Key Points
NK/T-cell lymphomas failing L-asparaginse, generally fatal, showed a high CR rate to PD1 blockade with pembrolizumab.
Comprehensive clinical, radiologic, pathologic, and molecular assessments showed different patterns of CRs and PRs.
Abstract
Natural killer (NK)/T-cell lymphomas failing L-asparaginse regimens have no known salvage and are almost invariably fatal. Seven male patients with NK/T-cell lymphoma (median age, 49 years; range, 31-68 years) for whom a median of 2 (range, 1-5) regimens (including l-asparaginase regimens and allogeneic hematopoietic stem-cell transplantation [HSCT] in 2 cases) failed were treated with the anti–programmed death 1 (PD1) antibody pembrolizumab. All patients responded, according to various clinical, radiologic (positron emission tomography), morphologic, and molecular (circulating Epstein-Barr virus [EBV] DNA) criteria. Two patients achieved complete response (CR) in all parameters. Three patients achieved clinical and radiologic CRs, with two having molecular remission (undetectable EBV DNA) but minimal EBV-encoded RNA-positive cells in lesions comprising predominantly CD3+CD4+ and CD3+CD8+ T cells (which ultimately disappeared, suggesting they represented pseudoprogression) and one having detectable EBV DNA despite morphologic CR. Two patients achieved partial response (PR). After a median of 7 (range, 2-13) cycles of pembrolizumab and a follow-up of a median of 6 (range, 2-10) months, all five CR patients were still in remission. The only adverse event was grade 2 skin graft-versus-host disease in one patient with previous allogeneic HSCT. Expression of the PD1 ligand was strong in 4 patients (3 achieving CR) and weak in 1 (achieving PR). PD1 blockade with pembrolizumab was a potent strategy for NK/T-cell lymphomas failing l-asparaginase regimens.
Introduction
Natural killer (NK)/T-cell lymphomas are aggressive malignancies, with anthracycline regimens resulting in dismal outcome.1 L-asparaginse regimens,1 particularly SMILE (dexamethasone, methotrexate, ifosfamide, l-asparaginase, and etoposide), have significantly improved the outlook2,3 and are now the most commonly employed initial therapy.1,4 However, SMILE or SMILE-like regimens still fail in 20% to 40% of cases.1-3 There is no known effective salvage, and outcome is virtually always fatal.
NK/T-cell lymphomas are invariably infected by Epstein-Barr virus (EBV), which exists in a latency II state, expressing immunogenic antigens EBNA1, LMP1, and LMP2.5 Furthermore, EBV-infected lymphoma cells upregulate programmed death ligand 1 (PDL1), ligand of the inhibitory receptor programmed death 1 (PD1) on T cells.6 Ligation of PDL1 on lymphoma cells with PD1 on effector T cells suppresses T-cell cytotoxicity. The PDL1/PD1 axis is therefore a potential mechanism for NK/T-cell lymphomas to avert effector T-cell targeting.
Here we report retrospectively results of PD1 blockade in relapsed/refractory NK/T-cell lymphomas failing l-asparaginase regimens, which provide proof-of-concept data that the PDL1/PD1 axis6 is highly relevant in this malignancy.
Study design
Patients and treatment
Patients with relapsed/refractory NK/T-cell lymphomas failing l-asparaginase regimens from Hong Kong, Singapore, and Seoul, treated with the anti-PD1 antibody pembrolizumab and reported annually to the Asia Lymphoma Study Group since 2015, were analyzed. On a named-patient off-label basis, pembrolizumab at 2 mg/kg every 3 weeks (based on efficacy in Hodgkin lymphoma)7 was used in all but 1 patient, who received pembrolizumab every 2 weeks. Patients provided informed consent for therapy.
Response assessment and monitoring
[18F]Fluorodeoxyglucose (FDG) positron emission tomography computed tomography (PET/CT) was used to assess response according to standard criteria (5-point Deauville score).8 For lesions not observed in pretreatment or other preceding scans, accessible ones were biopsied, whereas inaccessible ones were evaluated by standard criteria8 and immune-related response criteria.9 Because this study was retrospective, schedule of PET/CT varied, but early assessment after 3 to 4 cycles10 was adopted in most cases. Circulating EBV DNA was quantified with quantitative polymerase chain reaction.11
Results and discussion
Patients
Seven men (median age, 49 years; range 31-68, years) were treated (Tables 1 and 2). Most had advanced-stage disease with widespread organ involvement (initial diagnosis: IE, n = 2; IV, n = 5; before pembrolizumab: IE, n = 1; IV, n = 6; Table 1). Systemic HPS was present in 5 patients before pembrolizumab treatment. SMILE or SMILE-like regimens had failed for all patients. Two patients had relapsed after allo-HSCT. The median number of prior regimens including allo-HSCT was 2 (range, 1-5; Table 2). Details of previous treatment are shown in the supplemental Data available on the Blood Web site.
Clinicopathologic features on presentation and at relapse/refractory disease of 7 patients with NK/T-cell lymphoma treated with pembrolizumab
| Case . | . | . | On initial presentation . | At relapse/refractory disease before pembrolizumab . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sex . | Age, y . | Primary sites . | Marrow . | Stage . | Sites . | ECOG score . | Marrow . | Stage . | HPS . | |
| 1 | M | 68 | Skin of lower limbs, nasal cavities | Negative | IV | Calf skin | 1 | Negative | IE | Nil |
| 2 | M | 49 | Nasal cavities, lymph nodes, liver, spleen, bone | Negative | IV | Liver, spleen | 1 | Positive | IV | Yes |
| 3 | M | 38 | Nasopharynx | Negative | IE | Nasopharynx, hard palate | 2 | Positive | IV | Yes |
| 4 | M | 50 | Liver | Positive | IV | Liver | 3 | Positive | IV | Yes |
| 5 | M | 31 | Nasal cavity, nasopharynx, masseter muscle, bone | Negative | IV | Nasal cavity, liver | 2 | Negative | IV | Yes |
| 6 | M | 35 | Nasal cavity | Negative | IE | Lung, esophagus | 2 | Negative | IV | Nil |
| 7 | M | 51 | Liver, spleen | Positive | IV | Liver | 1 | Positive | IV | Yes |
| Case . | . | . | On initial presentation . | At relapse/refractory disease before pembrolizumab . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sex . | Age, y . | Primary sites . | Marrow . | Stage . | Sites . | ECOG score . | Marrow . | Stage . | HPS . | |
| 1 | M | 68 | Skin of lower limbs, nasal cavities | Negative | IV | Calf skin | 1 | Negative | IE | Nil |
| 2 | M | 49 | Nasal cavities, lymph nodes, liver, spleen, bone | Negative | IV | Liver, spleen | 1 | Positive | IV | Yes |
| 3 | M | 38 | Nasopharynx | Negative | IE | Nasopharynx, hard palate | 2 | Positive | IV | Yes |
| 4 | M | 50 | Liver | Positive | IV | Liver | 3 | Positive | IV | Yes |
| 5 | M | 31 | Nasal cavity, nasopharynx, masseter muscle, bone | Negative | IV | Nasal cavity, liver | 2 | Negative | IV | Yes |
| 6 | M | 35 | Nasal cavity | Negative | IE | Lung, esophagus | 2 | Negative | IV | Nil |
| 7 | M | 51 | Liver, spleen | Positive | IV | Liver | 1 | Positive | IV | Yes |
ECOG, Eastern Cooperative Oncology Group; HPS, hemophagocytic syndrome.
Therapy and outcome at initial presentation and before and after pembrolizumab treatment of 7 patients with NK/T-cell lymphoma treated with pembrolizumab
| Case . | Primary treatment . | Before pembolizumab . | Pembrolizumab treatment . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTx (cycles) . | RT . | Outcome . | Other Rx . | DOR, months . | CTx (cycles) . | Outcome . | DOR, mo . | PDL1 . | Dose, mg . | Cycles . | Outcome (cycles) . | Survival, mo . | |
| 1 | SIMPLE (6) | Nil | CR | Nil | 17 | Nil | NA | NA | Strong | 100 | 6 | PET/CT, Deauville score, 3 (4) | 4+ |
| EBV DNA: negative (1) | |||||||||||||
| 2 | SMILE (5) | Nil | CR | Allo-HSCT | 83 | Nil | NA | NA | Strong | 120 | 7 | PR, DOD | 6 |
| 3 | m-BACOD (4) | Yes | CR | Nil | 63 | SIMPLE (6) | CR | 20 | Strong | 100 | 12 | Pseudoprogression (3, 6, and 9); CR (12) | 10+ |
| SMILE (3) | PD | NA | EBV DNA: negative (1) | ||||||||||
| 4 | GELOX (3) | Nil | CR | Nil | 4 | SMILE (5) | PD | NA | Strong | 200 | 13 | Morphologic CR (6) | 8+ |
| RV (2) | PD | PET/CT CR (6) | |||||||||||
| BB (2) | PD | EBV DNA: negative (3) | |||||||||||
| DaraRev (1) | PD | EBV DNA then became positive | |||||||||||
| 5 | SMILE (6) | Yes | CR | Nil | 7 | GEMOX (1) | PD | NA | 20% | 100 | 2 | EBV DNA: negative | 2 |
| RT | PD | Died as a result of gastric ulcer bleeding | |||||||||||
| 6 | SMILE (1) | Yes | NA | Nil | 2 | GELOX (6) | PR | 6 | ND | 100 | 12 | CR (6) | 9+ |
| 7 | SMILE (6) | Nil | CR | Allo-HSCT | 10 | Nil | NA | NA | ND | 100 | 4 | CR (3) | 3+ |
| Case . | Primary treatment . | Before pembolizumab . | Pembrolizumab treatment . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTx (cycles) . | RT . | Outcome . | Other Rx . | DOR, months . | CTx (cycles) . | Outcome . | DOR, mo . | PDL1 . | Dose, mg . | Cycles . | Outcome (cycles) . | Survival, mo . | |
| 1 | SIMPLE (6) | Nil | CR | Nil | 17 | Nil | NA | NA | Strong | 100 | 6 | PET/CT, Deauville score, 3 (4) | 4+ |
| EBV DNA: negative (1) | |||||||||||||
| 2 | SMILE (5) | Nil | CR | Allo-HSCT | 83 | Nil | NA | NA | Strong | 120 | 7 | PR, DOD | 6 |
| 3 | m-BACOD (4) | Yes | CR | Nil | 63 | SIMPLE (6) | CR | 20 | Strong | 100 | 12 | Pseudoprogression (3, 6, and 9); CR (12) | 10+ |
| SMILE (3) | PD | NA | EBV DNA: negative (1) | ||||||||||
| 4 | GELOX (3) | Nil | CR | Nil | 4 | SMILE (5) | PD | NA | Strong | 200 | 13 | Morphologic CR (6) | 8+ |
| RV (2) | PD | PET/CT CR (6) | |||||||||||
| BB (2) | PD | EBV DNA: negative (3) | |||||||||||
| DaraRev (1) | PD | EBV DNA then became positive | |||||||||||
| 5 | SMILE (6) | Yes | CR | Nil | 7 | GEMOX (1) | PD | NA | 20% | 100 | 2 | EBV DNA: negative | 2 |
| RT | PD | Died as a result of gastric ulcer bleeding | |||||||||||
| 6 | SMILE (1) | Yes | NA | Nil | 2 | GELOX (6) | PR | 6 | ND | 100 | 12 | CR (6) | 9+ |
| 7 | SMILE (6) | Nil | CR | Allo-HSCT | 10 | Nil | NA | NA | ND | 100 | 4 | CR (3) | 3+ |
Allo-HSCT, allogeneic hematopoietic stem-cell transplantation; BB, brentuximab vedotin and bendamustine; CR, complete response; CTx, chemotherapy; DaraRev, daratumomab and lenalidomide; DOD, died of disease; GELOX, gemcitabine, l-asparaginase, and oxaliplatin; m-BACOD, methotrexate, bleomycin, doxorubicin, cyclophosphamide, vincristine, and dexamethasone; NA, not applicable; ND, not done; PR, partial response; RT, radiotherapy; RV, romidepsin and bortezomib; SIMPLE, dexamethasone, ifosfamide, gemcitabine, cisplatin, L-asparaginse, and etoposide; SMILE, dexamethasone, methotrexate, ifosfamide, l-asparaginase, and etoposide.
Response to pembrolizumab
A median of 7 (range, 2-13) cycles of pembrolizumab were administered. Objective response was observed in every patient. After a median follow-up of 6 (range, 2-10) months, 5 patients remained in CR.
CR with concordant clinical, radiologic, histologic, and molecular responses
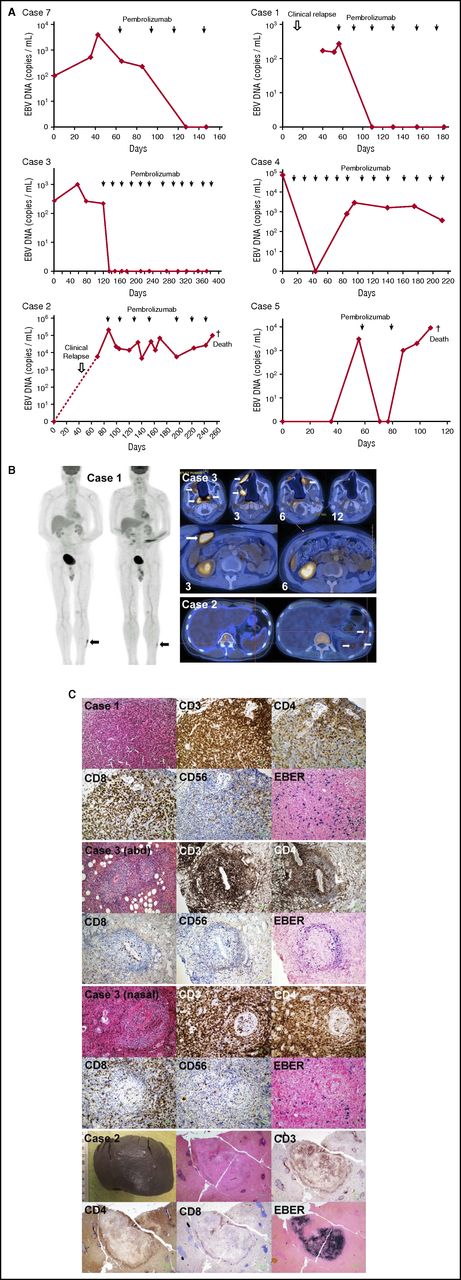
Patient 6 had biopsy-proven esophageal involvement and radiologic pulmonary infiltration. Pembrolizumab treatment led to immediate improvement, and PET/CT scan after 6 cycles showed metabolic CR. He has since received 6 more cycles. EBV DNA monitoring was not performed. Patient 7 relapsed after allo-HSCT, presenting with marrow involvement, cytopenia, and HPS. Symptoms and laboratory abnormalities resolved after the first cycle. EBV DNA fell from 3.9 × 103 copies/mL to undetectable after 2 cycles (Figure 1A). Marrow examination and PET/CT after 3 cycles confirmed CR. He has since received 5 cycles of treatment.
Treatment of 7 patients with relapsed/refractory NK/T-cell lymphomas treated with pembrolizumab. (A) Changes in circulating EBV DNA with pembrolizumab treatment. Case 7 relapsed after allogeneic HSCT. Undetectable EBV DNA correlated with CR on PET/CT scan and histologic examination of marrow after 3 cycles and onward. Case 1 had biopsy-proven cutaneous relapse 1.5 years after SMILE-induced CR. His skin lesions responded after the first cycle, paralleled by a decrease of EBV DNA to undetectable. After 4 cycles, with EBV DNA remaining undetectable, a biopsy of the index skin lesion still showed minimal EBER+ cells. He continued to remain asymptomatic with undetectable plasma EBV DNA after 2 more cycles. Case 3 had relapsed nasal lymphoma with marrow involvement and HPS. After the first cycle, symptoms and laboratory abnormalities resolved, with a concomitant fall of EBV DNA to undetectable. Despite repeated appearance of apparently new lesions on PET/CT in the nasal area and abdomen (abd) after 3, 6, and 9 cycles, his EBV DNA remained undetectable. He finally achieved metabolic CR on PET/CT after 12 cycles, demonstrating the prognostic importance of undetectable EBV DNA on disease remission. Case 4 presented with liver and marrow involvement, associated with HPS. After the first cycle, there was rapid improvement and resolution of HPS, paralleled by a fall of EBV DNA to undetectable after 4 cycles. After 6 cycles, PET/CT and marrow examination showed CR. EBV DNA then rose back to 102 to 103 copies/mL. He has to date received 13 cycles, remaining in clinical and radiologic remission. Case 2 relapsed with involvement of liver, spleen, and marrow and HPS 7 years after allogeneic HSCT. Symptoms and laboratory abnormalities resolved with initiation of treatment. After 3 cycles, EBER+ cells were cleared from the bone marrow. However, plasma EBV DNA remained detectable, heralding reappearance of EBER+ cells in the marrow when assessed after 3 more cycles. He then developed a chest infection and died as a result of sepsis. Case 5 presented with HPS on relapse. PET/CT showed hepatic involvement. After 1 cycle, symptoms and HPS resolved, with EBV DNA falling to undetectable. However, after the second cycle, there was an increase in EBV DNA. He then died as a result of sepsis consequent on gastrointestinal bleeding caused by gastric ulcers. (B) PET/CT results. Case 1: pretreatment scan showed a hypermetabolic lesion in left leg (left panel; arrow). Posttreatment scan showed that the lesion (arrow) was metabolically less active than the liver, hence qualifying for a Deauville score of 3. Case 3: evolution of nasal lesions in 4 consecutive scans (upper panel). Note complete destruction of all midline nasal structures. Leftmost was the PET/CT before pembrolizumab treatment, showing several hypermetabolic foci (arrows). After 3 cycles of pembrolizumab treatment, all pretreatment index lesions disappeared, but apparently new lesions appeared (arrows). After the sixth cycle of treatment, apparently new lesions developing after the third cycle had disappeared, but new lesions appeared again (arrow). After the 12th cycle, all hypermetabolic lesions had disappeared, and metabolic CR was achieved. Apparently new abdominal wall lesion, not present before treatment, appeared after 3 cycles of pembrolizumab therapy (lower panel; arrow). After the sixth cycle of pembrolizumab, the lesion completely disappeared. Case 2: pretreatment scan showed diffuse liver and spleen uptake. Posttreatment scan showed that uptake was confined to 3 small splenic foci (arrows). (C) Histopathologic evaluation of index lesions after pembrolizumab treatment. Histologic and immunophenotypic evaluations were performed by standard protocols. EBV early RNA (EBER) was detected by in situ hybridization. Case 1: left leg lesion. Histologic evaluation showed a lymphoid infiltrate without nuclear atypia. Immunohistochemical staining showed that the lymphoid infiltrate comprised predominantly CD3+ T cells, which were mainly CD4+ and CD8+. CD56+ cells and EBER+ cells constituted a minor proportion of cells. Case 3: abd lesion that appeared after the third cycle of pembrolizumab. Lymphoid cells without nuclear atypia appeared to be arranged in an angiocentric fashion. These were CD3+ T cells, which were predominantly CD4+, with a minor proportion being CD8+. CD56+ and EBER+ cells were scanty. Case 3: an apparently new nasal lesion that appeared after the third cycle of pembrolizumab. Histologic features similar to the abdominal lesion were observed. Infiltrating cells were CD3+, being predominantly CD4+ and with a minor proportion CD8+. CD56+ and EBER+ cells were scanty. Case 2: splenectomy specimen. Grossly, there were only a few small whitish nodules. Histologic examination of one of the nodules showed a lymphoid infiltrate that was CD3+. In the center of the lesion, the lymphoid cells were EBER+, and rimming the lesion were CD4+ and CD8+ cells. The rest of the spleen was not involved.
Treatment of 7 patients with relapsed/refractory NK/T-cell lymphomas treated with pembrolizumab. (A) Changes in circulating EBV DNA with pembrolizumab treatment. Case 7 relapsed after allogeneic HSCT. Undetectable EBV DNA correlated with CR on PET/CT scan and histologic examination of marrow after 3 cycles and onward. Case 1 had biopsy-proven cutaneous relapse 1.5 years after SMILE-induced CR. His skin lesions responded after the first cycle, paralleled by a decrease of EBV DNA to undetectable. After 4 cycles, with EBV DNA remaining undetectable, a biopsy of the index skin lesion still showed minimal EBER+ cells. He continued to remain asymptomatic with undetectable plasma EBV DNA after 2 more cycles. Case 3 had relapsed nasal lymphoma with marrow involvement and HPS. After the first cycle, symptoms and laboratory abnormalities resolved, with a concomitant fall of EBV DNA to undetectable. Despite repeated appearance of apparently new lesions on PET/CT in the nasal area and abdomen (abd) after 3, 6, and 9 cycles, his EBV DNA remained undetectable. He finally achieved metabolic CR on PET/CT after 12 cycles, demonstrating the prognostic importance of undetectable EBV DNA on disease remission. Case 4 presented with liver and marrow involvement, associated with HPS. After the first cycle, there was rapid improvement and resolution of HPS, paralleled by a fall of EBV DNA to undetectable after 4 cycles. After 6 cycles, PET/CT and marrow examination showed CR. EBV DNA then rose back to 102 to 103 copies/mL. He has to date received 13 cycles, remaining in clinical and radiologic remission. Case 2 relapsed with involvement of liver, spleen, and marrow and HPS 7 years after allogeneic HSCT. Symptoms and laboratory abnormalities resolved with initiation of treatment. After 3 cycles, EBER+ cells were cleared from the bone marrow. However, plasma EBV DNA remained detectable, heralding reappearance of EBER+ cells in the marrow when assessed after 3 more cycles. He then developed a chest infection and died as a result of sepsis. Case 5 presented with HPS on relapse. PET/CT showed hepatic involvement. After 1 cycle, symptoms and HPS resolved, with EBV DNA falling to undetectable. However, after the second cycle, there was an increase in EBV DNA. He then died as a result of sepsis consequent on gastrointestinal bleeding caused by gastric ulcers. (B) PET/CT results. Case 1: pretreatment scan showed a hypermetabolic lesion in left leg (left panel; arrow). Posttreatment scan showed that the lesion (arrow) was metabolically less active than the liver, hence qualifying for a Deauville score of 3. Case 3: evolution of nasal lesions in 4 consecutive scans (upper panel). Note complete destruction of all midline nasal structures. Leftmost was the PET/CT before pembrolizumab treatment, showing several hypermetabolic foci (arrows). After 3 cycles of pembrolizumab treatment, all pretreatment index lesions disappeared, but apparently new lesions appeared (arrows). After the sixth cycle of treatment, apparently new lesions developing after the third cycle had disappeared, but new lesions appeared again (arrow). After the 12th cycle, all hypermetabolic lesions had disappeared, and metabolic CR was achieved. Apparently new abdominal wall lesion, not present before treatment, appeared after 3 cycles of pembrolizumab therapy (lower panel; arrow). After the sixth cycle of pembrolizumab, the lesion completely disappeared. Case 2: pretreatment scan showed diffuse liver and spleen uptake. Posttreatment scan showed that uptake was confined to 3 small splenic foci (arrows). (C) Histopathologic evaluation of index lesions after pembrolizumab treatment. Histologic and immunophenotypic evaluations were performed by standard protocols. EBV early RNA (EBER) was detected by in situ hybridization. Case 1: left leg lesion. Histologic evaluation showed a lymphoid infiltrate without nuclear atypia. Immunohistochemical staining showed that the lymphoid infiltrate comprised predominantly CD3+ T cells, which were mainly CD4+ and CD8+. CD56+ cells and EBER+ cells constituted a minor proportion of cells. Case 3: abd lesion that appeared after the third cycle of pembrolizumab. Lymphoid cells without nuclear atypia appeared to be arranged in an angiocentric fashion. These were CD3+ T cells, which were predominantly CD4+, with a minor proportion being CD8+. CD56+ and EBER+ cells were scanty. Case 3: an apparently new nasal lesion that appeared after the third cycle of pembrolizumab. Histologic features similar to the abdominal lesion were observed. Infiltrating cells were CD3+, being predominantly CD4+ and with a minor proportion CD8+. CD56+ and EBER+ cells were scanty. Case 2: splenectomy specimen. Grossly, there were only a few small whitish nodules. Histologic examination of one of the nodules showed a lymphoid infiltrate that was CD3+. In the center of the lesion, the lymphoid cells were EBER+, and rimming the lesion were CD4+ and CD8+ cells. The rest of the spleen was not involved.
CR with discordant clinical, radiologic, histologic, and molecular responses
Patient 1 had biopsy-proven cutaneous relapse. Skin lesions responded after the first cycle, and EBV DNA became undetectable (Figure 1A). On PET/CT after 4 cycles, metabolic CR was achieved (Figure 1B). However, biopsy of the index lesion (scar-like by that time) showed scanty CD56+, EBER+ cells among CD3+CD8+ T cells (Figure 1C). He remained in clinical and molecular remission after 2 more cycles, with EBV DNA remaining undetectable. Patient 3 on relapse had complete destruction of hard palate and all midline nasal structures (Figure 1B), associated with marrow involvement and HPS. After the first cycle, HPS resolved, with gradual normalization of cell counts and liver biochemistry. EBV DNA fell from 2 × 102 copies/mL to undetectable (Figure 1A). On PET/CT scan after 3 cycles, preexisting nasal/nasopharyngeal lesions disappeared, but new lesions unexpectedly appeared in other areas (Figure 1B). A new lesion in the abdominal wall (Figure 1B) was biopsied, showing a predominance of CD3+CD4+ T cells with very scanty CD56+EBER+ cells (Figure 1C). Biopsy of an apparently new nasal lesion showed similar findings (Figure 1C). These lesions disappeared after 6 cycles, but newer nasal lesions appeared. The same phenomenon was seen after 9 cycles. After 12 cycles, FDG-avid lesions were no longer detected, and clinical, molecular, and metabolic CR was finally achieved. Patient 4 presented with involvement of liver and marrow and concomitant HPS. After the first cycle, symptoms and laboratory abnormalities resolved. EBV DNA fell from 6.9 × 104 copies/mL to undetectable after 4 cycles (Figure 1A). After 6 cycles, marrow examination and PET/CT confirmed CR. Afterward, EBV DNA slowly rose to 102 to 103 copies/mL. After 13 cycles of pembrolizumab, he was still in clinical and radiologic remission.
Partial response with discordant clinical, radiologic, pathologic, and molecular features
Patient 2 presented with marrow infiltration and HPS 7 years after allo-HSCT. Clinical and biochemical improvement were immediate after the first cycle. After 3 cycles, marrow was in morphologic remission. However, EBV DNA remained persistently detectable (Figure 1A). PET/CT showed 3 residual hypermetabolic splenic foci (Figure 1B), which on splenectomy showed EBER+ cells confined by CD4+ and CD8+ T cells (Figure 1C). After 3 more cycles, marrow showed reappearance of EBER+ cells. He then succumbed to a chest infection. Patient 5 presented with HPS. PET/CT showed diffuse hepatic involvement. After the first cycle, HPS resolved, with normalization of laboratory findings, and EBV DNA fell from 3 × 103 copies/mL to undetectable (Figure 1A). However, after a second cycle, there was an increase in EBV DNA. Its significance could not be assessed, because he died as a result of sepsis consequent on gastrointestinal bleeding caused by gastric ulcers.
Adverse events
Patient 7 (post–allo-HSCT) developed grade 2 rash, which on biopsy was consistent with acute graft-versus-host disease. There was a quick response to corticosteroid, and further pembrolizumab therapy was unhindered. Otherwise, no treatment-related adverse events were seen in other patients.
PDL1 expression on lymphoma cells
In 4 patients (cases 1-4), there was uniformly strong PDL1 expression (supplemental data). In case 5, weaker staining was found in ∼20% of cells. Data were not available for 2 patients (cases 6 and 7).
Responses after pembrolizumab treatment require clinical, radiologic, pathologic, and molecular assessments. Histologic evaluation might show residual EBER+ cells among infiltrating CD4+ and CD8+ T cells, appearing as FDG-avid lesions on PET/CT. With EBV DNA remaining undetectable, these lesions resolved after further treatment, consistent with pseudoprogression12-14 observed in solid tumors treated with immune checkpoint inhibitors. Similarly, in the presence of continued clinical remission, detectable EBV DNA was not necessarily associated with disease progression. This is different from chemotherapy treatment, where persistently positive EBV DNA portends a poor prognosis.15-18 Strong PDL1 expression correlated with excellent responses.13 The low-dose pembrolizumab regimen (2 mg/kg)7 was not associated with significant adverse events, even after allo-HSCT, consistent with safety of immune-checkpoint blockade after allo-HSCT.19-21
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Annie Pang for performing quantification of EBV DNA and Siok-Bian Ng for histopathologic evaluation of a patient.
The Singapore National Medical Research Council Translational and Clinical Research Flagship Programme provided funding for PDL1 staining.
The authors thank members of the Asia Lymphoma Study Group for constructive comments.
Authorship
Contribution: Y.-L.K. designed the study, treated the patients, and wrote and approved the manuscript; T.S.Y.C., D.T., S.J.K., L.-M.P., and B.M. treated the patients and approved the manuscript; P.-L.K. performed the PET/CT scans and approved the manuscript; F.L, R.A.-Y., and J.I. performed the histopathological examination and approved the manuscript; C.P. treated the patients and approved the manuscript; and E.T. designed the study, treated the patients, and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yok-Lam Kwong, Department of Medicine, Professorial Block, Queen Mary Hospital, Pokfulam Rd, Hong Kong, China; e-mail: ylkwong@hkucc.hku.hk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal