In this issue of Blood, Bujisic and colleagues identify that the inositol-requiring enzyme 1 (IRE1)-X-box–binding protein 1 spliced (XBP1s) arm of the unfolded protein response (UPR) is repressed specifically in germinal center B-cell–like (GCB) diffuse large B-cell lymphoma (DLBCL) by the histone methyltransferase enhancer of zeste homolog 2 (EZH2) and that recovery of XBP1s expression, either directly or with EZH2 inhibitors, hinders lymphoma growth.1
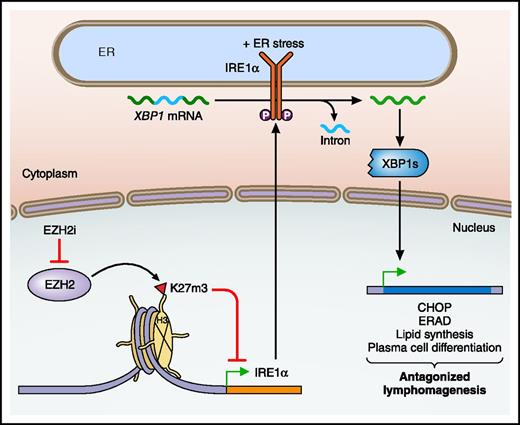
Regulation of the IRE1α branch of the UPR by EZH2 in GCB DLBCL. Within GCB DLBCL, the histone methyltransferase EZH2 trimethylates the tail of histone H3 at lysine 27 (H3K27m3), which represses the expression of IRE1α. The use of an EZH2 inhibitor (EZH2i) relieves the repression of IRE1α, which with ER stress converts XBP1 to its active, spliced form (XBP1s). XBP1s then initiates a transcriptional program, which in the GCB DLBCL context, antagonizes lymphomagenesis. ERAD, ER-associated degradation; P, phosphorylation. Professional illustration by Patrick Lane, ScEYEnce Studios.
Regulation of the IRE1α branch of the UPR by EZH2 in GCB DLBCL. Within GCB DLBCL, the histone methyltransferase EZH2 trimethylates the tail of histone H3 at lysine 27 (H3K27m3), which represses the expression of IRE1α. The use of an EZH2 inhibitor (EZH2i) relieves the repression of IRE1α, which with ER stress converts XBP1 to its active, spliced form (XBP1s). XBP1s then initiates a transcriptional program, which in the GCB DLBCL context, antagonizes lymphomagenesis. ERAD, ER-associated degradation; P, phosphorylation. Professional illustration by Patrick Lane, ScEYEnce Studios.
His eyebrow dark, and eye of fire, showed spirit proud, and prompt to ire—Walter Scott
With at least one third of all proteins translated in the cell improperly folded, the UPR is essential for cellular homeostasis.2 In cancer, the stress on the endoplasmic reticulum (ER) is greatly magnified due to both intrinsic stresses, such as increased proliferation, oncogene activation, and somatic mutations, and the extrinsic stresses of foreign environments, such as hypoxia, amino acid deprivation, and lactic acidosis. The UPR is initiated by three ER transmembrane proteins, which can sense misfolded proteins: IRE1α, protein kinase R–like ER kinase (PERK), and activating transcription factor 6α (ATF6).2 Whereas the initial goal of the UPR is to reestablish homeostasis by decreasing protein production and increasing protein-folding ability, the terminal UPR abandons the hope of repair and actively stimulates cell death.
IRE1α is a particularly interesting sentinel of ER stress, as it has both kinase and endoribonuclease functions. At low levels of kinase autophosphorylation, IRE1α’s endoribonuclease specifically excises an intron in the XBP1 messenger RNA (mRNA), leading to a functional XBP1s transcription factor that activates genes encoding proteins that help fold and transport proteins from the ER.2 At high levels of autophosphorylation, IRE1α’s endoribonuclease mediates regulated IRE1α-dependent decay (RIDD), a process by which many cargo- and protein-folding–encoding mRNAs at the ER membrane are degraded, which under certain conditions can exacerbate ER stress and lead to cell death.3 The duality of the UPR as both a survival and death-inducing pathway underlies the fact that in different contexts, UPR signaling can be either harmful or advantageous to cancer growth. One of the best examples is myeloma, where 50% of myeloma patients have high levels of XBP1s expression and mice with transgenic overexpression of XBP1s develop myeloma.4 However, treatment-refractory myelomas contain XBP1-inactivating mutations and knockdown of either IRE1α or XBP1 leads to bortezomib resistance.5
In this issue of Blood, Bujisic and colleagues reveal that activation of the IRE1α-XBP1s arm of the UPR is detrimental specifically to the growth of GCB type of DLBCL. Based on prior expression profiling data that revealed a selective enhancement of XBP1s target genes in activated B-cell (ABC) DLBCL,6 the authors interrogated the expression of various components of the UPR. Strikingly, they found that IRE1α mRNA expression is decreased in GCB DLBCL primary patient specimens and cell lines relative to normal germinal center B cells and ABC DLBCLs. The reduction of IRE1α and XBP1s protein levels in a panel of GCB DLBCL cell lines increased their susceptibility to ER stress. Cognizant of the frequent gain-of-function mutations in the histone H3K27 methyltransferase EZH2, which drives lymphomagenesis through gene repression,7 the authors queried if IRE1α might be an EZH2 target gene. Indeed, decreased IRE1 expression was seen not only in patients with mutant EZH2, but in patients with higher levels of EZH2 expression. Furthermore, the IRE1α promoter was enriched for histone 3 lysine 27 trimethylation (H3K27me3) marks in GCB compared with ABC DLBCL. Importantly, EZH2 inhibitors decreased the H3K27me3 marks in the IRE1α promoter, increased IRE1α and XBP1s levels, and killed GCB DLBCL cell lines in an IRE1α-dependent manner, suggesting that the IRE1α-XBP1s UPR pathway partially contributes to the antilymphoma activity of EZH2 inhibitors. Finally, inducible expression of XBP1s impaired the growth of a GCB, but not an ABC, DLBCL xenograft.
These novel findings suggest a new mechanism that links chromatin modifications with the UPR in the control of GCB DLBCL growth (see figure). Thus, the ER stress response pathway now joins proliferation checkpoints and plasma cell differentiation as a key target of EZH2-mediated repression. One caveat is that both the relative importance of the IRE1α-XBP1s pathway for EZH2 function and the mechanism of IRE1α-XBP1s’s antilymphoma activity are not completely clear. The modest inhibition that the authors observed upon overexpression of XBP1s in the GCB DLBCL xenograft model raises the possibility that additional IRE1α effector pathways, such as RIDD, may be contributory. Although both EZH2 inhibitors and XBP1s are known to induce plasma cell differentiation, there was no difference in the induction of plasma cell markers by XBP1s in the GCB and ABC DLBCL xenografts. In contrast, the upregulation of CHOP (C/EBP homologous protein) suggests activation of the terminal UPR and apoptosis specifically in the GCB DLBCL xenografts expressing XBP1s.
Bujisic and colleagues’ study has significant clinical implications for both therapeutic design and biomarkers, as EZH2 inhibitors have entered clinical trials.8 The fact that IRE1α levels correlated inversely with EZH2 levels irrespective of mutation status may explain why some patients with wild-type EZH2 appear to have responded in a phase 1 clinical trial. Moreover, if reactivation of IRE1α is the main target for EZH2 inhibitors, then ABC DLBCLs may not respond and GCB DLBCLs may be stratified by IRE1α expression. In addition, combining EZH2 inhibitors with ER stress agents and UPR inhibitors (currently under development) may be a fruitful approach. However, these combinations will need to be designed carefully to avoid the cytoprotective effects of the early UPR and to ensure activation of the terminal UPR with apoptosis through the BCL-2 family.9,10 Moreover, given the tumor-promoting role of XBP1s in plasma cell myeloma as opposed to this new tumor-antagonizing role uncovered in GCB DLBCL, careful consideration will be needed for the context-dependent activation of the UPR.
Several important future directions are raised by this study, including the importance of this pathway in normal development, during normal ER stress, and in other tumors with EZH2 mutation or IRE1α activity. In addition, what impact do other chromatin modifiers (eg, MLL2), which are mutated in DLBCL, have on the UPR? Nonetheless, as Walter Scott noted, the stimulation of IRE might be key in our proud fight against DLBCL.
Conflict-of-interest disclosure: S.G.K. is co-founder of Gene-in-Cell.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal