Key Points
The single-chain form of FXII, a component of the plasma contact system, has proteolytic activity.
Single-chain FXII activity suggests a mechanism of contact activation initiation when blood is exposed to physiologic/artificial surfaces.
Abstract
When blood is exposed to variety of artificial surfaces and biologic substances, the plasma proteins factor XII (FXII) and prekallikrein undergo reciprocal proteolytic conversion to the proteases αFXIIa and α-kallikrein by a process called contact activation. These enzymes contribute to host-defense responses including coagulation, inflammation, and fibrinolysis. The initiating event in contact activation is debated. To test the hypothesis that single-chain FXII expresses activity that could initiate contact activation, we prepared human FXII variants lacking the Arg353 cleavage site required for conversion to αFXIIa (FXII-R353A), or lacking the 3 known cleavage sites at Arg334, Arg343, and Arg353 (FXII-T, for “triple” mutant), and compared their properties to wild-type αFXIIa. In the absence of a surface, FXII-R353A and FXII-T activate prekallikrein and cleave the tripeptide S-2302, demonstrating proteolytic activity. The activity is several orders of magnitude weaker than that of αFXIIa. Polyphosphate, an inducer of contact activation, enhances PK activation by FXII-T, and facilitates FXII-T activation of FXII and FXI. In plasma, FXII-T and FXII-R353A, but not FXII lacking the active site serine residue (FXII-S544A), shortened the clotting time of FXII-deficient plasma and enhanced thrombin generation in a surface-dependent manner. The effect was not as strong as for wild-type FXII. Our results support a model for induction of contact activation in which activity intrinsic to single-chain FXII initiates αFXIIa and α-kallikrein formation on a surface. αFXIIa, with support from α-kallikrein, subsequently accelerates contact activation and is responsible for the full procoagulant activity of FXII.
Introduction
The cascade/waterfall hypotheses proposed by Macfarlane and by Davie and Ratnoff in 1964 describe plasma coagulation as a series of proteolytic steps culminating in thrombin-mediated conversion of fibrinogen to fibrin.1,2 Coagulation is initiated in these models by activation of factor XII (FXII) when blood is exposed to a suitable surface.1-3 These investigators postulated that FXII undergoes changes upon surface binding that form or expose an active site. It is now clear that a variety of substances promote reciprocal proteolytic conversion of FXII and prekallikrein (PK) to the proteases αFXIIa and α-kallikrein by a process called contact activation.4-6 αFXIIa then promotes coagulation by converting FXI to FXIa. Although patients lacking FXII do not have a bleeding disorder,7 the protein contributes to pathologic coagulation particularly when blood is exposed to artificial surfaces, such as during cardiopulmonary bypass, extracorporeal membrane oxygenation, and after placement of intravascular devices.8-15 In animal models, FXII contributes to thrombosis,16-20 with anionic polymers such as polyphosphate (Poly-P) and nucleic acids probably serving as counterparts to the nonbiologic surfaces used to induce contact activation in vitro.17,21-25
Despite progress in our understanding of the pathophysiology of contact activation, the processes that initiate FXII activation remain uncertain. Several triggering mechanisms have been proposed that are not mutually exclusive. Contact activation could be initiated by traces of αFXIIa, α-kallikrein, or other proteases that may always be present in plasma.26-28 Alternatively, single-chain FXII may have activity, either intrinsically or after binding to a surface, which starts the process.29-31 Here, we show that a form of FXII that is not converted to αFXIIa has proteolytic activity, and that the activity is enhanced in the presence of Poly-P. The results suggest a mechanism for initiation of surface-induced coagulation that does not depend on preexisting αFXIIa or α-kallikrein.
Experimental procedures
Materials
Normal plasma was obtained from Precision BioLogic. FXII-deficient plasma was obtained from George King Biomed. FXII, FXIIa, PK, α-kallikrein, and corn trypsin inhibitor (CTI) were obtained from Enzyme Research Laboratory. S-2302 (H-d-prolyl-l-phenylalanyl-l-arginine-p-nitroaniline) was obtained from DiaPharma. PTT-A reagent was obtained from Diagnostica Stago. Synthetic Poly-P (ILC Performance Products) of 60- to 100-unit chain length (not calcium saturated) was provided by Thomas Renné and Katrin Nikel (Karolinska Institute, Solna, Sweden). Monoclonal immunoglobulin Gs (IgGs) to FXI (O1A6),32 FXIIa (559C-X181-D06 [D06]),33 and kallikrein (559A-M202-H03 [H03])33,34 have been described. Goat polyclonal IgGs against FXII, PK, and FXI were obtained from Affinity Biologicals.
Recombinant proteins
Schematic diagrams of human FXII, PK, and FXI and their cleaved forms are shown in Figure 1A. A FXI complementary DNA (cDNA) with the active site serine replaced with alanine (FXI-S557A) was expressed in HEK293 cells using vector pJVCMV, purified by IgG affinity chromatography, and stored in 4 mM Tris-HCl, 150 mM NaCl, pH 7.4.35 FXII36 and PK37 cDNAs were introduced into pJVCMV and expressed in HEK293 cells under serum-free conditions (supplemental Figure 1, available on the Blood Web site). In addition to wild-type (WT) FXII (FXII-WT), variants were made with alanine replacing arginine at each known cleavage site (FXII-R334A, FXII-R343A, or FXII-R353A) or all 3 cleavage sites (FXII-T for “triple” mutant), or replacing the active site serine (FXII-S544A). PK-WT and variants with alanine replacing arginine at the activation site (PK-R371A) or the active site serine (PK-S559A) were also prepared. FXII and PK were purified by anion exchange chromatography (Figure 1B) as described under supplemental Figure 1.
Recombinant contact factors. (A) Schematic diagrams of contact factors showing noncatalytic (white boxes) and catalytic (shaded boxes) domains. Positions of active site serine residues are indicated by black bars. Sites of proteolysis during activation are indicated by arrows, with black arrows indicating sites of cleavage required for full protease activity. FXII is an 80-kDa polypeptide that may be cleaved at 3 locations. Cleavage after Arg353 converts FXII to αFXIIa. Cleavage of αFXIIa after Arg334 separates the noncatalytic and catalytic domains, forming βFXIIa. The importance of cleavage after Arg343 is not clear. The FXII noncatalytic domains are the fibronectin type 2 (F2), epidermal growth factor (EGF), fibronectin type 1 (F1), and kringle (K) domains, and a proline-rich region (PRR). PK is a 93-kDa polypeptide that is cleaved after Arg371 to form α-kallikrein (α-Kal). A second cleavage after Arg140 produces β-kallikrein (β-Kal). FXI is a homodimer of 80-kDa polypeptides. It is converted to FXIa by cleavage after Arg369. The noncatalytic portions of PK and FXI contain 4 apple domains, designated A1 to A4. (B) Coomassie blue–stained nonreducing SDS-PAGE of purified FXII (left panel) and PK (right panel) containing ∼2-μg samples per lane. Positions of molecular mass standards in kilodaltons are shown to the left of the images.
Recombinant contact factors. (A) Schematic diagrams of contact factors showing noncatalytic (white boxes) and catalytic (shaded boxes) domains. Positions of active site serine residues are indicated by black bars. Sites of proteolysis during activation are indicated by arrows, with black arrows indicating sites of cleavage required for full protease activity. FXII is an 80-kDa polypeptide that may be cleaved at 3 locations. Cleavage after Arg353 converts FXII to αFXIIa. Cleavage of αFXIIa after Arg334 separates the noncatalytic and catalytic domains, forming βFXIIa. The importance of cleavage after Arg343 is not clear. The FXII noncatalytic domains are the fibronectin type 2 (F2), epidermal growth factor (EGF), fibronectin type 1 (F1), and kringle (K) domains, and a proline-rich region (PRR). PK is a 93-kDa polypeptide that is cleaved after Arg371 to form α-kallikrein (α-Kal). A second cleavage after Arg140 produces β-kallikrein (β-Kal). FXI is a homodimer of 80-kDa polypeptides. It is converted to FXIa by cleavage after Arg369. The noncatalytic portions of PK and FXI contain 4 apple domains, designated A1 to A4. (B) Coomassie blue–stained nonreducing SDS-PAGE of purified FXII (left panel) and PK (right panel) containing ∼2-μg samples per lane. Positions of molecular mass standards in kilodaltons are shown to the left of the images.
Chromogenic assays
Continuous assay.
Assays were conducted in microtiter plate wells coated with PEG-20 000 to prevent induction of contact activation. Activation of FXII (200 nM) and/or PK (200 nM) in 100 μL of standard buffer (20 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid [HEPES], pH 7.4, 100 mM NaCl, 0.1% PEG-8000, 10 μM ZnCl2) was at 37°C. Reactions contained 200 μM S-2302, and changes in optical density at 405 nm (OD 405 nm) were monitored on a microplate reader. Some reactions contained Poly-P (70 μM) or 10% PTT-A reagent. S-2302 (1.6-1000 μM) cleavage by 5 nM αFXIIa or 200 nM FXII-T was studied under similar conditions. Rates of ΔOD405 nm were converted to p-nitroanaline formed (extinction coefficient for pNA 9920 M−1 cm−1). Km and kcat were determined by nonlinear least squares fitting using Scientist Software (Micromath).
Discontinuous assay.
FXII activation (200 nM) with Poly-P (70 μM) or α-kallikrein (50 nM), and PK (12.5-600 nM) cleavage by αFXIIa (25 pM) or FXII-T (15 nM) were performed in standard buffer at 37°C. For FXII activation, reactions were stopped with Polybrene (0.1 mg/mL) with or without H03 (10 nM). For PK cleavage, reactions were stopped with CTI (1.6 μM final). For all reactions, cleavage of 200 μM S-2302 (ΔOD405 nm) was monitored. Amounts of FXIIa or kallikrein formed were determined with standard curves prepared with pure protease.
Western blots
Reactions were conducted in polypropylene tubes coated with PEG-20 000. FXII (200 nM) with or without PK (200 nM), FXI (30 nM), or α-kallikrein (50 nM) was incubated at 37°C in standard buffer at 37°C. Some reactions contained 70 μM Poly-P or PTT-A reagent (25% final volume). At various times, aliquots were removed and mixed with sodium dodecyl sulfate (SDS) sample buffer. Samples were size fractionated by SDS–polyacrylamide gel electrophoresis (PAGE) (12% acrylamide) and transferred to nitrocellulose membranes. Blots of reduced proteins were probed with polyclonal IgG to FXII, PK, or FXI. Nonreducing blots were probed with antibodies that preferentially recognize FXIIa (D06) or kallikrein (H03). For all blots, detection was with an horseradish peroxidase–conjugated secondary antibody and chemiluminescence.
Clotting assays
FXII-deficient plasma (30 μL) was mixed with 30 μL of FXII (400 nM) in phosphate-buffered saline (PBS). PTT-A reagent (30 μL) was added followed by incubation for 5 minutes at 37°C. Twenty-five mM CaCl2 (30 μL) was added, and time to clot formation was measured on an ST-4 Analyzer (Diagnostica Stago). Results were compared with those for normal plasma. Means ± 1 standard deviation (SD) were compared by the Mann-Whitney test.
Thrombin generation
Thrombin generation was measured in 96-well polypropylene plates on a Fluoroskan Ascent analyzer as described.35 FXII-deficient plasma was supplemented with FXII (200 nM), 415 μM Z-Gly-Gly-Arg-AMC, 1.6 μM CTI or vehicle, 20 μg/mL IgG O1A6 or vehicle, and PTT-A reagent (13% final volume). Supplemented plasma (40 μL) was mixed with 10 μL of 20 mM HEPES, pH 7.4, 100 mM CaCl2, 6% bovine serum albumin, and fluorescence (excitation λ 390 nm, emission λ 460 nm) was monitored.
Carotid artery thrombosis model
Procedures with mice were approved by the Vanderbilt University Animal Care and Use Committee. FXII-deficient C57Bl/6 mice16 were anesthetized with 50 mg/kg intraperitoneal pentobarbital. The right common carotid artery was exposed and fitted with a Doppler probe (Transonic System). FXII (25 μg in 100 μL of PBS) was infused through the internal jugular vein. Five minutes later, two 1 × 1.5 mm filter papers (Schleicher & Schuell,) saturated with 5% FeCl3 were applied to opposite sides of the artery for 3 minutes.18 Flow was monitored for 30 minutes.
Results
FXII autoactivation
FXII undergoes autocatalytic activation in the presence of several polyanions.17,21,26-29 The Coomassie-stained reducing gel in Figure 2A shows the 80-kDa FXII polypeptide undergoing autocatalysis in the presence of Poly-P, forming the 50-kDa heavy and 30-kDa light chains of αFXIIa. The changes are accompanied by a rapid increase in amidolytic activity (Figure 2B). FXII is cleaved at up to 3 sites during activation (after Arg334, Arg343, or Arg353; Figure 1A). Cleavage after Arg353 is required for αFXIIa generation.36,38 FXII species with Arg353 (FXII-WT, FXII-R334A, FXII-R343A) undergo autocatalysis in the presence of Poly-P, whereas species lacking Arg353 (FXII-R353A and FXII-T) do not (Figure 2C middle column). Loss of the catalytic active site serine (Ser544),36 as expected, also prevents autocatalysis (Figure 2C middle column). Experiments examining cleavage of the tripeptide S-2302 support the western blot data, with amidolytic activity increasing in reactions with species that undergo conversion to αFXIIa, but not in reactions with FXII-R353A, FXII-T, or FXII-S544A (Figure 2D). These data show that cleavage after Arg353 is required for full elaboration of FXII amidolytic activity.
FXII autoactivation and activation by kallikrein. (A) Plasma FXII (200 nM) was incubated in standard buffer without Poly-P (−Poly-P) or in the presence of 70 μM Poly-P (+Poly-P). At the indicated times, samples were removed from the reaction into reducing sample buffer, size fractionated on a 12% polyacrylamide-SDS gel and stained with Coomassie blue. Positions of standard for single-chain FXII and the heavy chain (HC) and light chain (LC) of αFXIIa are shown at the right of each panel. (B) Chromogenic substrate assay for FXIIa activity for the fractions shown in the gel in panel A. Reactions were run in the presence (△) or absence (♦) of 70 μM Poly-P. (C) Recombinant FXII species (200 nM) incubated in the absence of an activator (control, left column), in the presence of 70 μM Poly-P (center column), or in the presence of 50 nM α-kallikrein without a surface (right column). At the indicated times, samples were removed into reducing sample buffer. Samples were size fractionated by SDS-PAGE, followed by western blot analysis with a polyclonal anti-human FXII IgG. For panels A and C, positions of standards for FXII and the heavy chain (HC) and light chain (LC) of αFXIIa are indicated at the right of each image. (D) Recombinant FXII species (200 nM) were incubated in the presence of 70 μM Poly-P and 200 μM S-2302. Changes in OD 405 nm were continuously monitored on a microplate reader. (E) FXII (200 nM) was incubated with 50 nM α-kallikrein for 120 minutes at 37°C in the absence of a surface. Kallikrein was inhibited with IgG H03 and FXIIa cleavage of S-2302 (200 μM) was measured.
FXII autoactivation and activation by kallikrein. (A) Plasma FXII (200 nM) was incubated in standard buffer without Poly-P (−Poly-P) or in the presence of 70 μM Poly-P (+Poly-P). At the indicated times, samples were removed from the reaction into reducing sample buffer, size fractionated on a 12% polyacrylamide-SDS gel and stained with Coomassie blue. Positions of standard for single-chain FXII and the heavy chain (HC) and light chain (LC) of αFXIIa are shown at the right of each panel. (B) Chromogenic substrate assay for FXIIa activity for the fractions shown in the gel in panel A. Reactions were run in the presence (△) or absence (♦) of 70 μM Poly-P. (C) Recombinant FXII species (200 nM) incubated in the absence of an activator (control, left column), in the presence of 70 μM Poly-P (center column), or in the presence of 50 nM α-kallikrein without a surface (right column). At the indicated times, samples were removed into reducing sample buffer. Samples were size fractionated by SDS-PAGE, followed by western blot analysis with a polyclonal anti-human FXII IgG. For panels A and C, positions of standards for FXII and the heavy chain (HC) and light chain (LC) of αFXIIa are indicated at the right of each image. (D) Recombinant FXII species (200 nM) were incubated in the presence of 70 μM Poly-P and 200 μM S-2302. Changes in OD 405 nm were continuously monitored on a microplate reader. (E) FXII (200 nM) was incubated with 50 nM α-kallikrein for 120 minutes at 37°C in the absence of a surface. Kallikrein was inhibited with IgG H03 and FXIIa cleavage of S-2302 (200 μM) was measured.
FXII activation by α-kallikrein
During contact activation, α-kallikrein cleaves FXII after Arg353 forming αFXIIa.36,38 Substitutions for Arg334 or Arg343 do not prevent this (Figure 2C right column). α-kallikrein also cleaves FXII-R353A (Figure 2C right column); however, this does not increase amidolytic activity (Figure 2E), indicating cleavage is after a residue other than 353. Because Arg353 replacement did not prevent FXII cleavage, we prepared FXII-T, which lacks all 3 known FXII cleavage sites. The failure of α-kallikrein to cleave FXII-T (Figure 2C right column) indicates α-kallikrein cleaves FXII-R353A after Arg334 and/or Arg343. FXII-S544A, which has Arg353, is cleaved by α-kallikrein (Figure 2C right column) but, as expected, also lacks amidolytic activity because of the absence of Ser544 (Figure 2E). We performed nonreducing western blots for reactions similar to those in the right column of Figure 2C using IgG D06, which preferentially recognizes fully formed FXIIa (supplemental Figure 2). Signals are detected for forms of FXII that have arginine at position 353, but not for forms with alanine at this position. This confirms that when FXII-R353A is cleaved by kallikrein, it does not assume a conformation similar to αFXIIa. Cumulatively, the results support the premise that full αFXIIa amidolytic activity requires cleavage after Arg353 and an active catalytic mechanism (ie, Ser544).
Reciprocal FXII and PK activation
Mixing FXII and PK in the absence of a surface leads to cleavage of both proteins as revealed by reducing western blots (Figure 3A). Each protein undergoes >1 cleavage, but at least 1 involves the respective activation sites (after FXII Arg353 or PK Arg371).38 This is shown in the nonreducing blots in Figure 3B, which use antibodies that preferentially recognize fully formed FXIIa (D06) and kallikrein (H03).33,34 Taken as a whole, the reducing and nonreducing blots indicate FXII is converted first to αFXIIa (80 kDa) by cleavage after Arg353, then more slowly to βFXIIa (30 kDa) by cleavage after Arg334 (Figure 1A). PK is converted to α-kallikrein by cleavage after Arg371, and then β-kallikrein by cleavage after Arg140 (Figure 1A), both of which are 90-kDa proteins (Figure 3B).37,38 Mixing FXII-WT and PK-WT generates patterns similar to their plasma counterparts (Figure 3C).
Reciprocal activation of FXII and PK in the absence of a surface. (A-C) FXII (200 nM) and PK (200 nM) species were incubated at 37°C. At indicated time points, samples were removed into reducing (A,C) or nonreducing (B) sample buffer, size fractionated by SDS-PAGE, and analyzed by western blot using (A,C) polyclonal IgG to FXII (XII) or PK or (B) monoclonal IgGs that preferentially recognize the activated forms of FXIIa (D06) and kallikrein (H03). (A-B) Reciprocal activation of plasma FXII and PK. (C) Activation of PK-WT by recombinant FXII species. For panels A and C, positions of standards for FXII (XII) and the heavy chain (HC) and light chain (LC) of FXIIa; and standards for PK, the heavy chain and light chain of α-kallikrein, and a fragment of the heavy chain of β-kallikrein (β) are indicated at the right of each image. For panel B, positions of standards for αFXIIa, βFXIIa, α-kallikrein (α-kal), and β-kallikrein (β-kal) are indicated on the right.
Reciprocal activation of FXII and PK in the absence of a surface. (A-C) FXII (200 nM) and PK (200 nM) species were incubated at 37°C. At indicated time points, samples were removed into reducing (A,C) or nonreducing (B) sample buffer, size fractionated by SDS-PAGE, and analyzed by western blot using (A,C) polyclonal IgG to FXII (XII) or PK or (B) monoclonal IgGs that preferentially recognize the activated forms of FXIIa (D06) and kallikrein (H03). (A-B) Reciprocal activation of plasma FXII and PK. (C) Activation of PK-WT by recombinant FXII species. For panels A and C, positions of standards for FXII (XII) and the heavy chain (HC) and light chain (LC) of FXIIa; and standards for PK, the heavy chain and light chain of α-kallikrein, and a fragment of the heavy chain of β-kallikrein (β) are indicated at the right of each image. For panel B, positions of standards for αFXIIa, βFXIIa, α-kallikrein (α-kal), and β-kallikrein (β-kal) are indicated on the right.
Unexpectedly, forms of FXII that do not form αFXIIa (FXII-R353A and FXII-T) induce PK cleavage (Figure 3C). The results could not be accounted for by endogenous FXII production by HEK293 cells, nor by non-FXII constituents of conditioned media (supplemental Figure 1). Furthermore, PK-WT was not cleaved by FXII-S544A (Figure 3C), indicating cleavage requires recombinant FXII with an intact FXII catalytic apparatus. The variant PK-S559A, which lacks an active site serine, was also cleaved by FXII-R353A and FXII-T, indicating α-kallikrein activity is not needed for PK cleavage in reactions with FXII-R353A and FXII-T (supplemental Figure 3). These data show that single-chain FXII has proteolytic activity.
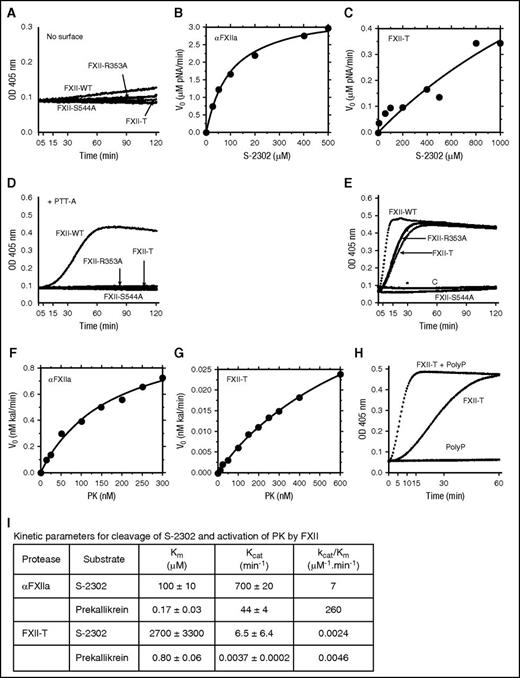
S-2302 cleavage by single-chain FXII
Single-chain FXII-WT cleaves S-2302 at a low rate (Figure 4A). This is usually interpreted as reflecting traces of contaminating αFXIIa, a possibility that cannot be ruled out. However, FXII-R353A and FXII-T also consistently demonstrated low-level amidolytic activity, whereas FXII-S544A does not (Figure 4A). S-2302 is cleaved by αFXIIa with Km 100 ± 10 μM and kcat 700 ± 20/minute (Figure 4B), and by FXII-T with Km 2700 ± 3300 μM, kcat 6.5 ± 6.4/minute (Figure 4C). The results indicate that catalytic efficiency (kcat/Km) for S-2302 cleavage by FXII-T is about 3000-fold lower than for αFXIIa (Figure 4I), a number that should be considered an approximation, as the apparent Km for the FXII-T-mediated reaction is greater than the achievable substrate concentration. Although addition of silica-based PTT-A reagent (Figure 4D) or Poly-P (Figure 2D) increased amidolytic activity with FXII-WT because of conversion to αFXIIa, neither compound enhanced the amidolytic activity of FXII-R353A or FXII-T.
Cleavage of S-2302 and PK by FXII species. (A) FXII-WT (WT), FXII-R353A, FXII-T (T), or FXII-S544A (200 nM) were incubated with S-2302 (200 μM) in standard buffer in the absence of a surface. Continuous formation of amidolytic activity was monitored at 405 nm. (B-C) Varying concentrations of S-2302 (1.6-1000 μM) were incubated with (B) 5 nM αFXIIa or (C) 200 nM FXII-T in the absence of a surface. Changes in OD 405 nm/minute were measured on a plate reader and converted to pNA generated per minute. Each point is a mean ± 1 SD for 3 separate experiments. (D) Same as reactions described in panel A except that incubations were conducted in the presence of a surface (PTT-A reagent, 10% final volume). Note that PTT-A reagent only increases the amidolytic activity of FXII-WT, compared with reactions in panel A because, of the 4 FXII species tested, it is the only 1 that is cleaved after Arg353 and has an active site serine at residue 544. (E) PK-WT (200 nM) was incubated with 200 nM FXII-WT, FXII-R353A, FXII-T, FXII-S544A, or control vehicle (C). Generation of kallikrein (or kallikrein and αFXIIa in the case of FXII-WT) was continuously monitored by cleavage of S-2302 (200 μM). (F-G) Varying concentration of PK were incubated with (F) 25 pM αFXIIa or (G) 15 nM FXII-T in the absence of a surface. Kallikrein generation was determined by measuring the rate of S-2302 (200 μM) cleavage. Each point is a mean ± 1 SD for 3 separate experiments. (H) Generation of kallikrein from PK-WT (200 nM) was followed by continuous monitoring of S-2302 (200 μM) cleavage in the presence or absence of a surface (70 μM Poly-P), and in the presence or absence of 200 nM FXII-T (T). (I) Kinetic parameters for cleavage of S-2302 and PK by αFXIIa or FXII-T determined from the curves in panels B, C, F, and G.
Cleavage of S-2302 and PK by FXII species. (A) FXII-WT (WT), FXII-R353A, FXII-T (T), or FXII-S544A (200 nM) were incubated with S-2302 (200 μM) in standard buffer in the absence of a surface. Continuous formation of amidolytic activity was monitored at 405 nm. (B-C) Varying concentrations of S-2302 (1.6-1000 μM) were incubated with (B) 5 nM αFXIIa or (C) 200 nM FXII-T in the absence of a surface. Changes in OD 405 nm/minute were measured on a plate reader and converted to pNA generated per minute. Each point is a mean ± 1 SD for 3 separate experiments. (D) Same as reactions described in panel A except that incubations were conducted in the presence of a surface (PTT-A reagent, 10% final volume). Note that PTT-A reagent only increases the amidolytic activity of FXII-WT, compared with reactions in panel A because, of the 4 FXII species tested, it is the only 1 that is cleaved after Arg353 and has an active site serine at residue 544. (E) PK-WT (200 nM) was incubated with 200 nM FXII-WT, FXII-R353A, FXII-T, FXII-S544A, or control vehicle (C). Generation of kallikrein (or kallikrein and αFXIIa in the case of FXII-WT) was continuously monitored by cleavage of S-2302 (200 μM). (F-G) Varying concentration of PK were incubated with (F) 25 pM αFXIIa or (G) 15 nM FXII-T in the absence of a surface. Kallikrein generation was determined by measuring the rate of S-2302 (200 μM) cleavage. Each point is a mean ± 1 SD for 3 separate experiments. (H) Generation of kallikrein from PK-WT (200 nM) was followed by continuous monitoring of S-2302 (200 μM) cleavage in the presence or absence of a surface (70 μM Poly-P), and in the presence or absence of 200 nM FXII-T (T). (I) Kinetic parameters for cleavage of S-2302 and PK by αFXIIa or FXII-T determined from the curves in panels B, C, F, and G.
PK activation by single-chain FXII
In mixtures of FXII and PK, amidolytic activity reflects FXIIa and kallikrein generation, as both proteases cleave S-2302. This is shown in Figure 4E for mixtures of FXII-WT and PK-WT. Mixing PK with FXII-R353A or FXII-T also generates amidolytic activity (Figure 4E). As these FXII species cleave S-2302 poorly (Figure 2E) and do not form αFXIIa, the amidolytic activity must be largely due to PK conversion to kallikrein by cleavage after Arg371. In support of this, neither FXII-R353A nor FXII-T cleaved PK lacking Arg371 (PK-R371A; supplemental Figure 4). Reactions containing PK alone or PK mixed with FXII-R544S generate little amidolytic activity (Figure 4E), demonstrating the importance of a functional FXII catalytic domain. αFXIIa activates PK with Km 0.17 ± 0.03 μM and kcat 44 ± 4/minute (Figure 4F), whereas FXII-T activates PK with Km 0.80 ± 0.06 μM and kcat 0.0037 ± 0.0002/minute (Figure 4G). The catalytic efficiency (kcat/KmFigure 4I) of FXII-T–mediated PK activation is ∼50 000-fold lower than for αFXIIa. Consistent with observations that polyanions accelerate reciprocal FXII/PK activation,31,33 PK activation by FXII-T is enhanced by Poly-P (Figure 4H).
Single-chain FXII catalyzes surface-dependent FXII activation
Neither FXII-T nor FXII-S544A undergo autocatalysis in the presence of Poly-P (Figure 2C), the former because it lacks cleavage sites and the latter because it lacks catalytic activity. When these proteins are mixed in the presence of a silica-based activated partial thromboplastin time (aPTT) reagent or Poly-P (Figure 5A), there is a time-dependent increase in a signal on western blots using IgG D06, indicating formation of a species with a catalytic domain with similar conformation to αFXIIa. Incubation of either protein alone with Poly-P does not produce a signal, consistent with results in Figure 2C (middle column). Of the 2 proteins, only FXII-S544A cleaved after Arg353 is recognized well by IgG D06 (supplemental Figure 2). Therefore, the results are most consistent with surface-induced FXII-T cleavage of FXII-S544A after Arg353. The results indicate that single-chain FXII can initiate FXII activation after the protein is bound to an activating surface.
Activation of FXII and FXI by single-chain FXII. (A) Western blots of a mixture of FXII-T (200 nM) and FXII-S544A (200 nM) in the absence of a surface (No Surface), or in the presence of PTT-A reagent (25% final volume) or Poly-P (70 μM). At indicated times, samples were removed into nonreducing sample buffer, size fractionated by SDS-PAGE, and analyzed by western blot using IgG D06 which recognizes formation of the FXIIa active site. The bottom row shows results for FXII-S544A and FXII-T incubated separately with 70 μM Poly-P. (B) FXI-S557A (30 nM) was incubated with 200 nM FXII-WT, FXII-T, or FXII-S544A in the absence (top row) or presence (bottom row) of 70 μM Poly-P. At indicated times, samples were removed into reducing sample buffer, size fractionated by SDS-PAGE, and analyzed by western blot using a goat-anti-human FXI polyclonal IgG.
Activation of FXII and FXI by single-chain FXII. (A) Western blots of a mixture of FXII-T (200 nM) and FXII-S544A (200 nM) in the absence of a surface (No Surface), or in the presence of PTT-A reagent (25% final volume) or Poly-P (70 μM). At indicated times, samples were removed into nonreducing sample buffer, size fractionated by SDS-PAGE, and analyzed by western blot using IgG D06 which recognizes formation of the FXIIa active site. The bottom row shows results for FXII-S544A and FXII-T incubated separately with 70 μM Poly-P. (B) FXI-S557A (30 nM) was incubated with 200 nM FXII-WT, FXII-T, or FXII-S544A in the absence (top row) or presence (bottom row) of 70 μM Poly-P. At indicated times, samples were removed into reducing sample buffer, size fractionated by SDS-PAGE, and analyzed by western blot using a goat-anti-human FXI polyclonal IgG.
Single-chain FXII catalyzes surface-dependent FXI activation
αFXIIa-mediated conversion of FXI to FXIa is a key step in contact activation-initiated coagulation. In the following experiments, FXI-S557A is used instead of FXI because the latter undergoes autoactivation when exposed to polyanions35 making it difficult to assess the FXII contribution to FXI activation. In the absence of Poly-P, mixing FXI-S557A with FXII-WT, FXII-T, or FXII-S544A does not result in detectable FXI-S557A cleavage (Figure 5B top row). With Poly-P (Figure 5B bottom row), FXII-WT converts FXI-S557A to a 2-chain species representing FXIa. This is consistent with FXII-WT undergoing autoactivation, and αFXIIa-WT then cleaving FXI-S557A. In the presence of Poly-P, FXII-T but not FXII-S544A cleaved FXI-S557A to the 2-chain form, indicating that single-chain FXII possesses activity that activates FXI on a surface.
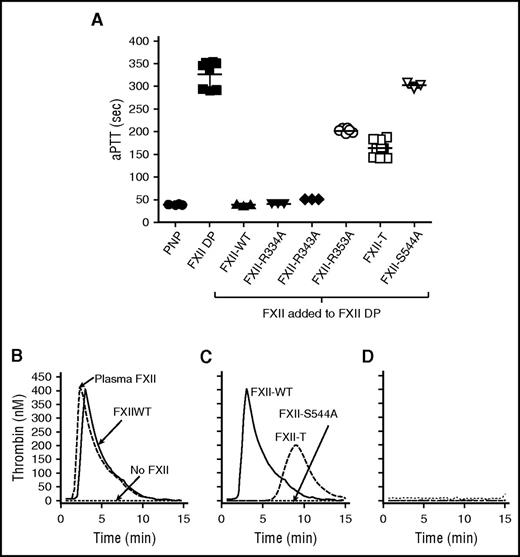
Single-chain FXII activity in plasma
In aPTT assays, the clotting time of normal and FXII-deficient plasma was 40 ± 1 seconds and 327 ± 30 seconds, respectively. Adding forms of FXII that can be converted to αFXIIa substantially shortened the aPTT of FXII-deficient plasma (FXII-WT [40 ± 2 seconds], FXII-R334A [41 ± 1 seconds], or FXII-R343A [51 ± 1 seconds]; Figure 6A). FXII-R353A and FXII-T, which do not form αFXIIa, also shortened the aPTT of FXII-deficient plasma (202 ± 5 seconds and 164 ± 19 seconds, respectively, P < .0001 for both compared with FXII-deficient plasma), but not to the same extent as FXII-WT. FXII-S544A did not affect the clotting time significantly (302 ± 6 seconds; P = .2). FXII-WT and plasma FXII support thrombin generation in FXII-deficient plasma supplemented with PTT-A reagent comparably (Figure 6B). FXII-T also supports thrombin generation, but the peak is smaller and delayed compared with FXII-WT (Figure 6C), whereas FXII-S544A did not support thrombin generation. The effects of FXII-WT and FXII-T on thrombin generation were blocked by the FXII inhibitor CTI and the FXIa inhibitor O1A6 (Figure 6D), showing that single-chain FXII promotes thrombin generation by activating FXI in the presence of a surface.
Surface-initiated clotting and thrombin generation in human plasma. (A) Clotting times in an aPTT assay for pooled normal plasma (PNP), FXII-deficient plasma (FXII DP), or FXII-deficient plasma supplemented with recombinant FXII species (FXII added to FXII DP). Each symbol indicates 1 clotting time and the horizontal bars indicate means for each group ± 1 SD. (B) Thrombin generation in FXII-deficient plasma supplemented with PTT-A reagent in the absence of FXII (No XII dotted line) or in the presence of plasma FXII (dashed line) or FXII-WT (solid line). (C) Thrombin generation in FXII-deficient plasma supplemented with PTT-A reagent and FXII-WT (solid line), FXII-T (dashed line), or FXII-S544A (dotted line). (D) Reactions for FXII-WT and FXII-T shown in panel C run in the presence of CTI or anti-FXI IgG 01A6. For all panels, results represent means for 3 runs.
Surface-initiated clotting and thrombin generation in human plasma. (A) Clotting times in an aPTT assay for pooled normal plasma (PNP), FXII-deficient plasma (FXII DP), or FXII-deficient plasma supplemented with recombinant FXII species (FXII added to FXII DP). Each symbol indicates 1 clotting time and the horizontal bars indicate means for each group ± 1 SD. (B) Thrombin generation in FXII-deficient plasma supplemented with PTT-A reagent in the absence of FXII (No XII dotted line) or in the presence of plasma FXII (dashed line) or FXII-WT (solid line). (C) Thrombin generation in FXII-deficient plasma supplemented with PTT-A reagent and FXII-WT (solid line), FXII-T (dashed line), or FXII-S544A (dotted line). (D) Reactions for FXII-WT and FXII-T shown in panel C run in the presence of CTI or anti-FXI IgG 01A6. For all panels, results represent means for 3 runs.
FXII and arterial thrombosis
FXII-deficient mice are protected from carotid artery thrombosis induced by application of FeCl3 to the vessel exterior (Figure 7).18 Infusion of FXII-WT, as expected, restored FeCl3-induced carotid artery occlusion in FXII-deficient mice. In contrast, FXII-R353A, FXII-T, and FXII-S544A failed to support thrombosis, with the exception of 1 of 9 animals supplemented with FXII-T. These data are consistent with results from plasma assays, and indicate that the relatively small amount of thrombin generated through the proteolytic activity of single-chain FXII is not sufficient to promote thrombosis in this model, and that αFXIIa is required for full elaboration of protease activity.
Mouse carotid artery thrombosis model. FXII-deficient C57Bl/6 mice were infused with 100 μL of PBS (C) or 100 μl of PBS containing 25 μg of FXII-WT, FXII-R353A, FXII-T, or FXII-S544A. Thrombus formation was induced by application of 2 pads saturated with 5% FeCl3 to opposite sides of the carotid artery for 3 minutes. Flow through the artery was recorded for 30 minutes. The percentages of animals with occluded arteries 30 minutes after FeCl3 application are shown in the bar graph (n = 9 for each bar). Representative plasma samples from test mice were analyzed by western blot to make certain that FXII was still in the circulation at the end of the study. Each number indicates a separate animal. C, FXII control; DP, FXII-deficient mouse plasma; NP, normal mouse plasma.
Mouse carotid artery thrombosis model. FXII-deficient C57Bl/6 mice were infused with 100 μL of PBS (C) or 100 μl of PBS containing 25 μg of FXII-WT, FXII-R353A, FXII-T, or FXII-S544A. Thrombus formation was induced by application of 2 pads saturated with 5% FeCl3 to opposite sides of the carotid artery for 3 minutes. Flow through the artery was recorded for 30 minutes. The percentages of animals with occluded arteries 30 minutes after FeCl3 application are shown in the bar graph (n = 9 for each bar). Representative plasma samples from test mice were analyzed by western blot to make certain that FXII was still in the circulation at the end of the study. Each number indicates a separate animal. C, FXII control; DP, FXII-deficient mouse plasma; NP, normal mouse plasma.
Discussion
FXII becomes active when blood is exposed to a variety of biologic and nonbiologic substances, however, the mechanism that initiates activation is debated. Given the range of surfaces that support contact activation, different processes may operate in different situations.39 In 1976, Griffin and Cochrane raised the question of whether a proteolytic or nonproteolytic process best explained initial FXII activity.40 Several groups proposed that FXII gains activity once surface-bound, and postulated that conversion to a 2-chain form is not required in all circumstances.29-31 Silverberg et al and Tans et al came to a different conclusion.26-28 In their opinions, αFXIIa was the active form of FXII, and the sigmoidal progress curves for surface-dependent FXII autoactivation were most compatible with a process initiated by traces of αFXIIa in FXII preparations. However, recognizing the difficulty of eliminating other possibilities with assays based on plasma-derived FXII, Silverberg and Kaplan suggested that if single-chain FXII had activity, it is weak, and calculated a limit on its ability to cleave the tripeptide S-2302 at <4200-fold that of αFXIIa.27
In vivo, polymeric anions may serve as counterparts to the nonbiologic substances traditionally used to study contact activation. One of the best characterized is Poly-P, polymers of inorganic phosphate that produce procoagulant effects in plasma including induction of contact activation.17,21,25,41 Engel et al recently reported that FXII bound to Poly-P chains of the size released by platelets cleaves S-2302 and activates PK and FXI without undergoing conversion to αFXIIa,31 indicating that Poly-P binding induces conformational changes in single-chain FXII that confer activity. A striking finding in their study, which conflicts with the data presented here, was that the amidolytic activities of αFXIIa and Poly-P–bound FXII were comparable, suggesting that conversion to αFXIIa is not necessary for full (or substantial) elaboration of protease activity. These experiments, and earlier work, used plasma FXII, making it difficult to exclude effects from αFXIIa, either as an initial contaminant or as a product generated during reactions. We addressed this issue with recombinant FXII that cannot be converted to αFXIIa. Our results show that single-chain FXII has activity and that Poly-P enhances that activity toward the macromolecular substrates PK, FXII, and FXI. However, there are important differences between our results and those reported by Engel et al, and they support different mechanisms for the role of cofactors such as Poly-P in FXII activity. Differences between recombinant and plasma-derived FXII, either as a consequence of the substitutions we introduced or subtler factors such as variance in posttranslational modification, may partly explain the weaker activity we detected for single-chain FXII, compared with the activity for plasma FXII reported by Engel et al. However, this would not explain the different behaviors observed for plasma FXII in the presence of Poly-P.
Members of the trypsin-like protease family are usually secreted as inactive single-chain zymogens that require internal proteolysis after Arg15 (chymotrypsin numbering) for activity.42 Upon cleavage, the new catalytic domain N terminus (residue 16) forms a salt bridge with Asp194, creating a functional substrate recognition site. Consistent with this, we observed that increased rates of S-2302 cleavage by FXII (plasma or recombinant) in the presence of Poly-P are associated with conversion to αFXIIa. Arg353 in human FXII corresponds to chymotrypsin Arg15,36,38 and replacing it blocks Poly-P–mediated FXII autocatalysis and the associated increase in amidolytic activity. These findings support the premise that polyanion-induced elaboration of full FXII activity involves cleavage after Arg353.27,28 Our data with plasma-derived and recombinant FXII-WT are in disagreement with those of Engel et al on this point. Perhaps differences in preparative techniques for Poly-P, or methods for detecting FXIIa contribute to the discrepancy. We observed that Poly-P–based reactions behave differently depending on buffer conditions, which may be relevant. However, it is worth noting that forms of FXII that do not form αFXIIa did not reconstitute FXII-deficient mice in a thrombosis model in which FXII and Poly-P play prominent roles.17,25 This argues against a mechanism in which binding of a cofactor converts FXII to a fully or substantially active protease without cleavage after Arg353, and is consistent with the marked decrease in activity of the natural variant FXII Locarno, in which proline replaces Arg353.43
Single-chain FXII-T cleaves S-2302 and activates PK without adding Poly-P or a surface, suggesting it has a low level of proteolytic activity when not surface-bound. Poly-P does, however, enhance PK activation, and facilitates FXII and FXI activation by FXII-T. There may be multiple effects that contribute to the mechanism involved. First, Poly-P–bound FXII-T may assume a conformation that enhances extended interaction with macromolecular substrates. It is also likely that the substrate (PK, FXII, or FXI) is altered upon binding to Poly-P, exposing activation cleavage sites. Furthermore, the size dependence of the Poly-P effect41,44 points to facilitation of protease and substrate binding in proximity to each other through a bridging (template) mechanism.
There is precedent for plasma serine proteases having activity in their single-chain forms, a property referred to as low zymogenicity. Single-chain tissue plasminogen activator (tPA) is eightfold less active against tripeptide substrates and 20- to 50-fold less active toward plasminogen than 2-chain tPA,45 but in the presence of fibrin both forms cleave plasminogen similarly. Renatus et al proposed that a salt bridge between Lys156 and Asp194 in tPA (Figure 8A) not found in most trypsin-like proteases contributes to formation of a functional active site in the single-chain protein.46 Single-chain urokinase also has activity toward plasminogen in the presence of fibrin and, like tPA, has lysine at position 156.47 Gln156 in FXII cannot form a stabilizing salt bridge with Asp194, but it may provide weak stabilization through hydrogen-bonding interactions. In addition to forming a salt bridge with Lys156, the carboxylate group of Asp194 in tPA forms hydrogen bonds with the main-chain nitrogen atoms of Gly142 and Cys191 (Figure 8A). A homology model of FXII-T based on the tPA structure reveals that the FXII Asp194 carboxylate can form the same hydrogen bonds (Figure 8B).48 Furthermore, because of an additional hydrogen bond with the side chain of Gln156, there is no impediment to oxyanion hole formation. Gln156 would be less effective at stabilizing Asp194 in FXII-T than the N-terminal Val16 in αFXIIa, consistent with the lower catalytic activity of FXII-T. Recently, the crystal structure of FXII catalytic domain with Arg-Ser blocking the N terminus (FXIIc) was reported.49 FXIIc has a zymogen-like conformation, lacking the oxyanion hole, and Asp194 interacts with Arg73 in a configuration similar to the zymogen triad described for chymotrypsinogen (Figure 8C).50,51 This suggests that a complex interplay of factors, including conformational changes within FXII induced by interactions with substrates, determine formation of a productive FXII-T enzyme-substrate complex, as has been proposed for other members of the trypsin-like protease family.52
Comparison of tPA and FXII structures. Shown are stick diagrams of S1 pocket structures with hydrogen bonds and electrostatic interactions shown as dotted lines (purple). In panels A and B, the position of the oxyanion hole is indicated by the juxtaposed blue spheres that represent the nitrogen atoms of Ser195 and Gly193. (A) Single-chain tPA active S1 pocket crystal structure (pdb:1BDA) is shown with Asp194 stabilized by the salt bridge formed with Lys156 (indicated by + and − symbols), and also by hydrogen bonds with the main-chain nitrogens of Gly142 and Cys191. The cyan stick figure represents the side chain of the arginine P1 residue of the tPA inhibitor dansyl-Glu-Gly-Arg-chloromethylketone. (B) Homology model (SWISS-MODEL48 ) of the S1 pocket of FXII-T based on the tPA crystal structure where Gln156 forms a hydrogen bond to the Asp194 carboxylate group. The side chain shown in cyan represents the P1 arginine of a substrate (PK or FXI). (C) Crystal structure (pdb:XDE) showing the inactive zymogen conformation of FXIIc where the oxyanion hole is absent (all figures prepared with PyMOL Molecular Graphics System, version 1.8; Schrödinger, LLC).
Comparison of tPA and FXII structures. Shown are stick diagrams of S1 pocket structures with hydrogen bonds and electrostatic interactions shown as dotted lines (purple). In panels A and B, the position of the oxyanion hole is indicated by the juxtaposed blue spheres that represent the nitrogen atoms of Ser195 and Gly193. (A) Single-chain tPA active S1 pocket crystal structure (pdb:1BDA) is shown with Asp194 stabilized by the salt bridge formed with Lys156 (indicated by + and − symbols), and also by hydrogen bonds with the main-chain nitrogens of Gly142 and Cys191. The cyan stick figure represents the side chain of the arginine P1 residue of the tPA inhibitor dansyl-Glu-Gly-Arg-chloromethylketone. (B) Homology model (SWISS-MODEL48 ) of the S1 pocket of FXII-T based on the tPA crystal structure where Gln156 forms a hydrogen bond to the Asp194 carboxylate group. The side chain shown in cyan represents the P1 arginine of a substrate (PK or FXI). (C) Crystal structure (pdb:XDE) showing the inactive zymogen conformation of FXIIc where the oxyanion hole is absent (all figures prepared with PyMOL Molecular Graphics System, version 1.8; Schrödinger, LLC).
Our results support a model for contact activation that does not require preexisting αFXIIa or α-kallikrein. Single-chain FXII would catalyze initial FXII and PK conversion to αFXIIa and α-kallikrein upon surface exposure. The relatively low activity of single-chain FXII suggests that it is αFXIIa and α-kallikrein that are responsible for subsequent acceleration of contact activation. This is consistent with the observation that progress curves for FXII autoactivation fit models based on initiation by traces of αFXIIa.27,28 Single-chain FXII activity is relevant early in contact activation when αFXIIa concentration is low, but is progressively less important as αFXIIa accumulates. Finally, our plasma-based studies indicate that although surface-bound single-chain FXII activates FXI, αFXIIa is a considerably better activator, supporting the premise that the procoagulant and prothrombotic activities of FXII are mediated largely, if not exclusively, through αFXIIa. The observation that single-chain FXII activates PK has interesting implications. Although the reaction is several orders of magnitude less efficient than for αFXIIa activation of PK, the plasma PK concentration (∼600 nM) is near Km for fluid-phase activation by FXII-T. Furthermore, under normal conditions, the plasma concentration of FXII (∼400 nM) is probably several orders of magnitude higher than that of αFXIIa. This suggests that the relatively high plasma FXII level could compensate for the weak specific activity of single-chain FXII toward PK. Given this, single-chain FXII activity may contribute to the basal reciprocal turnover of FXII and PK observed in vivo.19
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by awards HL58837 and HL81326 (D.G.) from the National Institutes of Health, National Heart, Lung, and Blood Institute, and Programme Grant RG/12/9/29775 and Project Grant PG/09/025/27136 (J.E.) from the British Heart Foundation. I.M.V. was supported by award HL130018 from the National Heart, Lung, and Blood Institute.
Authorship
Contribution: I.I. and A.M. conducted the in vitro experiments and contributed to writing the manuscript; M.-f.S. expressed, purified, and characterized recombinant proteins; Q.C. designed and performed mouse experiments; S.K.D. designed recombinant FXII variants and contributed to writing the manuscript; I.M.V. performed kinetic analysis and contributed to manuscript writing; J.E. performed structural analysis and contributed to writing the manuscript; and D.G. oversaw the project and writing of the manuscript.
Conflict-of-interest disclosure: D.G. is a consultant, and has received consultant fees, for several pharmaceutical companies (Aronora, Bayer, Dyax, Ionis, Merck, Novartis, Ono) with an interest in inhibition of contact activation proteases for therapeutic purposes. The remaining authors declare no competing financial interests.
Correspondence: David Gailani, Hematology/Oncology Division, Vanderbilt University, 777 Preston Research Building, 2220 Pierce Ave, Nashville, TN 37232; e-mail: dave.gailani@vanderbilt.edu.







This feature is available to Subscribers Only
Sign In or Create an Account Close Modal