM for each cohort (n = 4). ***P < .001 by Student t test. (C) Levels of sENG in leukemic cell lines Nalm-6, Semk-2, and HL-60. Supernatant of these cell cultures was collected on day 4. Error bars indicate SEM from 2 independent experiments. (D) Western blot analyses for MMP-14 in lysates from indicated cancer cell lines. Active form of MMP-14 is observed in Semk-2 as well as a positive control MDA-MB-231, but not in HL-60. GAPDH was used as loading control. (E) Schematic representation outlining the effect of sENG on TRC105 treatment. In AML, which is characterized by low levels of sENG, the monoclonal antibody TRC105 is free to bind to the membrane form of endoglin (left panel). However, in ALL (right panel), which exhibits high levels of sENG, TRC105 is decoyed, resulting in less TRC105 available to bind to mENG, and thereby less therapeutic effect.
Levels of sENG are higher in ALL and potentially interfere with TRC105 efficacy. (A-C) Levels of sENG in leukemic cells. Measurements were performed using standard quantitative ELISA. (A) Concentration of sENG in the plasma of AML (n = 11) and ALL (n = 18) patients. Each data point represents a single patient sample. **P < .01 by Student t test. (B) Levels of sENG in the serum of mice that had been injected with ALL or AML blasts, and subjected to TRC105 treatment, or not, for a period of 12 weeks. Error bars indicate SEM for each cohort (n = 4). ***P < .001 by Student t test. (C) Levels of sENG in leukemic cell lines Nalm-6, Semk-2, and HL-60. Supernatant of these cell cultures was collected on day 4. Error bars indicate SEM from 2 independent experiments. (D) Western blot analyses for MMP-14 in lysates from indicated cancer cell lines. Active form of MMP-14 is observed in Semk-2 as well as a positive control MDA-MB-231, but not in HL-60. GAPDH was used as loading control. (E) Schematic representation outlining the effect of sENG on TRC105 treatment. In AML, which is characterized by low levels of sENG, the monoclonal antibody TRC105 is free to bind to the membrane form of endoglin (left panel). However, in ALL (right panel), which exhibits high levels of sENG, TRC105 is decoyed, resulting in less TRC105 available to bind to mENG, and thereby less therapeutic effect.
To date, sENG/CD105 is mostly associated with microvessel density and angiogenesis,31 and thus, it is unknown whether leukemic cells may be a source of sENG. To address this question, we quantified levels of sENG on supernatant of different subtypes of leukemic cell line cultures. We found that higher levels of sENG are present in the supernatant of the lymphoblastic pre-B-cell lines Nalm-6 and SEMK-2 when compared with the promyeloblastic HL60 cell line (Figure 4C). This interesting finding points to differential cleavage of mENG in ALL compared with AML. Accordingly, MMP-14, which has been reported as the mediator of endoglin shedding,32 is distinctively expressed in the lymphoblastic pre-B-cell line, whereas it is not detected in HL60 myeloid cells (Figure 4D).
These data support the notion that sENG could mitigate the therapeutic activity of TRC105 by preventing the antibody from binding to the cell surface, as outlined in Figure 4E.
Effect of TRC105 combined with mild myeloablation
Because it is unlikely that TRC105 would be used as a monotherapy in AML and B-ALL, we next determined whether TRC105 could potentiate the effect of chemotherapeutic agents using a mild regimen. Once disease had been established, we began therapy with TRC105 (same manner as before) in combination with AraC for AML and cyclophosphamide (CPA) for ALL, which were injected only once a week. In the case of AML, AraC alone slowed down disease progression during the first 2 weeks, but this effect did not persist (supplemental Figure 9). On the other hand, combined therapy with TRC105 significantly reduced the levels of leukemic cells in the PB (twofold; supplemental Figure 9).
In ALL, both CPA alone or in combination with TRC105 suppressed leukemia development, but effect of combined therapy was more effective sooner (Figure 5A; supplemental Figure 10). Remarkably, BM analysis at 2 months of treatment revealed the lowest levels of leukemia infiltration in the TRC105 + CPA cohort (1.7 ± 0.5 vs 29.1 ± 15.5 in CPA alone), whereas the untreated and TRC105-alone cohorts were nearly completely positive for hCD45 (Figure 5B-C). Consistently, sENG was detected at very high levels in the serum of untreated ALL mice, and at lower levels in the TRC105 group, as observed in Figure 4B, but most importantly, no significant detection could be observed in the CPA and CPA+TRC105 cohorts (Figure 5D). These findings demonstrate that the inhibitory effect of sENG can be circumvented by suppressing tumor burden, resulting in the combined therapy having potent antileukemic activity (Figure 5B-C).
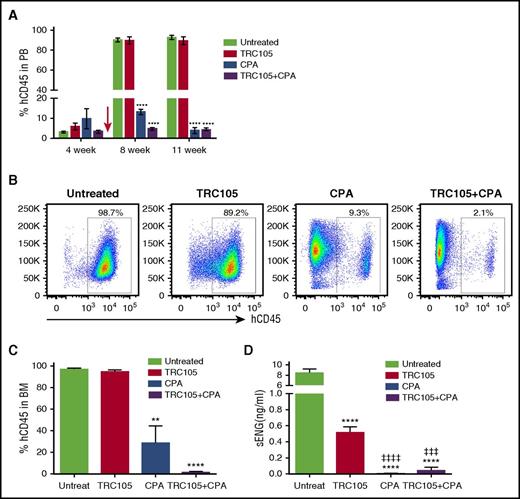
TRC105, in combination with mild myeloablation, suppresses the in vivo progression of ALL. (A-C) Effect of TRC105+CPA treatment on ALL progression. (A) Graph shows average percentage of hCD45 in PB. Error bars indicate SEM for each cohort (n = 4-6 each). Red arrow indicates beginning of treatment. ****P < .0001 by ANOVA. (B-C) Representative FACS plots show levels of hCD45 in the BM 11 weeks postinjection of ALL blasts (B), and graph shows average percentage of hCD45, with error bars indicating SEM (C). Leukemia progression was inhibited in the CPA-injected cohort, and this inhibition was significantly potentiated in the presence of combined therapy TRC105+CPA. **P < .01 and ****P < .0001 by ANOVA. (D) Levels of sENG 11 weeks after injection of ALL blast. Bars represent average concentration of sENG and error bars indicate SEM. Measurements were performed using standard quantitative ELISA. ****P < .0001 compared with untreated, ǂǂǂP < .0001 and ǂǂǂǂP < .0001, compared with TRC105 alone, by ANOVA.
TRC105, in combination with mild myeloablation, suppresses the in vivo progression of ALL. (A-C) Effect of TRC105+CPA treatment on ALL progression. (A) Graph shows average percentage of hCD45 in PB. Error bars indicate SEM for each cohort (n = 4-6 each). Red arrow indicates beginning of treatment. ****P < .0001 by ANOVA. (B-C) Representative FACS plots show levels of hCD45 in the BM 11 weeks postinjection of ALL blasts (B), and graph shows average percentage of hCD45, with error bars indicating SEM (C). Leukemia progression was inhibited in the CPA-injected cohort, and this inhibition was significantly potentiated in the presence of combined therapy TRC105+CPA. **P < .01 and ****P < .0001 by ANOVA. (D) Levels of sENG 11 weeks after injection of ALL blast. Bars represent average concentration of sENG and error bars indicate SEM. Measurements were performed using standard quantitative ELISA. ****P < .0001 compared with untreated, ǂǂǂP < .0001 and ǂǂǂǂP < .0001, compared with TRC105 alone, by ANOVA.
Discussion
Novel therapeutic targets for acute leukemia are urgently needed. Successful treatment of acute leukemia remains a clinical challenge due to the relatively heterogeneous responses to the current standard therapy and the associated toxicity, in particular in the elderly patient population.33,34 AML is associated with 5-year overall survival of <50%, and between 10% and 40% of newly diagnosed patients with AML do not achieve complete remission with intensive therapy.35,36 For ALL, although significant progress has been made in the last decade, in cases of refractory or relapsed ALL, second-line chemotherapy has shown poor effect, rarely resulting in long-term survival.37,38 Thus, there is a critical need for new therapeutic options. Monoclonal antibodies are promising agents because they deliver their therapeutic effects with minimal toxicity.38,39
Based on the expression of endoglin on HSCs, we investigated whether endoglin might also be expressed on leukemic blasts. We found that the majority of B-ALL and AML blasts express endoglin, and that leukemia-forming activity is detected uniquely in the endoglin-positive fraction of B-ALL, whereas in AML, this activity is enriched in this fraction, as leukemia develops faster when endoglin-positive cells are injected. Thus, removing endoglin-positive blasts delays AML disease onset (Figure 1D-E), but does not prevent it, as observed for B-ALL (Figure 1G-H). Of note, regardless of whether the mice received AML endoglin-positive or endoglin-negative blasts, by 12 weeks, the BM of engrafted mice was populated by blasts homogeneously positive for endoglin, suggesting that an endoglin-dim subpopulation was contained within the endoglin-negative fraction, or that injected endoglin-negative blasts acquired expression of this receptor in vivo. Most importantly, blocking endoglin by administrating TRC105 to AML-bearing mice suppressed the leukemic activity of these cells. Of clinical significance, treatment with TRC105 as monotherapy, upon disease onset, suppressed AML progression, resulting in increased survival of leukemic mice. Accordingly, reduced numbers of leukemic cells were observed in the PB and spleen of TRC-treated mice up to 12 weeks after transplantation. A similar suppressive effect was observed in the BM by 8 weeks, but TRC105 alone was not sufficient to avoid BM disease because by 12 weeks, there was no longer a difference between the TRC105-treated and the IgG control group. We believe this may be related to the antibody half-life and epitope occupancy. This warrants further studies, including testing the therapeutic effect in additional leukemic samples as well as different treatment regimens. Of note, the original primary AML sample (A0032) used in our xenotransplantation assays comes from a case of CML transformed, with blast crises, characterized by a complex cytogenetic profile (t(1;3);del(5);−7;idem;t(1;17)), with deletion on chromosomes 7, which has been reported to be associated with poor prognosis and resistance to standard therapy.40 Our encouraging results suggest the therapeutic benefit of targeting CD105 in AML with TRC105. This is underscored by the fact that phase 1 and 2 trials of TRC105 in solid tumors have shown it to be well tolerated at clinically relevant doses.8-10 When the maximum tolerated dose was exceeded, at 15 mg/kg every week, hypoproliferative anemia has been reported.9 This side effect was easily monitorable, reversible, and treatable without adverse sequelae,9 however, this would be important to monitor closely in leukemia patients receiving TRC105.
The concept of antibody targeting for malignancies, including acute leukemia, is well established.38,41 Several antigens have been identified as potential targets in AML. The best known is CD33, for which an anti-CD33 monoclonal antibody–conjugated gemtuzumab ozogamicin was approved by the US Food and Drug Administration for the treatment of relapsed AML. However, gemtuzumab ozogamicin was voluntarily removed from the US market after clinical trials showed no benefit in the improvement of survival outcomes, in addition to increased toxicity.42 Recently, monoclonal antibodies targeting CD44, CD123, and CD47 have demonstrated efficacy against AML leukemic stem cells in xenotransplantation models. However, these monoclonal antibodies showed limited efficacy in established disease treatment models,43-45 in contrast to TRC105, which effectively prolonged survival in AML, even when treatment started after establishment of truthful leukemia (20% of PB).
In the case of the ALL cohort, we observed only modest effects of TRC105 treatment. At week 8, massive leukemic cell infiltration was found in the PB, BM, and spleen of all experimental groups. It seems likely that this limited effect of TRC105 in the ALL model could be due to sENG, which arises from the proteolytic cleavage of the receptor extracellular domain by MMP-14.32,46 sENG has been shown to represent a biomarker for many solid cancers.47-49 Our results show for the first time shedding of sENG by lymphoblastic pre-B cells, which correlated with MMP-14 expression, indicating that this process is not restricted to angiogenic cells. Higher levels of sENG were detected in the plasma of B-ALL patients when compared with AML counterparts, indicating differences in endoglin shedding between ALL and AML. Consistent with this result, minimal levels of sENG were detected in the serum of AML-injected mice, whereas ALL-engrafted mice showed high levels of sENG, which were significantly decreased in the TRC105-treated cohort. These results support the premise that the soluble form of this receptor could be interfering with efficacy of TRC105 therapy by serving as a soluble decoy, preventing TRC105 from binding to the surface of target leukemic cells. Nevertheless, combined therapy with CPA circumvented this problem, and provided the most potent antileukemic activity. It is also plausible to hypothesize that in the setting of minimal residual disease, sENG may be present at lower levels, and thus TRC105 could be effective.
The mechanism by which TRC105 inhibits leukemia development warrants further investigation, but potential mechanisms include homing, antibody-dependent cellular cytotoxicity (ADCC), stimulation of complement-dependent cytotoxicity, inhibition of signal transduction and/or aberrant signal transduction, or direct induction of apoptosis.50 There are no natural killer cells in NSG mice, thus, ADCC does not underline the findings observed here, but considering that TRC105 mediates ADCC,9 it is therefore possible that efficacy might be even better in the immunocompetent setting. Because TGF-β, a well-established ligand for endoglin,51 is also known for its critical role in HSC regulation,52-54 we hypothesize that TRC105 may be functioning by modulating TGF-β signaling.
In conclusion, our results show that CD105 is a biomarker for acute leukemia that is necessary for leukemogenic activity. Our results strongly support the clinical evaluation of TRC105 in the context of AML and B-ALL.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are grateful to the Heme Malignancy Tissue Bank at the University of Minnesota. The authors thank Cynthia Dekay for assistance in graphic design.
This work was supported by a seed grant from the University of Minnesota Academic Health Sciences (R.C.R.P.), by funds from TRACON Pharmaceutical (R.C.R.P.), and by National Institutes of Health National Heart, Lung, and Blood Institute grant 2T32HL007062 (J.B.). The Heme Malignancy Tissue Bank at the University of Minnesota receives support from the National Cancer Institute (NCI no. 5P30CA077598-18), and from the Minnesota Masonic Charities and the Killebrew-Thompson Memorial Fund through the Cancer Experimental Therapeutics Initiative.
Authorship
Contribution: K.M.C.D. designed and conducted experiments, analyzed and interpreted the data, and wrote the paper; J.B. performed experiments and analysis, and contributed to interpretation of results and writing of the manuscript; V.K.P.O. conducted experiments and analyzed the data; M.B. performed analysis, contributed to interpretation of results and writing of the manuscript; A.Y. performed experiments; C.P.T. provided reagents, and contributed to data interpretation and writing of the manuscript; C.A.V.F. contributed to interpretation of results and writing of the manuscript; M.R.V. contributed to experiment design, interpretation of results, and writing of the manuscript; and R.C.R.P. supervised the overall project, designed experiments, analyzed the data, and wrote the paper.
Conflict-of-interest disclosure: R.C.R.P. has received research support from TRACON. C.P.T. is an employee of TRACON. The remaining authors declare no competing financial interests.
Correspondence: Rita C. R. Perlingeiro, Lillehei Heart Institute, University of Minnesota, 4-128 CCRB, 2231 6th St SE, Minneapolis, MN 55455; e-mail: perli032@umn.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal