In this issue of Blood, Kwong et al provide strong evidence that immune checkpoints inhibitors are remarkably efficient in treatment of relapsing natural killer (NK)/T-cell lymphoma.1
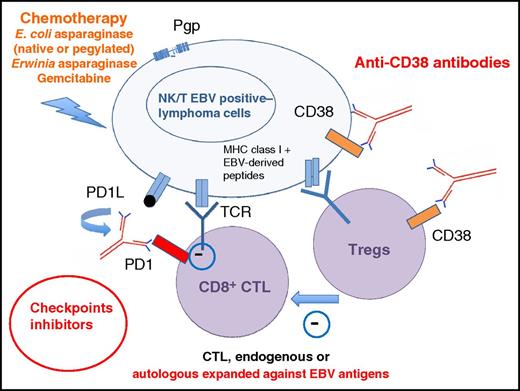
Multiapproach treatment of NK/T-cell lymphoma: l-asparaginase–based regimens (preferentially using pegylated forms or Erwinia asparaginase in patients with antibodies against Escherichia coli asparaginase and/or a low asparaginase activity), with Pgp-insensitive drugs, checkpoint inhibitors allowing cytotoxic T-cell–recognizing EBV antigens (EBNA1, LMP1, or LMP2), derived peptides to kill NK/T lymphoma cells (these T cells may be in vitro expanded against EBV antigens), and anti-CD38 antibodies able to kill NK/T lymphoma cells and to synergize with cytotoxic T cells (CTLs) by their action on regulatory T cells (Tregs). MHC, major histocompatibility complex; TCR, T-cell receptor.
Multiapproach treatment of NK/T-cell lymphoma: l-asparaginase–based regimens (preferentially using pegylated forms or Erwinia asparaginase in patients with antibodies against Escherichia coli asparaginase and/or a low asparaginase activity), with Pgp-insensitive drugs, checkpoint inhibitors allowing cytotoxic T-cell–recognizing EBV antigens (EBNA1, LMP1, or LMP2), derived peptides to kill NK/T lymphoma cells (these T cells may be in vitro expanded against EBV antigens), and anti-CD38 antibodies able to kill NK/T lymphoma cells and to synergize with cytotoxic T cells (CTLs) by their action on regulatory T cells (Tregs). MHC, major histocompatibility complex; TCR, T-cell receptor.
NK/T-cell lymphoma is a peculiar form of lymphoma frequent in Asian countries and very rare in the Western world that is characterized by a preponderant nasal localization (nasal type) and a constant association with Epstein-Barr virus (EBV) infection. Because of frequent expression of P-glycoprotein (Pgp), which confers a multidrug resistance phenotype, anthracycline-based regimens are not effective and are unable to cure either the localized form of NK/T-cell lymphoma without radiotherapy or the disseminated/relapsing form. The introduction of l-asparaginase in the treatment of this disease in the early 2000s has significantly improved the prognosis, formerly one of the worst among T-cell lymphomas. NK cells do not express asparagine synthase and, as a result, are particularly sensitive to asparagine starvation.
Case reports, retrospective studies, and prospective phase 2 trials have shown that asparaginase-based regimens may be highly and rapidly effective.2-4 Remarkably, about half of the patients with disseminated diseases as well as patients with systemic relapses could be cured even without consolidation with autologous or allogeneic stem cell transplantation. Patients with localized diseases already have a relatively good prognosis with radiotherapy alone. With the combination of asparaginase-based regimens, drugs insensitive to Pgp (eg, gemcitabine) and radiotherapy (eventually, along with concomitant chemotherapy using platinum derivatives), the survival curves for localized disease looks like those of Hodgkin disease, with more than 80% long-term survival.5 For patients relapsing after asparaginase-based regimens, which occurs in up to 50% of the patients with a disseminated disease, the prognosis remains poor, with a median survival of only 3 to 4 months. These relapses may occur because of anti-asparaginase antibodies that inhibit the drug.3 Some of these patients may respond to Erwinia asparaginase, another enzyme with asparaginase activity, but with no immune cross reactivity. Many different regimens, including those used in relapsing B- and T-cell lymphomas, have been tried without any clear success in these patients. Most studies have shown that allogeneic stem cell transplantation does not significantly improve the outcome, suggesting that a graft versus leukemia effect is not effective in this disease.
Taking advantage of EBV infection within tumor cells, 2 strategies have been tried to rescue relapsing patients. First, the combination of inhibitors of histone deacetylase (HDAC) to induce virus thymidine kinase and sensitization to antiviral drugs such as ganciclovir has been shown to be effective in some patients.6 HDAC should be used with extreme caution because of the high risk of tumor necrosis and toxicity resulting from virus reactivation.7 Second, autologous cytotoxic T cells expanded against EBV antigens have been tested, but the very frequent expression of programmed death ligand 1 (PDL1) and immunosuppressive cytokines by NK/T lymphoma cells could inhibit T-cell cytotoxicity.8,9 Indeed, in most cases, an immune deficiency is not observed in patients with NK/T-cell lymphoma, strongly suggesting that tumor cells have developed strategies to escape the strong immune response usually developed against EBV antigen–expressing cells. This expression of PDL1 by NK/T lymphoma cells is likely to be involved in this resistance, as seen in other EBV-associated neoplasia such as nasopharyngeal lesions, gastric carcinoma, and Hodgkin disease. This combination of frequent PDL1 expression and presence of foreign antigen expression on tumor cells makes the use of checkpoint inhibitors very attractive.
Kwong and colleagues, on behalf of the Asia Lymphoma Study Group, demonstrated in this small series of patients the effectiveness of pembrolizumab, an anti-PD1 antibody, in patients with NK/T-cell lymphoma. The 7 patients, previously treated using asparaginase-based regimens, all had advanced disease, with 5 patients having systemic hemophagocytic syndrome. All patients experienced a rapid response to pembrolizumab, with 5 complete responses (median follow-up, 6 months). Three of 4 patients with strong PDL1 expression achieved complete remission. Interestingly, residual lesions were biopsied after pembrolizumab treatment in 3 patients, and immunohistochemical staining showed that the infiltrating lymphoid cells were predominantly CD3+ T cells, with a mix of CD4+ and CD8+ and only a minority of CD56+ EBER+ cells. This finding is consistent with the hypothesis that pembrolizumab treatment allows T cells to recognize and kill EBV-infected NK/T lymphoma cells.
The outcome of NK/T-cell lymphoma patients with relapsing disseminated diseases after asparaginase-based regimens is dismal. The results of anti-inhibitory receptor programmed death 1 treatment in this very small series with short follow-up supports the use of this treatment in compassionate use programs. Clinical trials should be performed to extend the indications of checkpoint inhibitors in NK/T-cell lymphoma. However, it is still not known if these responses will be observed in all patients and what durations will be. As shown in this small group of patients, high expression of PDL1 might be a very strong predictor of response. This report, however, raises several questions. First, will we use immune checkpoint inhibitors in first-line treatment of NK/T-cell lymphoma? Second, will we be able in the near future to avoid radiotherapy and its deleterious side effects? Third, will we use combination therapy (see figure) to prevent relapses and selection of resistant cells? l-asparaginase, especially pegylated forms that are less immunogenic, will probably remain an important component to reduce tumor bulk without induction of a significant immunosuppressive effect. Combination with other immunotherapies such as anti-CD38 antibodies may present 2 advantages: that CD38 is expressed on NK cells and on a subtype of immunosuppressive regulatory T cells. A recent case report of a patient relapsing after bone marrow transplantation argues for this hypothesis.10 Hopefully, we are now approaching the time when we will see the vast majority of patients with this terrifying disease cured.
Conflict-of-interest disclosure: A.J. received honoraria from Jazz Pharmaceuticals and Janssen and research funding from Janssen. O.H. declares no competing financial interests.