To the editor:
Acute promyelocytic leukemia (APL) is a subtype of acute myeloid leukemia (AML), characterized by the t(15;17) translocation resulting in the PML-RARA fusion transcript. The use of all-trans retinoic acid (ATRA) and/or arsenic has revolutionized the treatment of this disease, which is now the most curable form of AML. However, APL is characterized by a high rate of early death (17%), mainly resulting from severe coagulopathy.1 Because this coagulopathy can be controlled with ATRA, cytological recognition of APL is of utmost importance to promptly start adequate therapy. Definitive confirmation of the diagnosis is later obtained with the detection of the hallmark translocation and/or the PML-RARA transcript using, respectively, cytogenetics or molecular biology.
Most of the time, cytological diagnosis of APL is straightforward because blast cells harbor primary azurophilic granulations and multiple bundles of Auer rods. Microgranular APL is a well-recognized cytological variant, whose identification is sometimes tricky, even for an experienced hematopathologist. In ambiguous cases, high myeloperoxidase (MPO) expression (assessed by flow cytometry) and activity (evidenced by cytochemistry) are a characteristic feature of APL, which can help in the early diagnostic procedure. We report here a case of APL without any expression or activity of MPO and describe the molecular mechanisms explaining this unusual presentation.
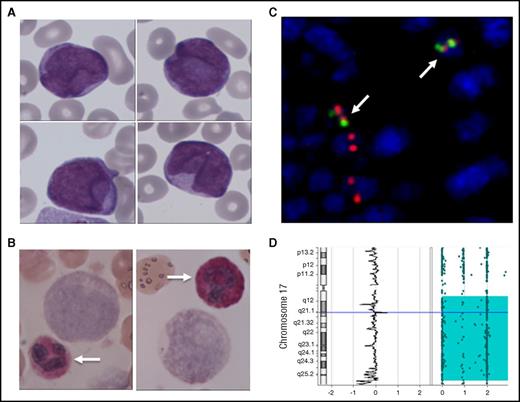
A 53-year-old white man, with no personal or familial medical history of APL, was referred to our institution for bleeding diathesis. Written informed consent was obtained from the patient. Blood cell count revealed marked leukocytosis (white blood cells, 36.1 × 109/L; neutrophils, 0.33 × 109/L; lymphocytes, 2.53 × 109/L; monocytes, × 109/L) with up to 95% of blasts (31 × 109/L), severe thrombocytopenia (16 × 109/L), and coagulopathy (prothrombin time, 50%; fibrinogen, 0.69 g/L; fibrinogen degradation products, 89 μg/mL). Peripheral blood and bone marrow aspirate examination showed heterogeneous medium-sized blast cells, most without granulations. No Auer rods were observed (Figure 1A). The blast cell phenotype was compatible with what is commonly observed in APL (CD45+lo/SSChi, CD34−, CD38+, CD1123+hi, CD13+ heterogeneous, CD33+, CD2+, CD9+) despite negative intracellular staining of MPO. This feature was further confirmed by cytochemistry that found no MPO enzymatic activity in blast cells, whereas neutrophil granulocytes used as internal control were strongly stained (Figure 1B). Negative toluidine blue staining and absence of CD203 expression excluded the diagnosis of acute basophilic leukemia. Of note, there was no detectable clonal abnormality on karyotype on 20 observed mitoses. Because the clinical presentation and the flow cytometry pattern strongly suggested APL diagnosis, we further investigated this case by fluorescence in situ hybridization analysis, which found a cryptic insertion of the chromosome 15q22 region within the chromosome 17q21 locus (Figure 1C), leading to a PML-RARA fusion transcript (bcr1) that was also detected by reverse transcription-quantitative polymerase chain reaction. Of note, 2 fusion signals were observed by nuclei, suggesting a duplication of the derivative chromosome 17. The patient was treated with a combination of ATRA and standard cytotoxic chemotherapy. He reached complete remission after induction and complete molecular remission after first consolidation therapy; flow cytometry evaluation of the MPO level was normal in polymorphonuclear leukocytes (data not shown). Each cycle was marked by severe infectious complication: 2 episodes of severe sepsis and a possible invasive pulmonary aspergillosis.
Cytological and molecular characterization of a MPO-negative APL. (A) May Grünwald Giemsa staining of a blood smear showing medium-sized myeloid blasts without granulation or Auer rods. (B) Cytochemical evidence for MPO negativity of blast cells (as compared with neutrophils; arrows). (C) Molecular evidence of the t(15;17) translocation by fluorescent in situ hybridization showing double fusion signals in blast cells (arrows). (D) Single nucleotide polymorphism analysis showing a uniparental disomy of the long arm of chromosome 17. Original magnification ×400 for panels A-B.
Cytological and molecular characterization of a MPO-negative APL. (A) May Grünwald Giemsa staining of a blood smear showing medium-sized myeloid blasts without granulation or Auer rods. (B) Cytochemical evidence for MPO negativity of blast cells (as compared with neutrophils; arrows). (C) Molecular evidence of the t(15;17) translocation by fluorescent in situ hybridization showing double fusion signals in blast cells (arrows). (D) Single nucleotide polymorphism analysis showing a uniparental disomy of the long arm of chromosome 17. Original magnification ×400 for panels A-B.
Further analyses were performed to understand the unusual characteristics of this APL (ie, unusual cytological presentation with MPO negativity). Comparative genomic hybridization and single nucleotide polymorphism array revealed a uniparental disomy (UPD) for the 17q chromosome (Figure 1D), where the MPO gene is located (17q22). We hypothesized that UPD of the MPO gene in blast cells might explain the phenotype of these cells. Indeed, at the constitutional genomic level, the patient harbored a heterozygous mutation of the intronic 3′ splice acceptor site of MPO intron 11 (c.2031-2A>C)(rs35897051) (Figure 2A), which has been previously reported in a family with hereditary myeloperoxidase deficiency.2 Because of the 17q UPD, the blast cells were homozygous for this mutant allele (Figure 2A). The consequence of this mutation is the retention of intron 11 on messenger RNA (mRNA), explaining an electrophoretic shift (Figure 2B). Whereas a small fraction of normal MPO mRNA from the residual normal hematopoiesis was detectable in the diagnostic sample, this mRNA was largely dominant in the complete remission sample, probably because of decreased stability of the mutated mRNA. Sanger sequencing of the complementary DNA confirmed intron 11 retention, leading to a premature STOP codon that prevents MPO expression at the protein level. Altogether, the blast cells of this APL were homozygous for MPO loss of function mutation because of UPD of the 17q chromosome in an asymptomatic MPO deficiency allele carrier (Figure 2C).
Molecular mechanism of MPO deficiency in blast cells. (A) Sanger sequencing of the intron 11 of MPO, showing a c.2031-2A>C mutation of the intronic 3′ splice acceptor site, which is heterozygous in germline (left) and homozygous in APL (middle). The right panel is a schematic representation of the 2 MPO alleles. (B) Polymerase chain reaction analysis of the MPO mRNA, showing intron 11 retention in the APL diagnosis sample (Diag) and not in the sample collected at the time of molecular complete remission(CR). A control mRNA (CTRL) was used to identify the normal size of MPO mRNA. The right panel is a schematic representation of the wild-type (WT) and mutant MPO mRNA. (C) Schematic overview of the molecular oncogenesis explaining this MPO-negative APL case. The patient is an asymptomatic heterozygote carrier of an MPO-deficient allele (MUT, left), who developed APL after t(15;17)(PML-RARA) translocation (middle). Because t(15;17) is located on chromosome 17 with the MPO-deficient allele, the 17q UPD results in homozygous MPO-deficient blast cells (right).
Molecular mechanism of MPO deficiency in blast cells. (A) Sanger sequencing of the intron 11 of MPO, showing a c.2031-2A>C mutation of the intronic 3′ splice acceptor site, which is heterozygous in germline (left) and homozygous in APL (middle). The right panel is a schematic representation of the 2 MPO alleles. (B) Polymerase chain reaction analysis of the MPO mRNA, showing intron 11 retention in the APL diagnosis sample (Diag) and not in the sample collected at the time of molecular complete remission(CR). A control mRNA (CTRL) was used to identify the normal size of MPO mRNA. The right panel is a schematic representation of the wild-type (WT) and mutant MPO mRNA. (C) Schematic overview of the molecular oncogenesis explaining this MPO-negative APL case. The patient is an asymptomatic heterozygote carrier of an MPO-deficient allele (MUT, left), who developed APL after t(15;17)(PML-RARA) translocation (middle). Because t(15;17) is located on chromosome 17 with the MPO-deficient allele, the 17q UPD results in homozygous MPO-deficient blast cells (right).
MPO is contained in the azurophilic granulations of neutrophils and plays a major role in their oxidative killing. MPO deficiency is the most common neutrophil biochemical defect, leading to higher susceptibility to infections.3 According to the Single Nucleotide Polymorphism database, about 0.7% of the population carries the mutant allele involved in this case. The resulting loss of exon 12 might prevent MPO expression because N-glycosylation of asparagine residues, including ASP 729 encoded by exon 12, is mandatory for MPO maturation. In particular, MPO glycosylation is essential for the interaction of MPO propeptide with calreticulin, which protects MPO from proteasomal degradation.4 MPO-negative APL that can lead to misdiagnosed APL has already been reported,5 but the underlying molecular mechanism has remained unknown so far. This case report illustrates how constitutional MPO mutant heterozygosity can lead to the absence of MPO expression, because of somatically acquired uniparental disomy leading to homozygosity of the defective allele in the leukemic cells.
Authorship
Contribution: M.H. and P.S. designed the study and performed the experiments; E.P., A.P., M.-O.G., D.M., S.G., I.T., E.C.-B., S. Huet, G.S., X.T., and S. Hayette were highly involved in the care of the patient or the routine diagnostic procedure; and all authors contributed to the redaction of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Pierre Sujobert, Hospices Civils de Lyon, Centre Hospitalier Lyon Sud, Laboratoire d’Hématologie, 165 Chemin du Grand Revoyet, 69695 Pierre Benite Cedex, France; e-mail: pierre.sujobert@chu-lyon.fr.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal