Key Points
The systematic review suggests that first-line antenatal management in FNAIT is weekly IVIG administration.
Noninvasive management is effective without the relatively high rate of adverse outcomes seen in invasive strategies.
Abstract
Several strategies can be used to manage fetal or neonatal alloimmune thrombocytopenia (FNAIT) in subsequent pregnancies. Serial fetal blood sampling (FBS) and intrauterine platelet transfusions (IUPT), as well as weekly maternal IV immunoglobulin infusion (IVIG), with or without additional corticosteroid therapy, are common options, but optimal management has not been determined. The aim of this systematic review was to assess antenatal treatment strategies for FNAIT. Four randomized controlled trials and 22 nonrandomized studies were included. Pooling of results was not possible due to considerable heterogeneity. Most studies found comparable outcomes regarding the occurrence of intracranial hemorrhage, regardless of the antenatal management strategy applied; FBS, IUPT, or IVIG with or without corticosteroids. There is no consistent evidence for the value of adding steroids to IVIG. FBS or IUPT resulted in a relatively high complication rate (consisting mainly of preterm emergency cesarean section) of 11% per treated pregnancy in all studies combined. Overall, noninvasive management in pregnant mothers who have had a previous neonate with FNAIT is effective without the relatively high rate of adverse outcomes seen with invasive strategies. This systematic review suggests that first-line antenatal management in FNAIT is weekly IVIG administration, with or without the addition of corticosteroids.
Introduction
Fetal and neonatal alloimmune thrombocytopenia (FNAIT) may lead to severe bleeding complications, such as intracranial hemorrhage (ICH), in the fetus or newborn. Thrombocytopenia is caused by maternal alloantibodies against human platelet (PLT) antigens (HPAs) resulting from maternal alloimmunization after exposure to paternally derived antigens on fetal PLTs. The most commonly involved are HPA-1a alloantibodies, which are responsible for ∼80% of FNAIT cases.1,2 Not only do these maternal alloantibodies cause destruction and inhibit the production of fetal PLTs, they are also thought to affect vascular integrity and angiogenesis, resulting in an increased risk of intracranial and extracranial bleeding complications in fetuses and neonates and potentially intrauterine and perinatal death.3-6
In the absence of population-based screening programs, the diagnosis of FNAIT is usually made after an incidental finding of neonatal thrombocytopenia or because of bleeding complications ranging from bruising or petechiae to intracranial hemorrhage in the fetus or newborn.
Consequently, with an estimated recurrence rate of 79% of severe bleeding complications, the current challenge is to determine the best management strategy for subsequent pregnancies in women with a history of FNAIT, with the goal of preventing these complications and avoiding maternal toxicities.7 To avoid unnecessary interventions and anxiety, paternal genotyping should always be performed for the HPA involved in the preceding FNAIT. In case of paternal heterozygosity, maternal-fetal incompatibility should be determined either by using amniocentesis or by assessing cell-free fetal DNA when HPA-1a is involved.
One of the first prenatal treatment strategies was ultrasound-guided fetal blood sampling (FBS) and intrauterine PLT transfusion (IUPT).8 This technique, used for the treatment of fetal anemia, was applied to fetuses with thrombocytopenia and involved the transfusion of PLTs. Cordocentesis in the presence of thrombocytopenia may, however, lead to fetal bradycardia, tamponade of the cord, and bleeding complications in the fetus, including exsanguination. In addition, given the short life span of transfused PLTs, transfusions are needed regularly, increasing the overall risk of fetal loss.9 The first noninvasive treatment, maternal infusion of intravenous immunoglobulin (IVIG), was reported in 1988, after which IVIG rapidly gained ground as a standard antenatal treatment strategy for FNAIT, as have corticosteroids.10 Prolonged use of IVIG and corticosteroids during pregnancy are associated with adverse effects as well. Although the side effects of IVIG are usually mild, hemolytic anemia, renal failure, aseptic meningitis, and thrombotic complications may occur.11,12 Corticosteroids are associated with hypertension and diabetes. Both agents can affect the quality of life of patients.12
No international consensus on the optimal antenatal management of FNAIT exists, and numerous strategies, noninvasive as well as invasive, are applied in different centers that specialize in antenatal therapy. Because FNAIT is a rare disease, systematically reviewing the literature to determine the evidence to support antenatal treatment options can inform practice. Hence, we performed a systematic review of all available literature on antenatal management strategies to inform and assist in the development of guidelines.
Methods
Data sources
This review was performed according to the PRISMA guidelines.13 With the assistance of a medical research library specialist, an electronic search strategy was developed and applied to Medline, EMBASE, and Cochrane Library databases from 1946 to December 2015 (supplemental Appendix, available on the Blood Web site). Reference lists were cross-checked for relevant citations.
Study selection and data extraction
Citations were reviewed by 2 reviewers to identify studies that met the following inclusion criteria: (1) original study; (2) included ≥5 pregnant women with pregnancies at risk for FNAIT or fetuses/neonates diagnosed with FNAIT; (3) treated with either IVIG, steroids, or IUPT; (4) included any of the outcomes: intracranial hemorrhage and fetal/neonatal PLT count; and 5) published in the English language. When there was a disagreement, the full report was retrieved and independent assessment was repeated. Disagreements for inclusion were resolved by consensus. For articles that were published more than once and contained the same FNAIT population, only the study with the largest number of women and the most complete data extraction was included. Data extraction was performed by 2 authors according to a predetermined standardized format of study characteristics, outcome data, and complications of interventions (Table 1).
Study outcomes
| Reference . | Study arms (risk group in case of stratification) . | N . | ICH in sibling, n (%) . | FBS, n (%) . | IUPT, n (%) . | FBS/IUPT-related AE, n (%) . | Duration IVIG, wk, mean (range) . | SE IVIG/ steroids, n (%) . | ICH, n (%) . | Mean PLTs × 109/L . | PLT < 50 × 109/L, n (%) . | Mortality, n (%) . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Randomized controlled trials | ||||||||||||
| 30 | IVIG 0.5 g | 12 | 0 | 0 | 0 | NA | 10 (7-11) | 0 | 0 | 81 | 3 (25) | 0 |
| IVIG 1 g | 11 | 0 | 0 | 0 | NA | 11 (7-12) | 0 | 0 | 110 | 4 (36) | 0 | |
| 37 | IVIG 2 g | 37 | 0 | All | 0 | 2 (5) | 16 (11-20) | 12 (32) | 1 | 169 | 5 (14) | 0 |
| IVIG 1 g + steroids | 36 | 0 | All | 0 | 2 (6) | 16 (12-19) | 13 (33) | 1 | 134 | 4 (11) | 0 | |
| 35 | IVIG (all) | 40 | 4 (10) | All | 0 | 11 (14) | NR | NR | 3 | 104 | NR | 4 (5) |
| IVIG + steroids (high) | 19 | 3 (16) | All | 0 | NR | NR | 99 | NR | ||||
| Steroids (standard) | 20 | 0 | All | 0 | NA | NR | 108 | NR | ||||
| 20 | IVIG* | 28 | 6 (21) | All | 0 | 5 (9) | 10 | 0 | 0 | 96 | <30 6 (21) | 5 (9)† |
| IVIG + steroids | 26 | 4 (15) | All | 0 | 11 | 2 (8) | 0 | 110 | <30 5 (19) | |||
| Prospective studies | ||||||||||||
| 17 | IVIG ± IUPT | 7 | 7 (100) | 3 (43) | 3 (43) | NR | 17 (8-21) | NR | 0 | 28‡ | 7 (100)‡ | 0 |
| 39 | IUPT predelivery | 2 | 0 | All | 2 (100) | NR | NA | NA | 0 | 210 | 0 | 0 |
| IVIG ± IUPT | 4 | 0 | 2 (50) | 2 (50) | NR | 17 (15-19) | NR | 0 | 204 | 0 | 0 | |
| IVIG + steroids | 13 | 2 (15) | 1 (8) | 1 (8) | NR | 12 (5-20) | NR | 0 | 118 | 4 (31) | 0 | |
| 18 | IVIG ± IUPT | 37 | 8 (19) | 26 (70) | 26 (70) | 0 | 5 (2-15) | NR | 0 | 67 | 17 (46) | 0 |
| FBS ± IUPT | 13 | All | 9 (69) | 2 (22) | NA | NA | 0 | 32 | 7 (54) | 1 (8) | ||
| 23 | IVIG | 8 | 3 (30) | All | NR | 2 (20) | 12 (3-16) | NR | 0 | NR | 1 (13) | 0 |
| Fetal IVIG | 2 | All | NR | 2 (1-2) | NR | 0 | NR | 2 (100) | 0 | |||
| 22 | IVIG | 9 | 5 (56) | All | 1 (11) | NR | NR | NR | 0 | 57 | 4 (44) | NR |
| IVIG + steroids | 9 | 3 (33) | 8 (89) | 0 | NR | NR | 5 (56) | 0 | 64 | 3 (33) | NR | |
| Retrospective studies | ||||||||||||
| 31 | IVIG 1 g (all) | 5 | 2 (40) | 0 | 0 | NA | NR | 0 | 1§ | 63 | 8 (67) | 0 |
| IVIG 0.5 g (standard) | 17 | 0 | 0 | 0 | NA | NR | 0 | 0 | 104 | 4 (33) | 0 | |
| 32 | IVIG | 27 | 9 (14) | 0 | 0 | NA | 14 | NR | 0 | 89 | 12 (44) | 0 |
| IVIG + steroids | 54 | 0 | 0 | NA | 14 | NR | 0 | 135 | 13 (27) | 0 | ||
| Steroids | 11 | 0 | 0 | NA | NA | NR | 0 | 46 | 8 (73) | 0 | ||
| 24 | IVIG | 17 | 5 (29) | 9 | 0 | 1 (11) | 12 | NR | 0 | 68 | 10 (59) | 0 |
| IVIG + steroids | 6 | 0 | 0 | 0 | NA | 7 | NR | 0 | 78 | 4 (67) | 0 | |
| 19 | IVIG 1 g (high + very high) | 5 | 5 (100) | All | 1 (17) | 3 (8) | 15 (7-25) | NR | 1 | 165 | 0 | 0 |
| IVIG 1 g + steroids (all) | 19 | 19 (100) | All | 0 | 12 (5-25) | NR | 3 | 85 | 6 (40) | 2 (11) | ||
| IVIG 2 g (all) | 4 | 4 (100) | All | 0 | 22 (18-25) | NR | 0 | 112 | 0 | 0 | ||
| IVIG 2 g + steroids (all) | 9 | 9 (100) | All | 0 | 23 (18-27) | NR | 1 | 135 | 0 | 0 | ||
| 21 | Fetal IVIG + IUPT | 10 | NR | All | 10 (100) | 0 | 10 (6-14) | NR | 0 | 189¶ | 0 | 0 |
| 26 | IVIG | 13 | 5 (38) | NR | 2 (15) | NR | NR | NR | 0 | 83 | 6 (46) | 0 |
| 34 | IVIG (all) | 53 | 5 (9) | 0 | 0 | NA | 8 (2-24) | NR | 0 | 125 | 10 (19) | 0 |
| FBS + IVIG (all) | 33 | 11 (33) | All | NR | 3 (7) | 6 (2-21) | NR | 0 | 174 | 0 | 1 (3) | |
| FBS + IUPT (standard) | 13 | 0 | All | 13 (100) | 0 | NA | NA | 0 | 145 | 3 (23) | 0 | |
| 41 | IUPT ± IVIG ± steroids | 40 | NR | All | 40 (100) | 3 (8) | NR | NR | 4 | 107 | NR | 6 (15) |
| IVIG and/or steroids | 7 | NR | NR | 0 | 1 (14) | NR | NR | 0 | 7-219 | NR | 1 (14) | |
| No treatment | 8 | NR | NA | NA | NA | NA | NA | 0 | 6-84 | NR | 0 | |
| 38 | IVIG | 24 | 0 | 4 (17) | 0 | NR | 15 (9-19) | NR | 0 | 118 | <30 2 (8) | 0 |
| No treatment | 6 | 0 | NA | NA | NA | NA | NA | 0 | 24 | <30 4 (67) | 0 | |
| 27 | IVIG* | 9 | 5 (56) | 0 | 0 | NA | NR | NR | 0 | 90 | 4 (44) | 0 |
| IUPT | 3 | 0 | All | 3 (100) | 3 (100) | NA | NA | 0 | 47 | 2 (100) | 1 (33) | |
| No treatment | 6 | 2 (34) | 0 | 0 | NA | NA | NA | 1 | 9 | 4 (80) | 1 (17) | |
| 28 | IVIG ± IUPT* | 18 | 6 (60) | All | 6 (33) | 3 (17) | 9 (1-19) | 1 (6) | 1§ | 81 | 6 (33) | 0 |
| IUPT weekly | 31 | 11 (42) | All | 31 (100) | 10 (30) | NA | NA | 2§ | NR | NR | 3 (10) | |
| FBS ± single IUPT | 7 | 0 | All | 5 (71) | 2 (29) | NA | NA | 0 | NR | NR | 0 | |
| 42 | IVIG ± IUPT* | 11 | 1 (9) | All | 9 (82) | 2 (18) | 6 (1-12) | 1 (9) | 0 | 109 | 5 (45) | 0 |
| IUPT | 4 | 0 | All | 4 (100) | 2 (50) | NA | NA | 0 | 76 | 2 (50) | 0 | |
| 29 | IVIG | 27 | 7 (26) | All | 1 (4) | NR | 7 (2-15) | NR | 2 | 69 | 13 (48) | 2 (7) |
| Steroids | 10 | NR | All | NR | NR | NA | NR | NR | NR | 6 (60) | NR | |
| 33 | IVIG + IUPT | 4 | 1 (25) | All | 4 (100) | 1 (25) | NR | 0 | 0 | 182 | 0 | 1 (25) |
| IVIG | 6 | 1 (17) | All | 0 | 1 (17) | NR | 0 | 0 | 98 | 2 (33) | 1 (17) | |
| 40 | IVIG + IUPT ± steroids | 8 | 6 (75) | All | 6 (100) | 1 (13) | 9 (4-17) | NR | 1§ | 340¶ | 0¶ | 2 (25) |
| IUPT ± steroids | 7 | 5 (71) | All | 7 (100) | 0 | NA | NR | 2§ | 305¶ | 0¶ | 1 (14) | |
| 36 | IVIG | 2 | NR | All | 0 | NR | 14 (13-14) | 0 | 0 | 60 | 1 (50) | 0 |
| IVIG + steroids | 4 | NR | All | 0 | NR | 11 (5-20) | 0 | 0 | 146 | 0 | 0 | |
| 25 | IUPT | 4 | 1 (20) | All | 5 (100) | 0 | NA | NA | 0 | 200 | 0 | 0 |
| IVIG (5 d) + IUPT | 1 | 0 | All | 1 (100) | 0 | 5 | 0 | 0 | 107 | 0 | 0 | |
| Reference . | Study arms (risk group in case of stratification) . | N . | ICH in sibling, n (%) . | FBS, n (%) . | IUPT, n (%) . | FBS/IUPT-related AE, n (%) . | Duration IVIG, wk, mean (range) . | SE IVIG/ steroids, n (%) . | ICH, n (%) . | Mean PLTs × 109/L . | PLT < 50 × 109/L, n (%) . | Mortality, n (%) . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Randomized controlled trials | ||||||||||||
| 30 | IVIG 0.5 g | 12 | 0 | 0 | 0 | NA | 10 (7-11) | 0 | 0 | 81 | 3 (25) | 0 |
| IVIG 1 g | 11 | 0 | 0 | 0 | NA | 11 (7-12) | 0 | 0 | 110 | 4 (36) | 0 | |
| 37 | IVIG 2 g | 37 | 0 | All | 0 | 2 (5) | 16 (11-20) | 12 (32) | 1 | 169 | 5 (14) | 0 |
| IVIG 1 g + steroids | 36 | 0 | All | 0 | 2 (6) | 16 (12-19) | 13 (33) | 1 | 134 | 4 (11) | 0 | |
| 35 | IVIG (all) | 40 | 4 (10) | All | 0 | 11 (14) | NR | NR | 3 | 104 | NR | 4 (5) |
| IVIG + steroids (high) | 19 | 3 (16) | All | 0 | NR | NR | 99 | NR | ||||
| Steroids (standard) | 20 | 0 | All | 0 | NA | NR | 108 | NR | ||||
| 20 | IVIG* | 28 | 6 (21) | All | 0 | 5 (9) | 10 | 0 | 0 | 96 | <30 6 (21) | 5 (9)† |
| IVIG + steroids | 26 | 4 (15) | All | 0 | 11 | 2 (8) | 0 | 110 | <30 5 (19) | |||
| Prospective studies | ||||||||||||
| 17 | IVIG ± IUPT | 7 | 7 (100) | 3 (43) | 3 (43) | NR | 17 (8-21) | NR | 0 | 28‡ | 7 (100)‡ | 0 |
| 39 | IUPT predelivery | 2 | 0 | All | 2 (100) | NR | NA | NA | 0 | 210 | 0 | 0 |
| IVIG ± IUPT | 4 | 0 | 2 (50) | 2 (50) | NR | 17 (15-19) | NR | 0 | 204 | 0 | 0 | |
| IVIG + steroids | 13 | 2 (15) | 1 (8) | 1 (8) | NR | 12 (5-20) | NR | 0 | 118 | 4 (31) | 0 | |
| 18 | IVIG ± IUPT | 37 | 8 (19) | 26 (70) | 26 (70) | 0 | 5 (2-15) | NR | 0 | 67 | 17 (46) | 0 |
| FBS ± IUPT | 13 | All | 9 (69) | 2 (22) | NA | NA | 0 | 32 | 7 (54) | 1 (8) | ||
| 23 | IVIG | 8 | 3 (30) | All | NR | 2 (20) | 12 (3-16) | NR | 0 | NR | 1 (13) | 0 |
| Fetal IVIG | 2 | All | NR | 2 (1-2) | NR | 0 | NR | 2 (100) | 0 | |||
| 22 | IVIG | 9 | 5 (56) | All | 1 (11) | NR | NR | NR | 0 | 57 | 4 (44) | NR |
| IVIG + steroids | 9 | 3 (33) | 8 (89) | 0 | NR | NR | 5 (56) | 0 | 64 | 3 (33) | NR | |
| Retrospective studies | ||||||||||||
| 31 | IVIG 1 g (all) | 5 | 2 (40) | 0 | 0 | NA | NR | 0 | 1§ | 63 | 8 (67) | 0 |
| IVIG 0.5 g (standard) | 17 | 0 | 0 | 0 | NA | NR | 0 | 0 | 104 | 4 (33) | 0 | |
| 32 | IVIG | 27 | 9 (14) | 0 | 0 | NA | 14 | NR | 0 | 89 | 12 (44) | 0 |
| IVIG + steroids | 54 | 0 | 0 | NA | 14 | NR | 0 | 135 | 13 (27) | 0 | ||
| Steroids | 11 | 0 | 0 | NA | NA | NR | 0 | 46 | 8 (73) | 0 | ||
| 24 | IVIG | 17 | 5 (29) | 9 | 0 | 1 (11) | 12 | NR | 0 | 68 | 10 (59) | 0 |
| IVIG + steroids | 6 | 0 | 0 | 0 | NA | 7 | NR | 0 | 78 | 4 (67) | 0 | |
| 19 | IVIG 1 g (high + very high) | 5 | 5 (100) | All | 1 (17) | 3 (8) | 15 (7-25) | NR | 1 | 165 | 0 | 0 |
| IVIG 1 g + steroids (all) | 19 | 19 (100) | All | 0 | 12 (5-25) | NR | 3 | 85 | 6 (40) | 2 (11) | ||
| IVIG 2 g (all) | 4 | 4 (100) | All | 0 | 22 (18-25) | NR | 0 | 112 | 0 | 0 | ||
| IVIG 2 g + steroids (all) | 9 | 9 (100) | All | 0 | 23 (18-27) | NR | 1 | 135 | 0 | 0 | ||
| 21 | Fetal IVIG + IUPT | 10 | NR | All | 10 (100) | 0 | 10 (6-14) | NR | 0 | 189¶ | 0 | 0 |
| 26 | IVIG | 13 | 5 (38) | NR | 2 (15) | NR | NR | NR | 0 | 83 | 6 (46) | 0 |
| 34 | IVIG (all) | 53 | 5 (9) | 0 | 0 | NA | 8 (2-24) | NR | 0 | 125 | 10 (19) | 0 |
| FBS + IVIG (all) | 33 | 11 (33) | All | NR | 3 (7) | 6 (2-21) | NR | 0 | 174 | 0 | 1 (3) | |
| FBS + IUPT (standard) | 13 | 0 | All | 13 (100) | 0 | NA | NA | 0 | 145 | 3 (23) | 0 | |
| 41 | IUPT ± IVIG ± steroids | 40 | NR | All | 40 (100) | 3 (8) | NR | NR | 4 | 107 | NR | 6 (15) |
| IVIG and/or steroids | 7 | NR | NR | 0 | 1 (14) | NR | NR | 0 | 7-219 | NR | 1 (14) | |
| No treatment | 8 | NR | NA | NA | NA | NA | NA | 0 | 6-84 | NR | 0 | |
| 38 | IVIG | 24 | 0 | 4 (17) | 0 | NR | 15 (9-19) | NR | 0 | 118 | <30 2 (8) | 0 |
| No treatment | 6 | 0 | NA | NA | NA | NA | NA | 0 | 24 | <30 4 (67) | 0 | |
| 27 | IVIG* | 9 | 5 (56) | 0 | 0 | NA | NR | NR | 0 | 90 | 4 (44) | 0 |
| IUPT | 3 | 0 | All | 3 (100) | 3 (100) | NA | NA | 0 | 47 | 2 (100) | 1 (33) | |
| No treatment | 6 | 2 (34) | 0 | 0 | NA | NA | NA | 1 | 9 | 4 (80) | 1 (17) | |
| 28 | IVIG ± IUPT* | 18 | 6 (60) | All | 6 (33) | 3 (17) | 9 (1-19) | 1 (6) | 1§ | 81 | 6 (33) | 0 |
| IUPT weekly | 31 | 11 (42) | All | 31 (100) | 10 (30) | NA | NA | 2§ | NR | NR | 3 (10) | |
| FBS ± single IUPT | 7 | 0 | All | 5 (71) | 2 (29) | NA | NA | 0 | NR | NR | 0 | |
| 42 | IVIG ± IUPT* | 11 | 1 (9) | All | 9 (82) | 2 (18) | 6 (1-12) | 1 (9) | 0 | 109 | 5 (45) | 0 |
| IUPT | 4 | 0 | All | 4 (100) | 2 (50) | NA | NA | 0 | 76 | 2 (50) | 0 | |
| 29 | IVIG | 27 | 7 (26) | All | 1 (4) | NR | 7 (2-15) | NR | 2 | 69 | 13 (48) | 2 (7) |
| Steroids | 10 | NR | All | NR | NR | NA | NR | NR | NR | 6 (60) | NR | |
| 33 | IVIG + IUPT | 4 | 1 (25) | All | 4 (100) | 1 (25) | NR | 0 | 0 | 182 | 0 | 1 (25) |
| IVIG | 6 | 1 (17) | All | 0 | 1 (17) | NR | 0 | 0 | 98 | 2 (33) | 1 (17) | |
| 40 | IVIG + IUPT ± steroids | 8 | 6 (75) | All | 6 (100) | 1 (13) | 9 (4-17) | NR | 1§ | 340¶ | 0¶ | 2 (25) |
| IUPT ± steroids | 7 | 5 (71) | All | 7 (100) | 0 | NA | NR | 2§ | 305¶ | 0¶ | 1 (14) | |
| 36 | IVIG | 2 | NR | All | 0 | NR | 14 (13-14) | 0 | 0 | 60 | 1 (50) | 0 |
| IVIG + steroids | 4 | NR | All | 0 | NR | 11 (5-20) | 0 | 0 | 146 | 0 | 0 | |
| 25 | IUPT | 4 | 1 (20) | All | 5 (100) | 0 | NA | NA | 0 | 200 | 0 | 0 |
| IVIG (5 d) + IUPT | 1 | 0 | All | 1 (100) | 0 | 5 | 0 | 0 | 107 | 0 | 0 | |
AE, adverse event; NA, not applicable; NR, not reported; SE, side effect.
One or 2 patients also received steroids (supplemental Table 1).
Five fetuses exsanguinated and were excluded from other analyses.
Platelet count before predelivery IUPT.
ICH occurred before start of therapy.
Platelet count after IUPT.
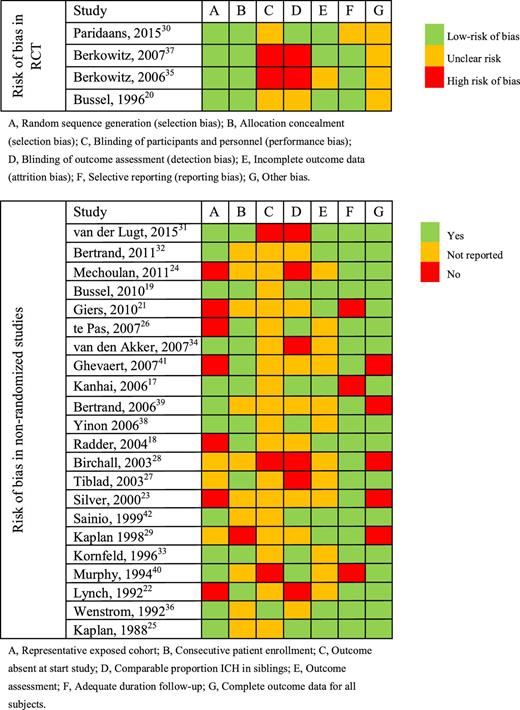
Risk of bias was assessed according to the Cochrane Collaboration’s tool14 for randomized studies and the Newcastle-Ottawa Scale15 for nonrandomized studies. The Newcastle-Ottawa Scale is based on 3 parameters: selection, comparability, and outcome (Table 2). For the parameter selection, we assessed whether the exposed cohort was representable for the FNAIT population (defined as HPA-incompatible pregnancies), if the patient enrollment was consecutive, and if ICH was absent at the start of the treatment. The parameter comparability was met if cohorts had a comparable proportion of siblings with ICH. For the parameter outcome of the Newcastle-Ottawa Scale, we assessed if the outcome of ICH was assessed by cranial ultrasound, if the follow-up was adequate (neonatal instead of fetal PLT count), and lastly, if neonatal PLT count and ICH data were available for all subjects.
Data analysis
Due to considerable methodological heterogeneity of the studies, a descriptive review of all included studies was performed rather than a meta-analysis. In 2011, a Cochrane review of part of the included RCTs was performed by Rayment et al,16 who also did not pool data.
Results
Study selection and characteristics
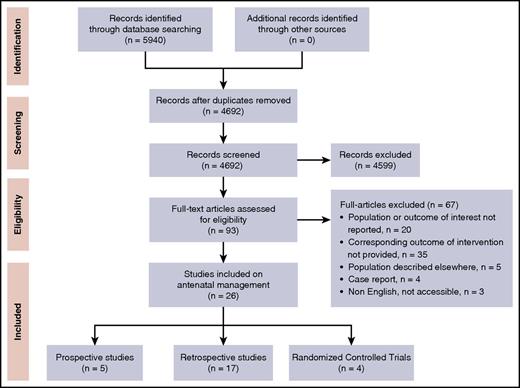
Our search strategy retrieved a total of 4692 single records that were screened for title and abstract, resulting in 93 full-text articles to be assessed for eligibility. Of those, 26 studies describing antenatal interventions in FNAIT were included (Figure 1), consisting of 4 RCTs, 5 prospective, and 17 retrospective studies (Table 1; supplemental Table 1).
Most studies included pregnancies at risk for FNAIT based on a history of FNAIT, additionally specified as with ICH,17-19 PLT count <100 × 109/L,20,21 PLT count <50 × 109/L,17,22 or with signs of bleeding,21,23,24 or based on another female family member with FNAIT25 or recurrent spontaneous miscarriages.21 One study identified postnatal FNAIT patients from a population of thrombocytopenic neonates.26 Five studies did not report testing for incompatibility between pregnant women and fetuses as a condition for inclusion in their study.21,22,27-29 FBS was performed in all but 3 studies.30-32 The earliest that fetal blood was sampled was in gestational week 16,33 but most commonly, sampling began in weeks 20 or 22. Of the 16 studies performing IUPT, 8 reported a fetal PLT count threshold to infuse PLTs.17-19,21-23,28,34 HPA-1a was the predominant cause of FNAIT in all articles, ranging from 72% to 100% of reported patients.
The overall quality of the RCTs was considered adequate, with lack of blinding presenting the highest risk of bias (Table 2). Comparing or pooling data from the nonrandomized cohort studies included in this review was hampered by differences in patient selection, in particular HPA type and severity of disease in the previous affected siblings.21,24 In addition, relevant data were lacking in several studies, such as exclusion of ICH by ultrasound before starting treatment35 and outcome data for all treatment arms (Table 2).
Antenatal management
IVIG and corticosteroids.
Of the 26 studies, 17 had a treatment arm with IVIG alone,19,20,22-24,26,27,29-38 3 studies had a treatment arm with corticosteroids alone,29,35 and 11 had a study arm that combined IVIG and corticosteroid treatment.19,20,22,24,32,35-37,39-41 There were 2 studies that compared all 3 arms.35,39 In most studies, IVIG was administered at a dose of 1 g/kg per week. Doses other than 1g/kg per week in ≥1 case were reported in 9 studies: 0.4 g/kg per day for 5 days,25,40 0.5 g/kg per week,22,30,31 0.8 g/kg per week,28 1 g/kg every 2 weeks,24,32 and 2 g/kg per week.19,37 Two studies did not report the IVIG dose.39,41 IVIG administration commenced as early as 10 weeks’ gestation19 and as late as 32 weeks’ gestation.18 Two studies administered IVIG directly to the fetus.21,23 Prednisone was used mainly at a dose of 0.5 mg/kg per day and dexamethasone was used at a dose of 1.5 mg/day. Specific dosages can be found in supplemental Table 1.
FBS/IUPT.
FBS was employed in 24 of the 26 studies. In 16 of these studies, FBS was combined with IUPTs. Five studies included a study arm with IUPT as the sole treatment.25,27,28,38,42 IUPT in combination with IVIG was used in three studies.21,33,40 The remainder of IUPTs were performed in addition to a maternal therapy strategy of IVIG and/or steroids.17-19,21,25,28,29,39,42 One study reported FBS and PLT transfusion in all fetuses prior to delivery.21 Three studies did not report the number of IUPTs performed for their study groups.22,29,34
Risk stratification.
Perinatal outcome
ICH.
All but 1 study described the occurrence of ICH for all study arms.29 In the 25 studies in which ICH was described, of the 839 pregnancies, a total of 24 ICHs were observed (3%). Seven of these occurred before treatment was started and 1 occurred in a group where no treatment was provided. Four ICHs were described by Heaver et al41 as part of a large retrospective analysis of patients with suspected FNAIT investigated at a reference laboratory. Unfortunately, no additional information on previously affected pregnancies or on the patients themselves was provided. Of the remaining 12 patients, 5 were described by Bussel et al,19 who reported different strategies of IVIG treatment in a high-risk population (all siblings suffered from an ICH). Three ICHs (2 grade III-IV hemorrhages resulting in fetal demise and 1 grade I hemorrhage) occurred after receiving 1 g/kg per week of IVIG and 1 mg/kg per day of prednisone, the fourth one was a grade II-III perinatal hemorrhage after delivery at 24 weeks’ gestation and the last one was a grade I hemorrhage, both after a combination of 2 g/kg per week of IVIG with 1 mg/kg per day of prednisone. Furthermore, Berkowitz et al35 described 2 neonates with ICHs that occurred in a low-risk population where none of the siblings suffered an ICH. Both ICHs were grade I subependymal hemorrhages, detected postnatally with normal neonatal PLT counts at birth (133 and 197 × 109/L) after treatment with 2 g/kg per week of IVIG and 1 g/kg per week of IVIG with 1 mg/kg per day of prednisone (treatment started at 20 weeks). Kaplan et al29 described 27 pregnancies treated with 1g/kg per week of IVIG, in which 2 fetuses had ICHs (1 resulting in death and 1 resulting in neurological sequela). Both were in the group of nine patients with persistently low PLTs despite treatment. Lastly, Berkowitz et al35 reported 3 ICHs, 2 grade I hemorrhages and 1 grade III hemorrhage, in a neonate that was delivered at 28 weeks’ gestation because of persisting fetal bradycardia after FBS. Overall, no remarkable or significant differences could be identified in the occurrence of ICHs between various study arms.
Mortality.
Two studies did not report mortality rates.22,29 In the 24 remaining studies, there was an overall mortality rate of 4% (30/821); of these, 17 were related to FBS/IUPT (53%) and 7 were due to an ICH (22%). In 6 fetuses/neonates, the cause of death could not be determined. Ghevaert et al41 described a fetal loss due to acute amnionitis at 16 weeks’ gestation (not related to treatment) and Murphy et al40 reported a fetal loss after a severe fall of the mother on icy pavement.
Neonatal PLT count.
Twenty studies reported neonatal PLT counts. Of the other 6 studies, 1 study reported the fetal PLT counts before predelivery IUPT,17 2 studies reported fetal PLT counts after predelivery IUPT,21,40 and 3 studies did not provide the neonatal PLT counts for all study arms.23,28,29 The mean neonatal PLT counts (× 109/L), as well as the proportion of neonates with PLT counts <50 × 109/L, varied widely between the studies, ranging from 0% to 100%, regardless of the intervention.
Three studies compared IVIG treatment alone to corticosteroids alone.29,32,35 Kaplan et al29 found a higher proportion of neonates with a PLT count <50 × 109/L in the group treated with steroids compared with the IVIG only group (60% vs 48%) as did Bertrand et al32 (73% vs 44%). Berkowitz et al35 found comparable mean PLT counts between those groups in patients; 104 × 109/L with IVIG only versus 108 × 109/L in the steroids only arm.
Three studies compared a noninvasive strategy (IVIG or IVIG and corticosteroids without FBS) to a strategy that included FBS and IUPT.27,33,34 Cornfield et al33 showed that IVIG treatment alone improved neonatal PLT counts in 4 of 6 patients, however, only 1 pregnancy was high risk. Tiblad et al27 reported a higher median PLT count of 90 × 109/L in the group treated with IVIG and a lower proportion of neonates with PLT counts <50 × 109/L (44% vs 100% in patients treated with IUPT). In addition, in the group treated with IVIG, 56% of the pregnancies were high risk, compared with 0% in the group treated with IUPT. Most recently, Van den Akker et al34 compared 53 women treated with IVIG only to 13 women treated with IUPT only; median neonatal PLT counts were 125 × 109/L and 145 × 109/L, respectively.
Of the 8 studies that compared IVIG only with IVIG and corticosteroids, Berkowitz et al35 identified comparable PLT counts between groups treated with IVIG only and IVIG with steroids (104 × 109/L and 99 × 109/L, respectively). The same group of investigators19 described management in 37 high-risk pregnancies. Four regimens, based on the timing of an ICH occurring in a previous pregnancy, were compared (Table 1; supplemental Table 1). No differences in neonatal PLT count between the treatment groups were identified (Table 1). Although Bertrand et al32 reported a significant difference in the number of neonates that needed postnatal treatment (26% in the group treated with IVIG and steroids vs 59% in the group treated with IVIG only [P = .01]), no significant differences in mean neonatal PLT count or severe thrombocytopenia were observed. The remaining 5 studies reported comparable neonatal PLT counts in women treated with IVIG only and IVIG combined with corticosteroids as well.20,22,24,36,37
Of the 4 studies that compared different IVIG regimens, 2 found comparable neonatal PLT counts with doses of 0.5 g/kg per week, 1 g/kg per week, and 2g/kg per week.19,30 Van Der Lugt et al31 reported a nonsignificant, lower mean PLT count in 5 women treated with 1g/kg per week (63 × 109/L) compared with 17 women treated with 0.5 g/kg per week (104 × 109/L).
Treatment-related complications.
Of the 24 studies in which FBS was performed with or without IUPT, 2 studies reported no procedure-related complications and 12 studies reported a total of 53 complications with a frequency ranging from 3% to 39% per treated pregnancy (Table 3). One study reported complications in more detail elsewhere.20,43 Overall, the proportion of treated cases with complications due to either FBS or IUPT was 11% (54 complications in 497 treated pregnancies). The most frequently described complication was the performance of an emergency cesarean section, mainly due to fetal distress (persisting bradycardia or fetal decelerations), of which approximately half resulted in a delivery before 34 weeks’ gestation. Fourteen of the 54 complications resulted in fetal or neonatal death (26%).
Complications of antenatal treatment
| Reference . | AEs in FBS/IUPT n/N (%)* . | Complications after FBS or IUPT (n) . | SEs in IVIG n/N (%)* . | Reported SEs in IVIG treatment (n) . | SEs in steroids n/N (%)* . | Reported SEs in steroid treatment (n) . | |
|---|---|---|---|---|---|---|---|
| 24 | 1/9 (11) | Emergency CS due to fetal distress (1); <34 wk (0) | NR | NR | NR | NR | |
| 19 | 4/37 (11) | Emergency CS or delivery (4); <34 wk (NR); due to fetal distress (3); insertion bleeding (1) | NR | NR | NR | NR | |
| 34 | 3/99 (3) | Perinatal death (1); emergency CS due to fetal distress (3); <34 wk (0) | NR | NR | NA | NA | |
| 37 | 4/74 (5) | Emergency CS (4); <34 wk (3); due to fetal distress (2); PROM (2) | NR | Rash (1) discontinued IVIG, headache, fatigue† | NR | Gestational diabetes (7), insomnia, mood swings† | |
| 35 | 11/79 (14) | Fetal death (1); neonatal death (1); emergency CS or delivery (10); <34 wk (NR); due to fetal distress (8); streaming (1); PROM (1) | NR | NR | NR | NR | |
| 18 | 2/40 (5) | Neonatal death after fetal distress (1); emergency CS due to exsanguination (1) | NR | NR | NR | NR | |
| 28 | 15/38 (39) | Fetal death (2); after exsanguination (1); emergency CS/delivery (13); <34 wk (6); due to fetal distress (6), infection (1), technical difficulties (3), cord spasm or thrombosis (2), placental artery bleeding (1) | 1/18 (6) | Headache and tachycardia (1), continued IVIG | NR | NR | |
| 23 | 2/10 (20) | Emergency CS due to insertion bleeding (2), <34 wk (1) | NR | NR | NA | NA | |
| 42 | 4/15 (27) | Emergency CS or delivery (4); <34 wk (1); due to fetal distress (3); acute amnionitis after ROM (1) | 1/11 (9) | Headache and tachycardia (1), continued IVIG | NR | NR | |
| 20 | 5/59 (9)‡ | Fetal or neonatal death after exsanguination (5)‡ | 0/54 | None | 2/26 (8) | Oligohydramnios (2) dexamethasone 1.5 mg - dexamethasone 4.5 mg | |
| 33 | 2/10 (20) | Pregnancy loss at 16 wk’s gestation (1); neonatal death due to chorioamnionitis at 25 wk (1); | 0/10 | None | NA | NA | |
| 40 | 1/15 (7) | fetal death due to cord hematoma (1) | NR | NR | NR | NR | |
| 22 | NR | NR | NR | NR | 5/9 (56) | Oligohydramnios (4) dexamethasone 5 mg | |
| Reference . | AEs in FBS/IUPT n/N (%)* . | Complications after FBS or IUPT (n) . | SEs in IVIG n/N (%)* . | Reported SEs in IVIG treatment (n) . | SEs in steroids n/N (%)* . | Reported SEs in steroid treatment (n) . | |
|---|---|---|---|---|---|---|---|
| 24 | 1/9 (11) | Emergency CS due to fetal distress (1); <34 wk (0) | NR | NR | NR | NR | |
| 19 | 4/37 (11) | Emergency CS or delivery (4); <34 wk (NR); due to fetal distress (3); insertion bleeding (1) | NR | NR | NR | NR | |
| 34 | 3/99 (3) | Perinatal death (1); emergency CS due to fetal distress (3); <34 wk (0) | NR | NR | NA | NA | |
| 37 | 4/74 (5) | Emergency CS (4); <34 wk (3); due to fetal distress (2); PROM (2) | NR | Rash (1) discontinued IVIG, headache, fatigue† | NR | Gestational diabetes (7), insomnia, mood swings† | |
| 35 | 11/79 (14) | Fetal death (1); neonatal death (1); emergency CS or delivery (10); <34 wk (NR); due to fetal distress (8); streaming (1); PROM (1) | NR | NR | NR | NR | |
| 18 | 2/40 (5) | Neonatal death after fetal distress (1); emergency CS due to exsanguination (1) | NR | NR | NR | NR | |
| 28 | 15/38 (39) | Fetal death (2); after exsanguination (1); emergency CS/delivery (13); <34 wk (6); due to fetal distress (6), infection (1), technical difficulties (3), cord spasm or thrombosis (2), placental artery bleeding (1) | 1/18 (6) | Headache and tachycardia (1), continued IVIG | NR | NR | |
| 23 | 2/10 (20) | Emergency CS due to insertion bleeding (2), <34 wk (1) | NR | NR | NA | NA | |
| 42 | 4/15 (27) | Emergency CS or delivery (4); <34 wk (1); due to fetal distress (3); acute amnionitis after ROM (1) | 1/11 (9) | Headache and tachycardia (1), continued IVIG | NR | NR | |
| 20 | 5/59 (9)‡ | Fetal or neonatal death after exsanguination (5)‡ | 0/54 | None | 2/26 (8) | Oligohydramnios (2) dexamethasone 1.5 mg - dexamethasone 4.5 mg | |
| 33 | 2/10 (20) | Pregnancy loss at 16 wk’s gestation (1); neonatal death due to chorioamnionitis at 25 wk (1); | 0/10 | None | NA | NA | |
| 40 | 1/15 (7) | fetal death due to cord hematoma (1) | NR | NR | NR | NR | |
| 22 | NR | NR | NR | NR | 5/9 (56) | Oligohydramnios (4) dexamethasone 5 mg | |
AE, adverse event; CS, cesarean section; CTG, cardiotocogram; NR, not reported; PROM, prelabor rupture of membranes; SE, side effect.
Number of reported complications (n) versus the total number of patients treated with this specific strategy (N).
Number of side effects reported; the total number of patients that reported a side effect is unclear.
The complications occurring during this study were reported in detail elsewhere.40
Of the 26 studies that used either IVIG or corticosteroids, 11 reported the side effects of the treatment. The most commonly reported side effect of dexamethasone treatment was the occurrence of oligohydramnios. Headache and rash were the most frequently reported side effects of IVIG treatment, leading to discontinuing of the treatment in only 1 patient.37
Discussion
Main findings
A noninvasive management approach in pregnancies complicated by FNAIT was found to be equally effective as compared with IUPT in preventing fetal and neonatal bleeding due to thrombocytopenia. Our analysis revealed a relatively high complication rate of antenatal management by FBS and IUPT of 11%, with 1 in 3 of these leading to fetal or neonatal loss. The most common noninvasive treatment administered to pregnant women was IVIG, primarily in a weekly dose of 1 g/kg. IVIG only treatment had a 98.7% success rate for preventing ICH (4 ICHs occurred in 315 pregnancies).16,17,19-24,26-32,34,35 This is consistent with the rate of 97.3% found in the Cochrane analysis reported by Rayment et al,16 which included 37 pregnancies treated with IVIG only. However, none of the studies were powered to detect a significant difference in bleeding outcomes.
Strengths and limitations
Besides the obvious lack of randomized studies with an adequate control group (placebo or no treatment), the main limitation of our review is the heterogeneity of the extracted data from the primary studies. Although neonatal outcomes are generally well reported and appear quite homogenous, the crux of the heterogeneity is the diversity of the study designs. First, there was extensive variation in the treatment strategies used, especially with respect to the different combinations. For example, Sainio et al42 described 15 women treated with 6 different strategies (IVIG only, IVIG and steroids, IVIG and IUPT, IVIG and steroids and IUPT, as well as weekly IUPT or FBS only). Secondly, the dosage of specific treatments differed considerably (eg, prednisone was prescribed as 0.5 to 1 mg/kg per day as well as 10 mg, 20 mg, 30 mg, and 60 mg per day). The interval and duration of therapeutic strategies also differed considerably between studies. For example, the mean duration of IVIG treatment varied from 2 weeks18,23 to 22 weeks.19 Additionally, in 3 of the 4 RCTs, treatment intensification was applied to increase fetal PLT counts, which could have led to underestimation of the difference between treatment arms when comparing neonatal PLT counts.20,35,37 Lastly, there was great variability in the risk of ICH when determined by the proportion of siblings with an ICH not only between studies, but also between study arms.
The 2 most commonly used endpoints for studies are ICH and neonatal PLT counts. Whereas antenatal strategies target the prevention of bleeding complications in fetuses and neonates, preferably mortality and long-term neurodevelopmental impairment should be the gold standard outcomes. Because these outcomes are rare, most studies are not powered to detect significant differences between treatment strategies and must resort to using PLT counts as surrogate outcome measurements.
In this regard, there appears to be a correlation between PLT count and risk of bleeding, but this does not appear to be a linear relationship.41 Although the neonatal PLT count appears to be the logical and best available surrogate outcome in evaluating antenatal treatment strategies, this parameter has limitations. Comparing treatment modalities based on mean or median PLT counts may therefore show some effect, but may not be meaningful clinically.44 In addition, very low PLT counts were often found in fetuses or neonates without any bleeding. Although it is unclear to what extent animal studies can be used for understanding pathophysiology in humans, there is increasing evidence suggesting impairment of angiogenesis and endothelial integrity as a possible cause of increased bleeding tendency, leading to the assumption that thrombocytopenia is not the sole cause of bleeding complications in FNAIT.3,45,46
Our systematic review was designed to evaluate the effect of antenatal treatment options on neonatal outcomes, including neonatal PLT count, ICH, and mortality, but it did not facilitate any conclusions on the need for centralized care, the optimal timing or mode of delivery, nor whether predelivery FBS should be performed to determine the mode of delivery, neonatal brain imaging, or the need for matched PLTs.
Ultimately, to our knowledge, this is the first systematically performed review that considers all available evidence, including randomized as well as nonrandomized studies. Despite the size and heterogeneity of the studies limiting the strength of this evidence, we used predefined outcome measures of all available evidence on antenatal management in pregnancies complicated by FNAIT.
Interpretation
This review suggests that noninvasive treatment strategies are safe and effective options for the antenatal management of pregnancies complicated by FNAIT, with a lower risk of severe complications compared with FBS and/or IUPT. The gestational age at which to start antenatal IVIG treatment in FNAIT has, however, not been well defined. It is reasonable to consider the severity of the disease in previous pregnancies when making treatment decisions. An earlier start of IVIG treatment will not necessarily result in a linear increase in the amount of immunoglobulin g (IgG) transported to the fetus.47 The amount of IgG that traverses the placenta depends on gestational age (with the greatest placental transport taking place in the third trimester), the IgG subclass, maternal IgG levels, and placental integrity.47
In cohort analyses performed by Bussel et al19 and Van der Lugt et al,31 pregnancies were divided into risk groups based on the only established risk factor for recurrent ICHs: whether the sibling had (high risk) or did not have (standard risk) an ICH and when the ICH occurred in pregnancy (high risk, very high risk, and extremely high risk).48,49 The time of initiation of IVIG treatment was based on this stratification, and the dosage used relied on the presumption that ICH recurred in 79% of subsequent pregnancies.7 An analysis of 43 cases of ICH performed by Tiller et al44 suggested that in order to reduce the risk of recurrent ICHs in subsequent pregnancies, IVIG should be initiated before 20 weeks’ gestation.
Whether the commonly used dose of 1 g/kg per week is the best treatment for all FNAIT pregnancies, or whether this could be reduced or increased in certain subgroups remains unclear. Data from the previously described randomized controlled trial30 and retrospective data provided by Van Der Lugt et al31 showed that the lower dose of 0.5 g/kg per week appeared not to be inferior to the 1 g/kg per week IVIG in standard risk (ie, a previous sibling that did not have an ICH) populations. Given the dose-related side effects and costs, a dose of 0.5 g/kg per week could be regarded as suitable for these women. A limited number of patients were treated with the lower dose, and therefore more data are probably required to change practice. Conversely, higher doses (ie, 2 g/kg per week) have also been used, but the studies analyzed were limited by adequately comparable treatment arms.19,37
The use of IVIG in pregnancies at risk for FNAIT is still off-label, and the possible immunostimulative or immunosuppressive effect of exposing the maturing fetal immune system to IVIG has not been adequately addressed. One cohort study by Radder et al18 attempted to address this by examining the neurodevelopmental outcome of 50 children at a median age of 5 years, of which 37 were exposed to IVIG during fetal life. A higher incidence of otorhinolaryngological and hearing disability in the group that did not receive IVIG was found. IgG, IgG subclass, IgA, and IgM levels were comparable between groups. A trend was found between high plasma IgE levels and in utero IVIG exposure; nonetheless, no difference in eczema or allergies was observed between the 2 groups. Although, based on this small cohort study, in utero exposure to IVIG seems to have no clinically apparent adverse effects in early childhood, further immunological research with a larger group of patients is needed to fully answer this question.
The benefit of adding corticosteroids to IVIG is unclear. One study found improvement in PLT counts (defined as a PLT count >25 × 109/L at second sampling, an increase by >10 × 109/L compared with the first sampling, or a PLT count >40 × 109/L that was not decreased by >10 × 109/L).16,35 The remaining 8 studies that compared treatment with IVIG to IVIG with steroids did not show significant differences in the PLT count, ICH, or mortality.19,20,22,24,32,35-37 More data from randomized studies comparing IVIG to IVIG with steroids that include an adequate control group are needed to reach any firm conclusions.
To achieve a major improvement in the treatment and prevention of FNAIT, physicians need to be able to prevent index cases, a strategy that was proven to be highly successful in hemolytic disease of the fetus and newborn, caused by the red blood cell counterpart of FNAIT. In order to do so, population-based screening programs are needed to identify first pregnancies at risk in time to start effective antenatal prophylaxis or treatment.
In conclusion, this article represents a systematic review on the effectiveness of different antenatal treatment strategies in pregnancies complicated by FNAIT, aiming to prevent ICH and bleeding-related fetal/neonatal losses. Our summary provides the best available evidence that suggests that the optimal approach is a noninvasive approach, involving weekly administration of IVIG, with or without the addition corticosteroids. Regarding the optimal dose and start of the treatment, there are insufficient data to recommend a specific gestational age or a specific dose. However, the data support the treatment of high-risk pregnancies (ie, sibling suffered from an ICH) with a dose of 1 g/kg per week of IVIG, started between 12 and 20 weeks’ gestation. For standard risk pregnancies (ie, no sibling suffered from an ICH), the data support starting treatment between 20 and 24 weeks’ gestation and the use of IVIG at a dose of 1 g/kg per week with or without steroids. Additional data, especially a reliable biomarker of severity in a patient known to be affected, might allow the use of a lower dose of IVIG (ie, 0.5 g/kg per week) or, alternatively, a higher dose of IVIG (ie, 2 g/kg per week) with or without corticosteroids, depending upon severity.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Shuoyan Ning for her assistance with data abstraction, Elizabeth Uleryk for the search strategy, and Sylvia Torrance and Kimberly Figures for administrative assistance.
This work was supported in part by Canadian Blood Services. Canadian Blood Services did not have any role in the design, analysis, and interpretation of the data or preparation, review, and approval of the manuscript.
The authors thank the International Collaboration for Transfusion Medicine Guidelines members: Shubha Allard, Barts Health NHS Trust and NHS Blood and Transplant; Celso Bianco, formerly with America's Blood Centers; Jeannie Callum, University of Toronto; Veerle Compernolle, Belgian Red Cross; Anne Eder, American Red Cross Blood Services; Dean Fergusson, University of Ottawa; Mark Fung, Fletcher-Allen Health Care; Andreas Greinacher, University of Greifswald; Heather Hume, University of Montreal; Lani Lieberman, University of Toronto; Catherine Moltzan, University of Manitoba; Michael Murphy, University of Oxford; Susan Nahirniak, University of Alberta; Katerina Pavenski, University of Toronto; Joanne Pink, Australian Red Cross Blood Services; Arjuna Ponnampalam, University of Manitoba; Paolo Rebulla, Ospedale Maggiore Policlinico; Nadine Shehata, University of Toronto; Cynthia So-Osman, Groene Hart Ziekenhuis, The Netherlands; Simon J. Stanworth, University of Oxford; Zbigniew M. Szczepiorkowski, Dartmouth-Hitchcock Medical Center; Susano Tanael, Canadian Blood Services; Alan T. Tinmouth, University of Ottawa; Ralph Vassallo, Blood Systems; Erica Wood, Monash University.
Authorship
Contribution: D.W. drafted the initial manuscript; J. Baker, E.M., L.L., N.S., and T.B. performed the data search; D.W. extracted and analyzed the data, supported by N.S. and S.T.; M.F.M., A.G., H.H., D.M.A., S.B., G.B., J. Bussel, M.K., C.K., J.K-K., D.O., and G.R. participated in the interpretation and revision of critically important contents of the review and gave final approval of the version to be submitted.
Conflict-of-interest disclosure: M.K. and J.K. are two of the founders and owners of Prophylix Pharma AS, a Norwegian biotech company coordinating the European Union–funded PROFNAIT Consortium, which is developing a prophylaxis against FNAIT. N.S. is a consultant for Canadian Blood Services. D.O. has received research funding for the project “Towards Routine HPA-Screening in Pregnancy.” J.B. is a consultant of Baxalta, Superior Biologics. The remaining authors declare no competing financial interests.
C.K. is retired from the Institut National de la Transfusion Sanguine.
Correspondence: Dick Oepkes, Department of Obstetrics, Leiden University Medical Center, P.O. Box 9600, K6-35, 2300 RC Leiden, The Netherlands; e-mail: d.oepkes@lumc.nl.