Abstract
The incidence of early death in a large population of unselected patients with acute promyelocytic leukemia (APL) remains unknown because of the paucity of outcome data available for patients treated outside of clinical trials. We undertook an epidemiologic study to estimate the true rate of early death with data from the Surveillance, Epidemiology, and End Results (SEER) program. A total of 1400 patients with a diagnosis of APL between 1992 and 2007 were identified. The overall early death rate was 17.3%, and only a modest change in early death rate was observed over time. The early death rate was significantly higher in patients aged ≥ 55 years (24.2%; P < .0001). The 3-year survival improved from 54.6% to 70.1% over the study period but was significantly lower in patients aged ≥ 55 years (46.4%; P < .0001). This study shows that the early death rate remains high despite the wide availability of all-trans retinoic acid and appears significantly higher than commonly reported in multicenter clinical trials. These data highlight a need to educate health care providers across a wide range of medical fields, who may be the first to evaluate patients with APL, to have a major effect on early death and the cure rate of APL.
Introduction
Acute promyelocytic leukemia (APL) is a subtype of acute myeloid leukemia (AML) characterized by distinctive morphology of blast cells,1,2 a life-threatening coagulopathy,3 and a specific balanced reciprocal translocation t(15;17),4 which fuses the PML (promyelocyte) gene on chromosome 15 to the RARα (retinoic acid receptor-α) gene on chromosome 17.5 Since the introduction of the differentiating agent all-trans retinoic acid (ATRA), the disease has become the most curable subtype of adult AML. Despite this dramatic improvement in outcome, early death primarily because of hemorrhage before and during induction therapy remains the main cause for treatment failure.
Cooperative group multicenter clinical trials report an early death rate of ∼ 5%-10% within the first month of starting ATRA, mainly from intracranial bleeding.6-12 However, the incidence of early death in unselected patients with APL remains unknown because of the paucity of outcome data available for patients treated outside of the clinical trial setting. Furthermore, the excellent outcome reported in multicenter clinical trials may engender a sense of safety and complacency that may lead to an underestimation of the critical aspects of APL management at the time of initial presentation when most deaths occur. Therefore, we undertook an epidemiologic study to estimate the true rate of early death with the use of a U.S. population-based dataset.
Methods
Patients
Data from 13 population-based cancer registries that participate in the National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) program13 were used for the study. The 13 registries were San Francisco-Oakland, Connecticut, Detroit (Metropolitan), Hawaii, Iowa, New Mexico, Seattle (Puget Sound), Utah, Atlanta (Metropolitan), San Jose-Monterey, Los Angeles, Alaska Natives, and rural Georgia. The SEER registries routinely collect data on patient demographics, primary site, tumor structure, stage at diagnosis, first course of treatment, and follow-up information.
The recent public dataset available from 13 SEER registries includes all patients whose disease was diagnosed from 1992 to 2007. In this study, patients with APL diagnosed from 1992 to 2007 were identified with the use of the International Classification of Disease for Oncology, Third Edition coding (histology code = 9866).14 For incidence rate calculations, all patients with APL were included. However, the following patients were excluded from the survival analysis: (1) patients diagnosed through autopsy or death certificate (n = 9); (2) patients with second or later primaries (n = 149); (3) patients with unknown or missing cause of death (n = 5); or (4) patients with no survival time (n = 14). Three study time periods were predetermined according to year of diagnosis: 1992-1995, 1996-2001, and 2002-2007. The initial time period of 1992-1995 was selected to mark the time before ATRA was approved by the Food and Drug Administration (FDA) in November 1995. Subsequent time periods of 1996-2001 and 2002-2007 were determined to allow equal number of years between these periods.
All patients were followed from the date of diagnosis until death or end of follow-up (December 31, 2007), whichever occurred first. The covariates of interest included time period of diagnosis, age (< 35, 35-54, and > 54), sex, resident county (rural vs urban), race (white, black, or other), and region (East, Northern Plains, Pacific Coast, South West).
Outcome measures
Incidence rates for APL were calculated by time period of diagnosis, age, sex, resident county, race, and region. All rates were age-adjusted to the 2000 US standard population. SEER-defined cause-specific death15 was the end point of interest for the survival analysis. Early death was defined as death within 1 month of APL diagnosis, and the probability of early death was calculated by subtracting survival rate at 1 month from 1. Survival rates at 1 month, 1 year, 2 years, and 3 years were calculated for each covariate with the use of the actuarial method. The effect of each covariate on the probability of early death was assessed with a univariate χ2 test. The log-rank test was used to determine the statistical significance of the difference in the survival distributions for each covariate.
To evaluate the effect of all covariates on mortality risk simultaneously, we performed Cox proportional hazard regression analysis. We started with a full model that included all covariates plus time period of diagnosis. Variable selection was conducted with the stepwise method (P to entry = 0.2, P to stay = 0.15). Multiple logistic regression analysis was used to evaluate the effect of covariates on the probability of early death. SEER*Stat v6.6.216 and SAS v9.1.3 (SAS Institute) were used for statis-tical analysis. Statistical significance was determined with a 2-sided P value < .05.
Results
Incidence of APL
A total of 1400 patients with APL were identified in the study (Table 1). The overall annual age-adjusted incidence rate was 0.23 per 100 000. A moderate increase was observed in the incidence of APL during the study period, from 0.16 in 1992-1995 to 0.28 in 2002-2007. The incidence rate was much higher in the older age group (0.42 per 100 000) than in the younger age group (0.13 per 100 000), and the median age at diagnosis was 44 years. No large rate variations were observed by sex, race, resident county, or region.
Summary statistics according to patients whose disease was diagnosed from 1992 to 2007 in SEER13 coverage areas
| . | No of patients . | Incidence rate* . | Probability of early death, % . | P† . | Survival rate, % . | P‡ . | ||
|---|---|---|---|---|---|---|---|---|
| 1 Y . | 2 Y . | 3 Y . | ||||||
| Overall | 1400 | 0.23 | 17.3 | 70.7 | 67.0 | 65.7 | ||
| Sex | ||||||||
| Male | 659 | 0.23 | 19.4 | .052 | 70.6 | 66.9 | 66.4 | .895 |
| Female | 741 | 0.24 | 15.3 | 70.8 | 67.1 | 65.2 | ||
| Race§ | ||||||||
| White | 1136 | 0.24 | 17.2 | .897 | 69.9 | 66.3 | 65.2 | .543 |
| Black | 118 | 0.19 | 16.3 | 72.7 | 67.9 | 66.6 | ||
| Other | 144 | 0.20 | 18.6 | 74.2 | 70.1 | 68.0 | ||
| Age group | ||||||||
| < 35 y | 433 | 0.13 | 12.3 | < .0001 | 81.2 | 77.9 | 76.3 | < .0001 |
| 35-54 y | 465 | 0.26 | 16.0 | 75.6 | 72.4 | 72.0 | ||
| > 54 y | 502 | 0.42 | 24.2 | 53.2 | 48.4 | 46.4 | ||
| Time period of diagnosis | ||||||||
| 1992-1995 | 224 | 0.16 | 22.1 | .068 | 62.5 | 56.8 | 54.6 | .0002 |
| 1996-2001 | 495 | 0.22 | 14.7 | 70.9 | 67.2 | 66.0 | ||
| 2002-2007 | 681 | 0.28 | 17.5 | 73.4 | 70.7 | 70.1 | ||
| Resident county‖ | ||||||||
| Urban | 1287 | 0.23 | 17.2 | .728 | 71.0 | 67.3 | 66.2 | .239 |
| Rural | 109 | 0.21 | 18.6 | 66.8 | 63.2 | 60.2 | ||
| Region¶ | ||||||||
| East | 233 | 0.23 | 16.9 | .818 | 74.9 | 72.4 | 72.4 | .210 |
| Northern Plains | 251 | 0.22 | 18.7 | 68.8 | 64.7 | 62.1 | ||
| Pacific Coast | 796 | 0.24 | 16.6 | 70.6 | 66.7 | 65.4 | ||
| Southwest | 120 | 0.20 | 19.4 | 66.7 | 62.9 | 62.9 | ||
| . | No of patients . | Incidence rate* . | Probability of early death, % . | P† . | Survival rate, % . | P‡ . | ||
|---|---|---|---|---|---|---|---|---|
| 1 Y . | 2 Y . | 3 Y . | ||||||
| Overall | 1400 | 0.23 | 17.3 | 70.7 | 67.0 | 65.7 | ||
| Sex | ||||||||
| Male | 659 | 0.23 | 19.4 | .052 | 70.6 | 66.9 | 66.4 | .895 |
| Female | 741 | 0.24 | 15.3 | 70.8 | 67.1 | 65.2 | ||
| Race§ | ||||||||
| White | 1136 | 0.24 | 17.2 | .897 | 69.9 | 66.3 | 65.2 | .543 |
| Black | 118 | 0.19 | 16.3 | 72.7 | 67.9 | 66.6 | ||
| Other | 144 | 0.20 | 18.6 | 74.2 | 70.1 | 68.0 | ||
| Age group | ||||||||
| < 35 y | 433 | 0.13 | 12.3 | < .0001 | 81.2 | 77.9 | 76.3 | < .0001 |
| 35-54 y | 465 | 0.26 | 16.0 | 75.6 | 72.4 | 72.0 | ||
| > 54 y | 502 | 0.42 | 24.2 | 53.2 | 48.4 | 46.4 | ||
| Time period of diagnosis | ||||||||
| 1992-1995 | 224 | 0.16 | 22.1 | .068 | 62.5 | 56.8 | 54.6 | .0002 |
| 1996-2001 | 495 | 0.22 | 14.7 | 70.9 | 67.2 | 66.0 | ||
| 2002-2007 | 681 | 0.28 | 17.5 | 73.4 | 70.7 | 70.1 | ||
| Resident county‖ | ||||||||
| Urban | 1287 | 0.23 | 17.2 | .728 | 71.0 | 67.3 | 66.2 | .239 |
| Rural | 109 | 0.21 | 18.6 | 66.8 | 63.2 | 60.2 | ||
| Region¶ | ||||||||
| East | 233 | 0.23 | 16.9 | .818 | 74.9 | 72.4 | 72.4 | .210 |
| Northern Plains | 251 | 0.22 | 18.7 | 68.8 | 64.7 | 62.1 | ||
| Pacific Coast | 796 | 0.24 | 16.6 | 70.6 | 66.7 | 65.4 | ||
| Southwest | 120 | 0.20 | 19.4 | 66.7 | 62.9 | 62.9 | ||
Incidence rate was age-adjusted to the US 2000 standard population.
Chi-square test was used to test the early death rate difference among groups for each covariate.
Log-rank test was used to test survival curve difference among groups for each covariate. All survival data are used in this test.
Other race includes American Indians/Alaska Natives and Asian/Pacific Islanders. The patients with unknown race (n = 2) are not listed; therefore, the sum of white, black, and other was not equal to total.
The patients with unknown resident county (n = 4) are not listed; therefore, the sum of urban and rural was not equal to total.
Region is defined as follows: East includes Connecticut, Atlanta (Metropolitan), and Rural Georgia; Northern Plains includes Detroit (Metropolitan) and Iowa; Pacific Coast includes San Francisco-Oakland, Hawaii, Seattle (Puget Sound), San Jose-Monterey, and Los Angeles; and Southwest includes New Mexico and Utah. Because no patients with APL were reported from Alaska during 1992-2007, the region of Alaska is not listed.
Early death rate
A total of 177 patients were excluded from the survival and early death rate calculations, who met the exclusion criteria as defined in “Methods.” The overall early death rate was 17.3% during the study period (Table 1). The univariate χ2 test analysis (Table 1) showed that there was no significant change in early death rate when analyzed by each time period (P = .068). However, when more specific comparisons were made (Table 2), the patients with diagnosis in 1996-2001 showed a significantly lower early death rate than the patients with diagnosis in 1992-1995 (P = .012). Although the early death rate in 2002-2007 was also lower compared with 1992-1995, this did not reach statistical significance (P = .077).
Results of logistic regression models that evaluated the effects of various factors on probability of early death
| Independent variable* . | Full model . | Reduced model . | ||
|---|---|---|---|---|
| Adjusted odds ratio (95% CI) . | P . | Adjusted odds ratio (95% CI) . | P . | |
| Sex | ||||
| Male (ref) | 1.000 | 1.000 | ||
| Female | 0.749 (0.544-1.031) | .077 | 0.753 (0.572-0.993) | .044 |
| Race | ||||
| White (ref) | 1.000 | |||
| Black | 0.999 (0.553-1.802) | .997 | ||
| Other | 1.184 (0.715-1.960) | .511 | ||
| Age group | ||||
| < 35 (ref) | 1.000 | 1.000 | ||
| 35-54 | 1.306 (0.861-1.980) | .209 | 1.315 (0.918-1.885) | .136 |
| > 54 | 2.296 (1.537-3.431) | < .0001 | 2.287 (1.619-3.232) | < .0001 |
| Time period of diagnosis | ||||
| 1992-1995 (ref) | 1.000 | 1.000 | ||
| 1996-2001 | 0.601 (0.380-0.949) | .029 | 0.603 (0.406-0.897) | .012 |
| 2002-2007 | 0.722 (0.472-1.102) | .131 | 0.717 (0.497-1.036) | 0.077 |
| Resident county | ||||
| Rural (ref) | 1.000 | |||
| Urban | 1.067 (0.576-1.975) | .837 | ||
| Region† | ||||
| East (ref) | 1.000 | |||
| Northern Plains | 1.080 (0.620-1.882) | .786 | ||
| Pacific Coast | 0.918 (0.581-1.450) | .715 | ||
| Southwest | 1.122 (0.571-2.206) | .739 | ||
| Independent variable* . | Full model . | Reduced model . | ||
|---|---|---|---|---|
| Adjusted odds ratio (95% CI) . | P . | Adjusted odds ratio (95% CI) . | P . | |
| Sex | ||||
| Male (ref) | 1.000 | 1.000 | ||
| Female | 0.749 (0.544-1.031) | .077 | 0.753 (0.572-0.993) | .044 |
| Race | ||||
| White (ref) | 1.000 | |||
| Black | 0.999 (0.553-1.802) | .997 | ||
| Other | 1.184 (0.715-1.960) | .511 | ||
| Age group | ||||
| < 35 (ref) | 1.000 | 1.000 | ||
| 35-54 | 1.306 (0.861-1.980) | .209 | 1.315 (0.918-1.885) | .136 |
| > 54 | 2.296 (1.537-3.431) | < .0001 | 2.287 (1.619-3.232) | < .0001 |
| Time period of diagnosis | ||||
| 1992-1995 (ref) | 1.000 | 1.000 | ||
| 1996-2001 | 0.601 (0.380-0.949) | .029 | 0.603 (0.406-0.897) | .012 |
| 2002-2007 | 0.722 (0.472-1.102) | .131 | 0.717 (0.497-1.036) | 0.077 |
| Resident county | ||||
| Rural (ref) | 1.000 | |||
| Urban | 1.067 (0.576-1.975) | .837 | ||
| Region† | ||||
| East (ref) | 1.000 | |||
| Northern Plains | 1.080 (0.620-1.882) | .786 | ||
| Pacific Coast | 0.918 (0.581-1.450) | .715 | ||
| Southwest | 1.122 (0.571-2.206) | .739 | ||
Reference coding was used for each variable. The reference group for each variable was labeled with ref.
Region is defined as follows: East includes Connecticut, Atlanta (Metropolitan), and Rural Georgia; Northern Plains includes Detroit (Metropolitan) and Iowa; Pacific Coast includes San Francisco-Oakland, Hawaii, Seattle (Puget Sound), San Jose-Monterey, and Los Angeles; and Southwest includes New Mexico and Utah. Because no patients with APL were reported from Alaska during 1992-2007, the region of Alaska is not listed.
The early death rate rose with increasing age. The rate was ∼ 2-fold higher for patients aged ≥ 55 years (24.2%) compared with patients aged ≤ 34 years (12.3%; P < .0001). Female patients (15.3%) had a lower early death rate than male patients (19.4%). However, this was statistically significant only in the reduced regression model (P = .044). No significant differences in the early death rate were identified by race, rural/urban county, or region.
Overall survival
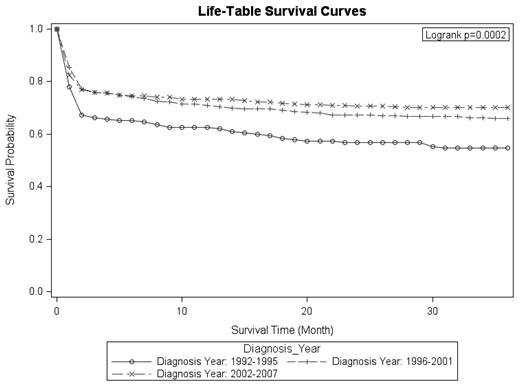
A continuous overall improvement in cause-specific survival was observed over the study period (Table 1). The 3-year survival rate improved from 54.6% in 1992-1995 to 66.0% in 1996-2001 and to 70.1% in 2002-2007. Survival decreased sharply in the first 2 months after the APL diagnosis (Figure 1). After 2 months, survival declined at a much lower rate for each time period of diagnosis.
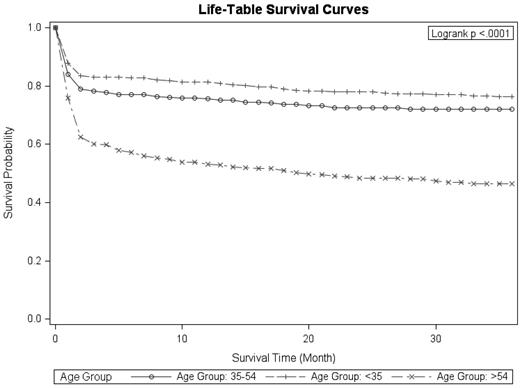
Survival among patients aged ≥ 55 years was considerably lower than among younger patients and appeared to decline at a higher rate than among younger patients throughout the first year after diagnosis (Figure 2). On the basis of the log-rank test, the survival distributions differ significantly by diagnostic time period (P = .0002) and by age group (P < .0001). Although the survival rates were lower for patients residing in rural counties, this difference was not statistically significant (P = .239). No differences were observed by sex, race, and region.
Results of the Cox proportional hazard regression analysis are shown in Table 3. Of the covariates studied, only diagnostic time period and age were significantly associated with mortality risk. Patients diagnosed in 1996-2007 had decreased risk of dying of the disease, compared with patients diagnosed in 1992-1995. Patients aged ≥ 55 years had increased risk of dying of the disease compared with younger patients (< 35 years). Mortality risk for patients aged 35-54 years was also higher in comparison to younger patients, but this difference was not statistically significant (adjusted hazard ratio = 0.694; 95% confidence interval = 0.540-0.892).
Results of Cox proportional hazard regression models evaluating the effects of various factors on mortality risk
| Independent variable* . | Full model . | Reduced model . | ||
|---|---|---|---|---|
| Adjusted hazard ratio (95% CI) . | P . | Adjusted hazard ratio (95% CI) . | P . | |
| Sex | ||||
| Male (ref) | 1.000 | |||
| Female | 1.030 (0.851-1.247) | .761 | ||
| Race | ||||
| White (ref) | 1.000 | |||
| Black | 0.997 (0.693-1.433) | .987 | ||
| Other | 0.875 (0.638-1.199) | .405 | ||
| Age group | ||||
| < 35 y (ref) | 1.000 | 1.000 | ||
| 35-54 y | 1.266 (0.974-1.646) | .078 | 1.258 (0.969-1.633) | .084 |
| > 54 | 2.648 (2.083-3.366) | < .0001 | 2.646 (2.086-3.356) | < .0001 |
| Time period of diagnosis | ||||
| 1992-1995 (ref) | 1.000 | 1.000 | ||
| 1996-2001 | 0.699 (0.544-0.899) | .005 | 0.694 (0.540-0.892) | .004 |
| 2002-2007 | 0.615 (0.479-0.788) | .0001 | 0.609 (0.475-0.781) | .0001 |
| Resident county | ||||
| Rural (ref) | 1.000 | |||
| Urban | 0.927 (0.650-1.322) | .677 | ||
| Region† | ||||
| East (ref) | 1.000 | |||
| Northern Plains | 1.169 (0.823-1.659) | .384 | ||
| Pacific Coast | 1.223 (0.914-1.636) | .176 | ||
| Southwest | 1.355 (0.893-2.057) | .154 | ||
| Independent variable* . | Full model . | Reduced model . | ||
|---|---|---|---|---|
| Adjusted hazard ratio (95% CI) . | P . | Adjusted hazard ratio (95% CI) . | P . | |
| Sex | ||||
| Male (ref) | 1.000 | |||
| Female | 1.030 (0.851-1.247) | .761 | ||
| Race | ||||
| White (ref) | 1.000 | |||
| Black | 0.997 (0.693-1.433) | .987 | ||
| Other | 0.875 (0.638-1.199) | .405 | ||
| Age group | ||||
| < 35 y (ref) | 1.000 | 1.000 | ||
| 35-54 y | 1.266 (0.974-1.646) | .078 | 1.258 (0.969-1.633) | .084 |
| > 54 | 2.648 (2.083-3.366) | < .0001 | 2.646 (2.086-3.356) | < .0001 |
| Time period of diagnosis | ||||
| 1992-1995 (ref) | 1.000 | 1.000 | ||
| 1996-2001 | 0.699 (0.544-0.899) | .005 | 0.694 (0.540-0.892) | .004 |
| 2002-2007 | 0.615 (0.479-0.788) | .0001 | 0.609 (0.475-0.781) | .0001 |
| Resident county | ||||
| Rural (ref) | 1.000 | |||
| Urban | 0.927 (0.650-1.322) | .677 | ||
| Region† | ||||
| East (ref) | 1.000 | |||
| Northern Plains | 1.169 (0.823-1.659) | .384 | ||
| Pacific Coast | 1.223 (0.914-1.636) | .176 | ||
| Southwest | 1.355 (0.893-2.057) | .154 | ||
Reference coding was used for each variable. The reference group for each variable was labeled with ref.
Region is defined as follows: East includes Connecticut, Atlanta (Metropolitan), and Rural Georgia; Northern Plains includes Detroit (Metropolitan) and Iowa; Pacific Coast includes San Francisco-Oakland, Hawaii, Seattle (Puget Sound), San Jose-Monterey, and Los Angeles; and Southwest includes New Mexico and Utah. Because no patients with APL were reported from Alaska during 1992-2007, the region of Alaska is not listed.
Discussion
The large number of patients with newly diagnosed APL and the long follow-up reported here confirm improvement in survival over time in a US population-based study. Disappointingly, the early death rate has decreased only modestly over time and appears significantly higher than that reported in contemporary multicenter clinical trials. This is despite the wide availability of ATRA after the approval by the FDA in November 1995.
Because almost every patient achieves complete remission with ATRA combined with anthracycline-based chemotherapy and the relapse rate once a patient is in complete remission is very low,17 early death primarily because of hemorrhage before and during induction therapy currently remains the main cause for treatment failure in APL.18 Previous studies that examined trends in outcome of APL have reported a dramatic reduction of the early death rate of 5%-10% since the introduction of ATRA.7-12,19 This compares favorably with the reported early death rate of ≤ 20% in patients treated before ATRA became available.20-22 However, these studies have predominantly been conducted in modest-sized cohorts of highly selected patients in a clinical trial setting and fail to account for patients who die of hemorrhagic complications before registration on the clinical trial. Furthermore, because only a small minority of patients with cancer are enrolled in clinical studies23,24 and most patients with APL are treated at institutions with limited experience,25 the information from such trials, although clearly important, cannot be generalized and may not represent the real-world incidence of the early death rate.
In fact, several albeit small single-institution observational studies suggest that the rate of early death may be higher than what is reported in cooperative group clinical trials.26-28 However, all of these single-center studies are limited by the same selection bias present in clinical trial data. More recently, a population-based study from the Swedish Adult Acute Leukemia Registry reported a high early death rate of 29% in 105 patients with diagnosis between 1997 and 2006.29 To our knowledge, our study is the largest population-based study to provide insight into the early death rate in a large population of unselected patients with newly diagnosed APL.
The higher than previously reported and only modestly improved early death rate after the approval of ATRA shown in our study indicates that the availability of ATRA per se has not eliminated the main cause of treatment failure in APL. The institution of an international effort to recognize and treat APL early has been shown to significantly reduce early mortality in developing countries.30 Similarly, for other diseases, such as acute coronary syndrome, that require a prompt initiation of the appropriate therapy the concerted effort and enforcement by professional organizations and hospitals to establish a specific management recommendation has dramatically improved the clinical outcome.31 Our study, therefore, shows a need to educate health care providers across a wide range of medical fields, including emergency department physicians, nurses, internists, and family practice physicians, who may be the first to evaluate such patients to have a major effect on early death and the cure rate of APL. More importantly, there is a clear need to provide the knowledge necessary to recognize APL as a medical emergency that requires specific and simultaneous actions, including a prompt initiation of ATRA, aggressive supportive care to counteract the coagulopathy, and transfer to experienced medical centers when the disease is first suspected.
To implement immediate institution of the appropriate therapy in APL, health care providers must maintain a high vigilance for the potential diagnosis. In most cases, the diagnosis of APL is suggested by the characteristic morphology of the leukemic cell population32 and clinical presentation with a hemorrhagic syndrome and severe coagulopathy. Patients at high risk of developing fatal hemorrhagic complications have been associated with the following factors: active bleeding, hypofibrinogenemia (< 100 mg/dL), increased fibrin degradation products or D-dimers combined with prolonged prothrombin time, or activated partial thromboplastin time, as well as increased white blood cell and peripheral blasts counts, abnormal levels of creatinine, or poor performance status.25 Because ATRA is known to rapidly improve the biochemical signs of the APL coagulopathy, treatment with ATRA should be initiated without waiting for genetic confirmation of the diagnosis because ATRA is unlikely to have any deleterious effect should genetic assessment fail to confirm the diagnosis of APL.
Although genetic confirmation of the specific lesion PML/RARA fusion is required to establish the definitive diagnosis of APL, test results from FISH and RT-PCR may not be immediately available at community hospitals and require a turnaround time of 1-2 days. Improvements in immunostaining with anti-PML mAb 33 on dry smears of BM or even peripheral blood samples have been shown to establish a rapid diagnosis in < 4 hours with high sensitivity and specificity.34 This test may be particularly valuable in smaller centers that lack immediate access to a molecular diagnostics laboratory. However, these tests should not delay the initiation of ATRA if APL is suspected, and patients should immediately be started on ATRA or transferred to more experienced centers equipped with ATRA and resources to provide aggressive support with blood products.
Although our study showed no significant difference in early death rate between patients treated in urban and rural counties, a higher rate of severe bleeding and early death has been reported in patients treated at nonspecialized or inexperienced institutions.18,25 This discrepancy may be because of the limited statistical analysis in patients treated in rural counties in our study because of the small sample size, or because treatment centers may not be as critical as the experience and expertise of treating health care providers. Because APL is a rare subtype of AML associated with a complex coagulopathy at presentation and treated differently from other AML subtypes, immediate and efficient access to experienced medical centers is essential to achieve the high cure rate that has been frequently reported in large clinical trials.
The study reported here also showed several noteworthy findings about long-term survival and incidence rates. First, the survival improved from the calendar period 1992-1995 to 1996-2001, but there has been no further improvement since 2001. The initial improvement in survival probably reflects the introduction of ATRA as standard of care during induction in mid-1990s, and the effect of the anthracycline-based consolidation chemotherapy as the standard treatment approach after remission. Second, the long-term survival rates appear lower than that reported in clinical trials,35,36 particularly in patients aged ≥ 55 years. In fact, > 50% of these patients are not cured of their disease, mainly driven by the significantly higher mortality within the first 2 months of diagnosis. The poor clinical outcome in these patients with what has been traditionally considered as a highly curable disease is sobering and, again, highlights the importance of reducing early death to improve the cure rate in APL. Furthermore, because only a minority of older patients are referred to tertiary care centers and enter clinical trials,37,38 it is even more critical to be aware of the increased risk of disease- and treatment-related complications in these patients.11,39 Third, the annual age-adjusted incidence rate of APL appears to have slightly increased over time, although the rate varied widely from year to year. The most significant increase occurred from 1992-1993 to 1995-1996, probably reflecting increased awareness of the diagnosis when ATRA became publicly accessible after the FDA approval and increased availability and use of cytogenetic and molecular tests to confirm the diagnosis. However, if the rise of the incidence rate is partly because of increased reporting of the disease, it is even more striking that the early death rate remains high despite the increased awareness of the disease and improved diagnostic tests.
Strengths of our study include a large number of patients available in a population-based setting, providing the real-world data of the early deaths and long-term survival in unselected patients with APL which were highly representative of the general patient population treated in the United States. Our study also has several limitations. Although we attempted to provide a systematic view of temporal trends in the rate of early death, the specific time points and causes of deaths within the first month of diagnosis are not captured in the SEER dataset. However, a recent study by the cooperative group Programa Español de Tratamientos en Hematología (PETHEMA) reported that, although most early deaths occurred in the first week of treatment, 44% of hemorrhagic deaths as well as most early deaths because of infection and the APL differentiation syndrome occurred in the next 3 weeks.40 Because the SEER dataset does not include information about treatment history and disease characteristics, further analysis of the early death rate and long-term survival by different risk groups, additional chromosomal abnormalities, and molecular mutations were unable to be performed in our study. Finally, the SEER dataset may not have captured patients who were treated with a presumptive diagnosis of “AML not otherwise specified” and subsequently died of hemorrhage without being recognized as having APL. Therefore, the early death rate may even be higher than that reported in our analysis.
In conclusion, our study shows that the early death rate remains high in APL despite ATRA. APL needs to be recognized by all clinicians as a medical emergency that necessitates the prompt initiation of ATRA and immediate referral to specialized and more experienced centers. Furthermore, major improvements in the early death and cure rates of APL will depend not on new drugs but on the education of the health care providers across a wide range of medical fields.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study used the SEER database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the New York State Cancer Registry, New York State Department of Health, and the SEER Program tumor registries in the creation of the SEER database.
Authorship
Contribution: J.H.P., B.Q., K.S.P., M.J.S., J.G.J, T.L.R., J.K.A., D.D., J.M.R., and M.S.T. conceived and designed the research; B.Q., K.S.P., and M.J.S. analyzed the data; and J.H.P. wrote the manuscript. All authors contributed to the revisions of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jae H. Park, Memorial Sloan-Kettering Cancer Center, 1275 York Ave, New York, NY 10065; e-mail: parkj6@mskcc.org.