Abstract
For more than 50 years, investigators have considered a malignant stem cell as the potential origin of and a key therapeutic target for acute myeloid leukemia (AML) and other forms of cancer.1-4 The nature and existence of tumor-initiating cells for leukemia and other malignancies have long been the subject of intense and rigorous study; indeed, the promise of the potential to eradicate such cells is clear. However, until recently, deficiencies in our understanding of the nature of these cell populations, coupled with a limited ability to therapeutically exploit their weaknesses, have been limiting factors in realizing the goal of targeting leukemic stem cells (LSCs). Exciting new insights into the fundamental underpinnings of LSCs are now being made in an era in which drug development pipelines offer the potential to specifically target pathways of significance. Therefore, the focus in this new era, characterized by the confluence of understanding LSCs and the ability to target them, is shifting from “if it can be done” to “how it will be done.” Moving from a theoretical stage to this hopeful era of possibilities, new challenges expectedly arise, and our focus now must shift to determining the best strategy by which to target LSCs, with their well-documented heterogeneity and readily evident intra- and interpatient variability. The purpose of this review is therefore both to summarize the key scientific findings pertinent to AML LSC targeting and to consider methods of clinical evaluation that will be most effective for identifying successful LSC-directed therapies.
Assumptions derived by analogy to normal hematopoiesis
Normal hematopoietic development is driven by a well-characterized hierarchical process that originates from a multipotent stem cell. Because the developmental processes and biological characteristics of normal hematopoiesis have been extensively investigated, those findings provide a useful intellectual framework by which to approach the study of malignant hematopoiesis. However, we note that several early assumptions made in the characterization of leukemic stem cells (LSCs) are not as clear as originally expected. Central to most studies of LSCs are the following premises.
LSCs are quiescent
Numerous articles have described LSCs as quiescent, with the attractive speculation that this property is central to the drug resistance that characterizes primitive leukemic cells.2,5,6 However, the data on this issue do not always support this hypothesis. Early ex vivo analyses of cells with a CD34+/CD38− immunophenotype showed that these LSC-enriched populations were mostly in the quiescent phase of the cell cycle (G0).7 However, those experiments assumed that other phenotypes did not contain LSCs, a premise subsequently proven to be incorrect. In fact, more recent studies clearly demonstrate LSCs can also be found in subpopulations of more actively cycling cells.8-10 For example, cycling LSC populations, characterized by CD93 expression, have been shown in acute myeloid leukemia (AML) with an MLL gene rearrangement.11 Furthermore, immune-deficient mice bearing primary human AML xenografts have recently shown that the relative reduction of functionally defined LSCs can be similar to the reduction of bulk blasts upon challenge with cytarabine.12 This finding suggests that LSCs are not always refractory to chemotherapy that targets dividing cells, which in practice has been known for many years, based on the observation that core binding factor AML is particularly sensitive to high doses of cytarabine13 and can be cured with this approach.14 Taken together, the collective data indicate that in some cases LSCs share the normal hematopoietic stem cell (HSC) property of cell-cycle quiescence, but that in a variety of circumstances they may also display a more active cell-cycle profile. Thus, incorporation of cycle-active agents into anti-LSC regimens may have a role in certain situations.
LSCs are rare
Seminal studies in the 1990s first described the prevalence of LSCs in primary human AML specimens using limiting-dilution transplantation assays, reporting LSC frequencies varying over a 500-fold range (from 1 in 10 000 to 1 in 5 million).15,16 The difference in frequency between specimens was striking in these studies and suggested that interpatient variability in stem-cell characteristics could be substantial. Notably, the Bonnet et al15 study was performed with NOD-SCID mice, a relatively early type of immune-deficient strain, which retains significant innate immune function. Subsequent studies have shown that engraftment of primary AML specimens can be greatly enhanced by using strains with more profound immune deficiency, as well as strains engineered to express human cytokines.17,18 The use of these improved models has shown that LSC frequency is often higher than first observed in earlier studies and that the LSC phenotype can vary much more than originally appreciated.9 Further, recent studies by Ho et al10 have shown a dramatic increase in LSC frequency as a function of chemotherapy treatment and subsequent relapse. Using paired specimens from patients at diagnosis and following relapse after conventional chemotherapy, these studies showed a 10- to 100-fold increase in LSC frequency at relapse. Perhaps even more ominous, the cell-surface phenotype at relapse had fractured, with functionally defined LSCs detectable in populations that contained all permutations of CD34 and CD38 expression.10 These data indicate that the frequency of LSCs correlates with disease pathogenesis19 and emphasize the need to intervene with regimens that directly target LSCs at as early a stage as possible.
AML mimics the hierarchical developmental structure of normal hematopoiesis
Numerous articles have depicted AML development as a close analog to the normal hematopoietic hierarchy, suggesting a so-called fossil record that can explain the ancestry of AML stem cells, progenitor cells, and bulk tumor.3,5,20 Although this may be a useful conceptual framework, it also substantially oversimplifies the biology of AML evolution and has proven to be limited in several instances. Attempts to directly correlate the development of LSCs and normal hematopoiesis underappreciate the dynamic and highly plastic nature of AML biology as a function of pathogenesis. Thus, although chronic myeloid leukemia (CML), various myeloproliferative neoplasms, and even de novo AML may retain a relatively clear developmental hierarchy, upon disease progression or relapse after therapy, there can be dramatic loss of developmental structure. This observation is clearly documented by the temporal analysis of AML specimens during the course of therapy,10 as well as by studies examining the hierarchical structure of diseases like CML.21,22 The increased developmental instability observed in advanced stages of AML pathogenesis also likely contributes to the variable cell-cycle status and LSC frequency noted in “LSCs are quiescent” and “LSCs are rare.”
Taken together, we conclude that although analogies to normal hematopoiesis can be instructive, it is important to consider the highly dynamic and unstable nature of LSCs to develop improved therapies for AML that can be effective despite this challenge. Further, we note that all of the therapeutic strategies discussed in “LSC characteristics that may provide opportunities for therapeutic targeting” are directed toward fully transformed AML LSCs, and the degree to which such approaches may be useful for pre-LSC populations is largely unknown.
LSC characteristics that may provide opportunities for therapeutic targeting
In the following sections, we describe several strategies for targeting of AML LSCs. We note that such strategies fall into two broad categories: therapies that selectively eradicate LSCs (termed LSC specific) and therapies that eradicate both the bulk AML population as well as the LSC compartment (termed LSC active). Where known, these two classes are noted in the following descriptions.
Aberrant immunophenotypes
Reports in 199416 and 199715 first described immunophenotypic and biological properties of primary human AML stem cells. With a phenotype in hand, investigators were able to physically isolate LSC-enriched populations and perform a variety of studies. However, given the observation that the initially described cell-surface phenotype of CD34+/CD38− was identical to that of normal HSCs, and noting that the quiescent cell-cycle status of AML CD34+/CD38− cells was analogous to that of their normal HSC counterparts,7 investigators wondered whether LSCs might be so similar to HSCs that there would be no therapeutic index, making their specific eradication impossible.
The first clearly defined LSC-specific immunophenotypic property was expression of the interleukin-3 receptor α (CD123) antigen within the CD34+/CD38− compartment.23 Functional studies using immune-deficient mice as hosts for primary AML xenografts showed that all detectable engraftment potential resided in this CD34+/CD38−/CD123+ population. Furthermore, expression of CD123 in the normal stem-cell population was negligible. Subsequent studies using similar approaches have described multiple additional immunophenotypic differences that may distinguish LSCs from HSCs (Table 1). Each of these antigens is aberrantly upregulated in at least some LSCs from some patients, although the relative degree of expression varies. However, the discovery of these distinct LSC markers has allowed for three potentially important capabilities: (1) the ability to monitor LSC populations during the course of disease pathogenesis and therapy; (2) the ability to isolate enriched populations for experimental analyses; and (3) opportunities for therapeutic intervention using antibody-based targeting strategies.
Strategies under investigation for targeting LSCs
| LSC antigen . | Description . | Antibody, if applicable . | Active and recruiting trials . |
|---|---|---|---|
| CD123 | IL-3 receptor α chain23,24 | SGN-123A, antibody–drug conjugate | NCT02848248 |
| JNJ-56022473, dual-specific antibody with CD16 | NCT02472145 | ||
| SL-401, antibody toxin chimeric protein using diphtheria toxin | NCT02113982 | ||
| NCT02270463 | |||
| XmAb14045, dual-specific antibody with CD3 | NCT02730312 | ||
| MGD006, dual-specific antibody with CD3 | NCT02152956 | ||
| KHK2823, IgG1 antibody | NCT02181699 | ||
| CLL-1 | C-type lectin-like molecule-125 | ||
| CD33 | Receptor of myeloid cells, member of the Ig superfamily26 | SGN-CD33A, antibody–drug conjugate | NCT02326584 |
| NCT02785900 | |||
| NCT02312037 | |||
| NCT02614560 | |||
| IMGN779, antibody drug conjugate | NCT02674763 | ||
| Gemtuzumab ozogamicin, antibody–drug conjugate | NCT01869803 | ||
| CD44 | Receptor for hyaluronic acid27,28 | ||
| CD47 | Receptor for signal regulatory protein α (SIRPα)29,30 | Hu5F9-G4, anti-CD47 monoclonal antibody | NCT02678338 |
| TTI-621, antibody against CD47 binding domain of SIRPα | NCT02663518 | ||
| INBRX-103 (CC-90002), anti-CD47 monoclonal antibody | NCT02367196 | ||
| NCT02641002 | |||
| CD96 | Tactile, member of Ig superfamily31 | ||
| CD93 | Marker of a nonquiescent LSC population in MLL-rearranged AML11 | ||
| CD25 | IL-2 receptor α chain32 | ADCT-301, antibody–drug conjugate | NCT02588092 |
| LMB-2, antibody toxin chimeric protein using Pseudomonas exotoxin | N/A for AML | ||
| CD32 | Fc-γ receptor II32 | ||
| Tim-3 | T-cell immunoglobulin mucin-333,34 | TSR-022, monoclonal antibody | N/A for AML |
| CD99 | MIC2 or single-chain type-1 glycoprotein (in press) | ||
| IL1-RAP | IL-1 receptor accessory protein35,36 | IL-1 receptor antagonist | N/A for AML |
| LSC antigen . | Description . | Antibody, if applicable . | Active and recruiting trials . |
|---|---|---|---|
| CD123 | IL-3 receptor α chain23,24 | SGN-123A, antibody–drug conjugate | NCT02848248 |
| JNJ-56022473, dual-specific antibody with CD16 | NCT02472145 | ||
| SL-401, antibody toxin chimeric protein using diphtheria toxin | NCT02113982 | ||
| NCT02270463 | |||
| XmAb14045, dual-specific antibody with CD3 | NCT02730312 | ||
| MGD006, dual-specific antibody with CD3 | NCT02152956 | ||
| KHK2823, IgG1 antibody | NCT02181699 | ||
| CLL-1 | C-type lectin-like molecule-125 | ||
| CD33 | Receptor of myeloid cells, member of the Ig superfamily26 | SGN-CD33A, antibody–drug conjugate | NCT02326584 |
| NCT02785900 | |||
| NCT02312037 | |||
| NCT02614560 | |||
| IMGN779, antibody drug conjugate | NCT02674763 | ||
| Gemtuzumab ozogamicin, antibody–drug conjugate | NCT01869803 | ||
| CD44 | Receptor for hyaluronic acid27,28 | ||
| CD47 | Receptor for signal regulatory protein α (SIRPα)29,30 | Hu5F9-G4, anti-CD47 monoclonal antibody | NCT02678338 |
| TTI-621, antibody against CD47 binding domain of SIRPα | NCT02663518 | ||
| INBRX-103 (CC-90002), anti-CD47 monoclonal antibody | NCT02367196 | ||
| NCT02641002 | |||
| CD96 | Tactile, member of Ig superfamily31 | ||
| CD93 | Marker of a nonquiescent LSC population in MLL-rearranged AML11 | ||
| CD25 | IL-2 receptor α chain32 | ADCT-301, antibody–drug conjugate | NCT02588092 |
| LMB-2, antibody toxin chimeric protein using Pseudomonas exotoxin | N/A for AML | ||
| CD32 | Fc-γ receptor II32 | ||
| Tim-3 | T-cell immunoglobulin mucin-333,34 | TSR-022, monoclonal antibody | N/A for AML |
| CD99 | MIC2 or single-chain type-1 glycoprotein (in press) | ||
| IL1-RAP | IL-1 receptor accessory protein35,36 | IL-1 receptor antagonist | N/A for AML |
Ig, immunoglobulin; IL, interleukin; N/A, not applicable.
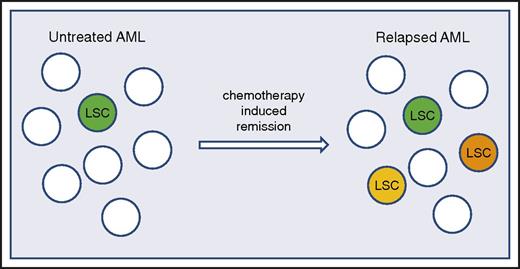
Although all of these capabilities have been pursued, the key caveat to such approaches is the relative fidelity of any specific marker. As noted, the cell-surface phenotype of LSCs can vary from patient to patient, or potentially even more challenging, distinct LSC populations with varying phenotypes can be detected within the same patient.8,9 Finally, in an extremely concerning response to chemotherapy, it has recently been reported that LSCs can diverge and evolve, with acquisition of new and different phenotypes upon relapse (Figure 1).10 Therefore, although pursuing antigenic targets to eradicate LSCs is an active area of research, the efficacy of this approach remains to be determined and, like many strategies, may be limited by the relative plasticity of LSC phenotypes.
LSC frequency and heterogeneity increase after unsuccessful treatment of AML. In de novo AML, the LSC population is relatively uniform and present at a low frequency (LSC represented by green colored cell). Upon treatment with chemotherapy, when patients attain a remission and subsequently relapse, the relapse is characterized by increased numbers of LSCs and a more heterogeneous LSC population (indicated by green, orange, and yellow colored cells).10
LSC frequency and heterogeneity increase after unsuccessful treatment of AML. In de novo AML, the LSC population is relatively uniform and present at a low frequency (LSC represented by green colored cell). Upon treatment with chemotherapy, when patients attain a remission and subsequently relapse, the relapse is characterized by increased numbers of LSCs and a more heterogeneous LSC population (indicated by green, orange, and yellow colored cells).10
There are several antibody-based strategies under investigation for targeting LSCs (Table 1). One approach involves linking antibodies to various toxins to create a targeted delivery vehicle. Gemtuzumab ozogamicin (Mylotarg; Pfizer), which utilizes an anti-CD33 antibody conjugated to the antitumor antibiotic calicheamicin, and vadastuximab talirine (SGN-33A; Seattle Genetics), which conjugates the DNA binding agent pyrrolobenzodiazepine to CD33, employ this approach. Although CD33 expression on LSCs shows significant variability,26,37,38 gemtuzumab ozogamicin demonstrated activity for some patients, particularly when combined with chemotherapy agents.39 Similar antibody–drug conjugate efforts are currently under way using anti-CD12340 and other antibodies.
Strategies that use the immunological properties of antibodies to enhance antibody-dependent cell-mediated toxicity are also being pursued. Bispecific CD3341 and CD12342 antibodies linked to CD3 are being developed, as is a CD123 antibody linked to CD16,43 meant to deliver cells expressing these targets into the vicinity of T cells or natural killer cells, respectively. Another novel approach involves targeting the CD47 antigen, preventing it from interacting with its cognate macrophage receptor SIRP1-α; preclinical studies have shown this disruption can lead to the activation of innate immunity and macrophage-mediated destruction of LSCs.30,44 Finally, the use of CD123 or other antigens as targets for newly developed chimeric antigen receptor mediated T-cell–based approaches is also an exciting opportunity that has shown promise in preclinical studies and in which clinical investigations are ongoing.45,46
LSC metabolic properties
Aside from immunophenotype, LSCs can also be distinguished from normal tissues by virtue of their distinct metabolism and response to various drugs (Table 2). For example, several lines of investigation point toward an increased reliance on cellular mechanisms related to control of oxidative state and management of cellular stress (eg, heat shock protein [Hsp90] and unfolded protein responses). A recent report showed that AML cells rely on a tumor-specific heat shock protein species (teHsp90) that is selectively activated under conditions of cell stress and signalosome activity.47 Additionally, a central component of stress response pathways, activation of NF-κB, is evident in LSCs but not in normal resting HSCs.7,48 Challenge of LSCs with agents that inhibit NF-κB, proteasome activity, HSP function, and glutathione balance has been demonstrated to selectively target LSCs and progenitor cells in comparison with normal HSC controls.49-51
Distinct metabolism of and response to drugs by LSCs
| Metabolic target . | Description . | Therapy, if applicable . | Active and recruiting trials . |
|---|---|---|---|
| Hsp90 | Heat shock protein; protective under cell stress conditions47 | AT13387 | N/A for AML |
| AUY922 (luminespib) | |||
| BIIB028 | |||
| STA-9090 (ganetespib) | |||
| MPC-3100 | |||
| IPI-493 | |||
| SNX-5422 | |||
| NF-κB | Nuclear factor κB; regulates stress response pathways7,48 | Bortezomib | NCT01736943 |
| NCT01534260 | |||
| Choline magnesium trisalicylate | N/A for AML | ||
| Pentoxifylline | N/A for AML | ||
| Glutathione | Redox balance regulator51 | Buthionine sulfoximine | N/A for AML |
| CB-839 | NCT02071927 | ||
| Bcl-2 | B-cell lymphoma 2; regulator of mitochondrial activity and antiapoptotic factor52 | Venetoclax | NCT02670044 |
| NCT02203773 | |||
| S 055746 | NCT02920541 | ||
| Oblimersen | N/A for AML | ||
| IDH1/2 | Isocitrate dehydrogenase; susceptible to perturbations in oxidative phosphorlyation53 | AG221 (IDH2 inhibitor) | NCT02632708 |
| NCT02677922 | |||
| NCT02577406 | |||
| AG120 (IDH1 inhibitor) | NCT02632708 | ||
| NCT02074839 | |||
| AG881 (dual IDH1 and IDH2 inhibitor) | NCT02492737 | ||
| IDH305 (IDH1 inhibitor) | NCT02826642 | ||
| NCT02381886 | |||
| FT-2102 (IDH1 inhibitor) | NCT02719574 | ||
| ALOX-5 | Arachidonate 5-lipoxygenase; potential LSC regulator54 | VIA-2291 | N/A for AML |
| PF-04191834 | |||
| GSK2190915 |
| Metabolic target . | Description . | Therapy, if applicable . | Active and recruiting trials . |
|---|---|---|---|
| Hsp90 | Heat shock protein; protective under cell stress conditions47 | AT13387 | N/A for AML |
| AUY922 (luminespib) | |||
| BIIB028 | |||
| STA-9090 (ganetespib) | |||
| MPC-3100 | |||
| IPI-493 | |||
| SNX-5422 | |||
| NF-κB | Nuclear factor κB; regulates stress response pathways7,48 | Bortezomib | NCT01736943 |
| NCT01534260 | |||
| Choline magnesium trisalicylate | N/A for AML | ||
| Pentoxifylline | N/A for AML | ||
| Glutathione | Redox balance regulator51 | Buthionine sulfoximine | N/A for AML |
| CB-839 | NCT02071927 | ||
| Bcl-2 | B-cell lymphoma 2; regulator of mitochondrial activity and antiapoptotic factor52 | Venetoclax | NCT02670044 |
| NCT02203773 | |||
| S 055746 | NCT02920541 | ||
| Oblimersen | N/A for AML | ||
| IDH1/2 | Isocitrate dehydrogenase; susceptible to perturbations in oxidative phosphorlyation53 | AG221 (IDH2 inhibitor) | NCT02632708 |
| NCT02677922 | |||
| NCT02577406 | |||
| AG120 (IDH1 inhibitor) | NCT02632708 | ||
| NCT02074839 | |||
| AG881 (dual IDH1 and IDH2 inhibitor) | NCT02492737 | ||
| IDH305 (IDH1 inhibitor) | NCT02826642 | ||
| NCT02381886 | |||
| FT-2102 (IDH1 inhibitor) | NCT02719574 | ||
| ALOX-5 | Arachidonate 5-lipoxygenase; potential LSC regulator54 | VIA-2291 | N/A for AML |
| PF-04191834 | |||
| GSK2190915 |
N/A, not applicable.
Strategies of targeting LSCs that modulate mitochondrial activity have also been described. Lagadinou et al52 reported that LSCs appeared to preferentially rely on oxidative phosphorylation and that pharmacological or genetic disruption of mitochondrial activity (via inhibition of B-cell lymphoma 2 [Bcl-2]) resulted in LSC-specific targeting.52 Notably, subsequent work demonstrated that patients with AML with IDH1 or IDH2 mutations are particularly sensitive to Bcl-2 inhibition and that the mechanism involved 2-hydroxyglutarate–mediated suppression of cytochrome c oxidase activity in the electron transport chain, further indicating a specific reliance on oxidative phosphorylation in LSCs.53 Subsequent studies of cancer stem-cell populations in melanoma and pancreatic cancer have indicated an analogous reliance on oxidative phosphorylation, suggesting that this characteristic may be a feature of multiple forms of cancer.55,56 Finally, unique features of mitochondrial biology distinguish LSCs, as reported by Skrtic et al,57 who demonstrated that inhibition of the mitochondrial translation machinery results in inhibition of human LSCs. Taken together, these findings suggest that central aspects of energy metabolism may represent opportunities for therapeutic interventions targeting LSCs. Because this approach relies on modulating fundamental aspects of cell biology, it also may represent a relatively comprehensive type of therapy that will be broadly active despite the intrapatient heterogeneity previously documented for human LSC populations.10
Other intriguing metabolic targets that tie closely to the early origins of leukemia as defined by the evolution of common mutations are the isocitrate dehydrogenase enzymes (IDH1 and IDH2). Mutations of IDH genes result in metabolic dysregulation through the massive overproduction of the oncometabolite 2-hydroxyglutarate, which subsequently induces epigenetic changes that favor the leukemic phenotype.58 Notably, mutations in IDH1/2 occur early in AML pathogenesis and are nearly always present at relapse, suggesting this gene represents a stable driver of stem-cell aberrancy.59-61 Ongoing studies with recently developed small-molecule inhibitors of IDH1 and IDH2 will determine their relative ability to target LSC populations.
LSCs that underlie CML are also susceptible to modulation of metabolism, and it may be possible to extrapolate these findings to AML. For example, the arachidonate 5-lipoxygenase gene (Alox5) was reported to be a critical regulator for LSCs in CML,54 and use of the Bcl-2 inhibitor venetoclax (ABT-199; AbbVie) was recently reported as a potent strategy for targeting CML stem cells in combination with dasatinib.62
Taken together, multiple lines of investigation support metabolic targeting as a potentially important means by which LSCs can be either directly targeted or at least sensitized to other agents. In some cases, fundamental metabolic activities such as oxidative phosphorylation appear to be preferentially required by LSCs in comparison with HSCs. Notably, such sensitivities may transcend specific mutational profiles, suggesting relatively broad applicability in leukemias arising via differing pathogenic processes.
Distinct epigenetic properties of LSCs
Given the prevalence of driver mutations in genes associated with epigenetic regulation, it is not surprising that relatively recent studies have identified several potential approaches to targeting LSCs based on epigenetic modulators (Table 3). For example, studies by Bernt et al63 in AMLs with MLL gene rearrangements have shown that the H3K79 methyltransferase DOT1L is a critical regulator of the transformed state. Both genetic and pharmacological inhibition of DOT1L impair LSCs, and notably, inhibition of DOT1L appears to be surprisingly leukemia selective, with little impairment of normal hematopoiesis. These and other findings have led to the development of the clinical-grade DOT1L inhibitor pinometostat (EPZ-5676; Epizyme) and associated early-phase clinical trials (NCT01684150 and NCT02141828). Based on preclinical models, these drugs are predicted to be LSC active. Similarly, the lysine-specific demethylase KDM1A has also been shown to be an important factor for murine LSCs derived from MLL-AF9 transformation,64 and its inhibition sensitizes primary human AML cells to treatment with all-trans-retinoic acid.65 Yet another example recently showed that treatment of MLL-AF9 murine leukemia with a bromodomain and extra terminal protein inhibitor resulted in the emergence of resistance to LSCs.66 This finding implies that the activity of such drugs is sufficiently LSC active to create selective pressure on the LSC compartment. Finally, targeting of epigenetic regulators has also recently shown efficacy toward CML stem cells, where intrinsic dysregulation of polycomb repressive complex 2 results in CML-specific sensitivity to EZH2 inhibition.67
Potential approaches to targeting LSCs based on epigenetic modulators
| Epigenetic target . | Description . | Therapy, if applicable . | Active and recruiting trials . |
|---|---|---|---|
| DOT1L | H3K79 methyltransferase; pathogenic in MLL-rearranged AML63 | Pinometostat | N/A for AML |
| LSD1 | Lysine-specific demethylase64,65 | IMG-7289 | NCT02842827 |
| GSK2879552 | NCT02177812 | ||
| Tranylcypromine | NCT02273102 | ||
| NCT02261779 | |||
| INCB059872 | NCT02712905 | ||
| BET | Bromodomain and extra terminal protien66 | CPI-0610 | NCT02158858 |
| FT-1101 | NCT02543879 | ||
| OTX015 | N/A for AML | ||
| TEN-010 | NCT02308761 | ||
| MK-8628 | NCT02698189 | ||
| BAY1238097 | N/A for AML | ||
| GSK525762 | NCT01943851 | ||
| INCB054329 | NCT02431260 | ||
| INCB057643 | NCT02711137 | ||
| EZH2 | Enhancer of zeste 2 polycomb repressive complex 2 subunit67 | Tazemetostat | N/A for AML |
| CPI-1205 | N/A for AML | ||
| GSK2816126 | N/A for AML | ||
| MAK683 | N/A for AML |
| Epigenetic target . | Description . | Therapy, if applicable . | Active and recruiting trials . |
|---|---|---|---|
| DOT1L | H3K79 methyltransferase; pathogenic in MLL-rearranged AML63 | Pinometostat | N/A for AML |
| LSD1 | Lysine-specific demethylase64,65 | IMG-7289 | NCT02842827 |
| GSK2879552 | NCT02177812 | ||
| Tranylcypromine | NCT02273102 | ||
| NCT02261779 | |||
| INCB059872 | NCT02712905 | ||
| BET | Bromodomain and extra terminal protien66 | CPI-0610 | NCT02158858 |
| FT-1101 | NCT02543879 | ||
| OTX015 | N/A for AML | ||
| TEN-010 | NCT02308761 | ||
| MK-8628 | NCT02698189 | ||
| BAY1238097 | N/A for AML | ||
| GSK525762 | NCT01943851 | ||
| INCB054329 | NCT02431260 | ||
| INCB057643 | NCT02711137 | ||
| EZH2 | Enhancer of zeste 2 polycomb repressive complex 2 subunit67 | Tazemetostat | N/A for AML |
| CPI-1205 | N/A for AML | ||
| GSK2816126 | N/A for AML | ||
| MAK683 | N/A for AML |
N/A, not applicable.
Collectively, there are significant data to support LSC-targeting strategies based on epigenetic modulation, particularly for leukemias arising from MLL translocation. Mechanistically, these approaches often appear to restore some degree of differentiation capability to primitive cells, thereby reducing self-renewal potential and/or increasing their sensitivity to other agents. Thus, building epigenetic modulators into more conventional regimens appears to be a promising direction for future therapies.
Microenvironmental interactions as potential targets for intervention
As with many forms of cancer, strategies that seek to disrupt supportive microenvironmental components of the malignant stem-cell niche are appealing potential approaches to targeting LSCs. The first clear evidence supporting this strategy for AML was the 2006 report by Jin et al,27 in which antagonist antibodies against the CD44 antigen were reported to strongly suppress the in vivo growth of human LSCs. Notably, a simultaneous report by Krause et al28 showed a role for CD44 in homing and engraftment of CML stem cells, suggesting that both acute and chronic forms of leukemia may use CD44 for adhesive interactions within the marrow niche. However, subsequent studies have suggested that CML and AML niches differ and that modulation of niche components to suppress LSC growth may require strategies specific to the disease and stage of pathogenesis.68
It has been reported that chemotherapy-resistant AML LSCs home to the endosteal region of the bone marrow,69 a characteristic frequently described for normal HSCs.70 LSCs have also been reported to reside in adipose tissue, where local microenvironmental factors drive altered metabolism, leading to chemotherapy resistance.71 Intriguingly, mechanisms that modulate LSC metabolism may go beyond secreted factors and cell–cell interactions. A recent study indicates that the physical transfer of mitochondria from mesenchymal cells can influence leukemic cells, making them more resistant to chemotherapy, a phenomenon also described for ovarian cancer cells.72,73 Collectively, these findings indicate that modulation of niche interactions may serve to increase the sensitivity of LSCs to therapeutic intervention.
Finally, there is also evidence that a proinflammatory state can influence LSC growth and survival. An intriguing study by Kagoya et al48 has recently shown that autocrine secretion of tumor necrosis factor-α drives the constitutive activation of NF-κB, a property of LSCs that is presumably associated with their intrinsic biology and malignant transformation. Further, a number of mutations associated with myeloid malignancy and stem-cell transformation support a proinflammatory milieu that likely favors growth of LSCs.74 Conversely, chronic inflammation has been shown to degrade the potential of normal HSCs.75,76 Hence, inhibition of proinflammatory factors may serve the dual function of inhibiting LSC activity and creating an environment more favorable for normal stem cells. This type of intervention could be particularly interesting in the context of postchemotherapy treatment, where the need for suppression of residual disease and promotion of normal cell regeneration is perhaps most acute.
Clinical development of LSC-directed therapies
As LSC-targeting therapies become available for clinical evaluation, among the most crucial considerations will be the timing of the therapy. It was once assumed that LSCs, the fundamental origin of AML, remained static through the entire disease course, which would suggest that an LSC-directed therapy should be effective no matter when it is employed. Recent data now suggest that LSCs, when exposed to chemotherapy that is not curative, evolve with substantial heterogeneity and complexity compared with their newly diagnosed, pretreatment predecessors (Figure 1).10 These findings indicate it is critical to intervene at the earliest possible stage of pathogenesis and that the ideal time to employ an LSC-directed therapy is at the time of diagnosis, before or concurrent with conventional therapies, when the LSC population is most vulnerable (ie, minimally complex and at its lowest prevalence10 ).
A real-world illustration of this concept comes from an analysis of early-phase clinical trials involving the Bcl-2 inhibitor venetoclax. Bcl-2 is overexpressed in LSCs but not HSCs,52 making it an attractive LSC-directed target. When used as a single agent in relapsed and refractory AMLs, venetoclax resulted in a complete remission (CR) or CR with incomplete count recovery rate of 19%.77 Subsequently, when administered in combination with azacitidine, decitabine, or low-dose cytarabine to previously untreated patients, this rate was 60% to 70%.78,79 Although it is possible that synergistic interactions with a backbone therapy accounted for this improvement in response, another possible explanation for the difference in outcome in the relapsed versus upfront treatment setting for this LSC-directed therapy is that LSCs were more amenable to eradication before the selective pressure of exposure to chemotherapy.
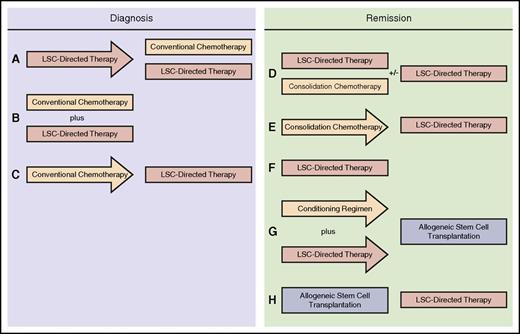
In the initial phases of using LSC-directed therapies in the setting of clinical trials, it would not be ethical to withhold conventional therapy for a newly diagnosed patient. Furthermore, given variations between the phenotype of LSCs and that of the descendent bulk population, LSC-directed therapies may be ineffective against the bulk disease, allowing a patient who is only administered an LSC-directed therapy to be overcome by the proliferative bulk disease. Therefore, at least in the near future, LSC-directed therapies will need to coexist with conventional treatments. In the interests of targeting LSCs before chemotherapy-induced evolution, clinical trials involving LSC-directed therapies should be written to administer the LSC-directed therapy either before or concurrent with chemotherapy (Figure 2A-B). The former scenario is appealing in the design of early-phase studies because it affords an opportunity, with well-designed protocol-mandated bone marrow aspirations, to study the impact of the LSC-directed therapy independent of the impact of chemotherapy on the LSC population, as well as to understand the adverse event profile of a drug that may be employed in a first-in-human setting. Designs in which chemotherapy precedes the LSC-directed therapy (Figure 2C) are less appealing, because chemotherapy has been shown to result in increased complexity of the LSC population,10 potentially mitigating the efficacy of an LSC-directed approach.
Potential clinical trial designs of LSC-directed therapies, administered both at diagnosis and in remission. (A) The optimal approach for use of an LSC-directed therapy at diagnosis, in which the LSC-directed therapy is administered first as a single agent, followed by conventional chemotherapy, usually with continuous concomitant use of the LSC-directed therapy. This scenario allows for the independent study of the impact of the LSC-directed therapy. (B) Concurrent administration of LSC-directed therapy and conventional chemotherapy during induction; this is feasible if the action of the drugs involved is not antagonistic or associated with significant overlapping toxicity. (C) LSC-directed therapies can be used after conventional therapy. The concern with this approach is that instituting conventional chemotherapy first may result in the development of a more complex, heterogeneous LSC population that will be less amenable to treatment with the LSC-directed therapy. During standard consolidation, LSC-directed therapies can be paired with conventional consolidation therapy, with the option of maintaining the LSC-directed therapy for a more extended period (D) or administering in sequence with consolidation chemotherapy (E). (F) LSC-directed therapies may also be administered as maintenance therapy, in the absence of chemotherapy, in some situations in which a patient who has achieved a remission may not be a candidate for consolidation chemotherapy. (G) LSC-directed therapies may be employed in conditioning regimens before allogeneic stem-cell transplantation. (H) LSC-directed therapies can be used in the posttransplantation setting, which is particularly appealing for those patients with risk factors for relapse after transplantation.
Potential clinical trial designs of LSC-directed therapies, administered both at diagnosis and in remission. (A) The optimal approach for use of an LSC-directed therapy at diagnosis, in which the LSC-directed therapy is administered first as a single agent, followed by conventional chemotherapy, usually with continuous concomitant use of the LSC-directed therapy. This scenario allows for the independent study of the impact of the LSC-directed therapy. (B) Concurrent administration of LSC-directed therapy and conventional chemotherapy during induction; this is feasible if the action of the drugs involved is not antagonistic or associated with significant overlapping toxicity. (C) LSC-directed therapies can be used after conventional therapy. The concern with this approach is that instituting conventional chemotherapy first may result in the development of a more complex, heterogeneous LSC population that will be less amenable to treatment with the LSC-directed therapy. During standard consolidation, LSC-directed therapies can be paired with conventional consolidation therapy, with the option of maintaining the LSC-directed therapy for a more extended period (D) or administering in sequence with consolidation chemotherapy (E). (F) LSC-directed therapies may also be administered as maintenance therapy, in the absence of chemotherapy, in some situations in which a patient who has achieved a remission may not be a candidate for consolidation chemotherapy. (G) LSC-directed therapies may be employed in conditioning regimens before allogeneic stem-cell transplantation. (H) LSC-directed therapies can be used in the posttransplantation setting, which is particularly appealing for those patients with risk factors for relapse after transplantation.
As outlined, the least optimal scenario in which to determine clinical efficacy of LSC eradication is in the floridly relapsed setting. A more promising approach would be to administer LSC-directed therapies after treatment has been administered for patients who achieve a morphological remission. This could involve a number of different scenarios, including pairing LSC-directed therapies with chemotherapy consolidation regimens (Figure 2D-E), using them alone as maintenance strategies (Figure 2F), incorporating them into conditioning regimens for allogeneic stem-cell transplantation (Figure 2G), or using them in the post–stem-cell transplantation period as maintenance therapy for patients at high risk for relapse (Figure 2H). This would be a particularly exciting strategy, because with access to effective LSC-directed therapies, the focus could shift from waiting for relapse to occur, when these treatments would be less likely to work, to making concerted efforts to prevent relapse from happening at all. New and exciting methods to detect minimal residual disease,80 particularly from accessible sources such as peripheral blood,81 provide a rationale for such an intervention with an LSC-targeting therapy before morphologic relapse and during a period in which the LSC population may still be sufficiently homogenous or few in number to respond.
When designing clinical trials using purported LSC-directed therapies, priority should be given to proving in vivo that the drugs are in fact hitting their targets. We have previously proposed doing this by collecting pre- and posttreatment bone marrow aspirates and at a later date performing limiting-dilution experiments, in which tumor cells are transplanted into xenograft models at decreasing dilutions.82 This would allow for quantification of the ability of a novel therapy to target LSCs and provide an opportunity to make inferences as to whether this is the mechanism of action in responding patients.
We believe it is possible that studying purported LSC-directed therapies in the clinic will require reassessments of how responses are defined. In the clinical study of hematological malignancies, there is precedent for the unique mechanism of novel therapies to result in changes in response assessments, such as widespread adoption of the use of CR without recovery of platelets for gemtuzumab ozogamicin in AML83 or partial response with lymphocytosis for ibrutinib in chronic lymphocytic leukemia.84 Similarly, we believe allowances for new end points of importance in clinical trials evaluating LSC-directed therapies will likely be required. In this instance, it may become important to recognize that CR, or response rates in general, is a poor surrogate for efficacy when analyzing LSC-directed therapies.
An example is provided through the targeting of CD33, which can be expressed by LSCs,37 albeit inconsistently.26,37,38 When targeting LSCs with the anti-CD33 immunoconjugate gemtuzumab ozogamicin, several randomized studies failed to show a benefit with respect to response rates, but did show improvements in other, arguably more meaningful, end points such as event-free survival, relapse-free survival, and overall survival.85-87 This pattern of a lack of appreciable improvements in response rates coupled with clear clinical benefit was confirmed in a meta-analysis using data from five different trials.39 This finding is analogous to that of an earlier AML study in which it was observed that patient survival, but not response, could be predicted by LSC burden at diagnosis in patients treated with conventional chemotherapy.19
We must be prepared for the possibility that the use of an LSC-directed therapy, alone or in combination with chemotherapy, will not increase response rates. The impact in the relatively small LSC population may not be perceptible in the initial treatment period, when eradicating the bulk disease may be the most important acute consideration. If AML is a fire, chemotherapy douses the flames, whereas an LSC-directed therapy extinguishes the embers. The focus is naturally on the conflagration at hand, but if there is any hope of controlling the fire over time, attention must also be paid to the embers, even during the active firefight.
Therefore, if chemotherapy treats today’s disease and an LSC-directed therapy treats tomorrow’s recurrence before it can occur, it follows that the activity of LSCs may not be recognizable until the future, when the disease does not relapse or relapses after a longer remission duration than would otherwise have been expected. Recognition of this caveat will be crucial for allowing the drug development process for LSC-directed therapies to proceed through its earliest stages, where conventional strategies usually rely on response rates as the primary end point to warrant continued investment and attention. Clinical trials evaluating these agents, even in early stages, must give importance to clinically relevant end points such as event-free, disease-free, progression-free, and overall survival.82
Finally, some consideration must be given to the duration of treatment with LSC-directed therapies. Clinical trials with non–LSC-directed small-molecule inhibitors are typically written so that patients can remain on a study indefinitely, until limited by tolerability issues or relapsed disease. In the setting of LSC-directed therapies, whether a patient would require continuous administration of a treatment, staving off disease on a daily basis but in constant need of replenishment, or be able to be cured would be a question worthy of investigation. Well-designed studies incorporating elements such as randomized discontinuation,88 as has been done in other oncology studies,89 may be required to answer these questions. We look forward to the day when these questions become relevant in the drug development process for AML and have reason to hope that they are around the corner.
Acknowledgments
The authors acknowledge critical comments and review of the manuscript by Clayton Smith and Martin Carroll.
Authorship
Contribution: Both D.A.P. and C.T.J. contributed equally with background research and writing of the article.
Conflict-of-interest disclosure: D.A.P. is a member of advisory boards for Celgene and Pfizer. C.T.J. is an equity holder in Leuchemix Inc.
Correspondence: Craig T. Jordan, University of Colorado, Anschutz Medical Campus, 12700 East 19th Ave, Research Complex 2, Room 10016, Aurora, CO 80045; e-mail: craig.jordan@ucdenver.edu.