To the editor:
Since the introduction of imatinib, tyrosine kinase inhibitors (TKIs) have become key drugs for treating chronic myeloid leukemia (CML).1 Although TKIs used for CML were specifically designed to inhibit Abelson murine leukemia viral oncogene homolog 1 (ABL) kinase, they have had some off-target effects.2,3 It is important to recognize and manage adverse events caused by TKIs,4-8 considering that patients take TKIs over a long period.
A positive fecal occult blood test (FOBT) has been occasionally observed in some CML patients treated with a TKI, especially dasatinib, during regular health checkups. There are several case reports concerning hemorrhagic colitis in CML patients treated with dasatinib9-11 ; however, its prevalence and its association with positive FOBTs are unknown.
To clarify the frequency of TKI-induced hemorrhagic colitis and the screening efficacy of an FOBT followed by a colonoscopy, we prospectively enrolled CML patients. Eligibility criteria were the identification of break point cluster region (BCR)–ABL fusion transcripts by reverse transcription polymerase chain reaction or t(9;22)(q34;q11) by G-banding or fluorescence in situ hybridization; administration of a TKI (imatinib, nilotinib, or dasatinib); and administration of a TKI for more than 1 month. Exclusion criteria were the coadministration of other anticancer drugs, platelet counts <50 × 109/L, history of any gastrointestinal cancer, impossibility of tissue biopsy because of antiplatelet or anticoagulant drugs, or presence of a bleeding disorder.
A first FOBT (FOBT1) was performed on all patients. Immunophenotyping of peripheral blood cells was also performed at the same time as the FOBT1 by flow cytometry. Upper gastrointestinal endoscopy and a first colon fiberscopy (CF1) were performed in patients with a positive FOBT1. When there were any lesions, tissue biopsies were performed. After confirmation of TKI-induced colitis by pathological analysis, the TKI was interrupted for 2 to 4 weeks, after which the FOBTs were reevaluated (FOBT2). A second CF (CF2) was performed after an additional patient agreement was obtained. The protocol was approved by the Institutional Review Board. Written informed consent was obtained from all patients before registration in accordance with the Declaration of Helsinki.
An FOBT was interpreted as positive when it was positive twice consecutively. Immunochemical FOBTs were performed for all patients. TKI-induced colitis was confirmed by (1) exclusion of other diseases that caused gastrointestinal bleeding, such as polyps, ischemic enteritis, inflammatory bowel disease, and cancers; and (2) inflammatory cell infiltration confirmed by pathological examination.
The immunohistochemical analysis of formalin-fixed, paraffin-embedded biopsy specimens was conducted using monoclonal antibodies against myeloperoxidase, CD3, CD20, CD68 (Dako, Glostrup, Denmark), CD4, CD8, CD19, CD38, CD56, CD163, and Granzyme B (Novocastra, Newcastle, United Kingdom).
Immunophenotyping of peripheral blood was performed with flow cytometry using antibodies against CD2, CD3, CD4, CD7, CD8, CD19, CD20, CD25, CD56, CD57, HLA-DR, and T-cell receptors αβ and γδ. All antibodies were purchased from Beckman Coulter (Brea, CA). The analyses were performed with a Cytomics FC500 cytometer (Beckman Coulter).
The primary endpoint of this study was to determine the frequency of TKI-induced hemorrhagic colitis. The secondary end points were utility of FOBT, histopathological analyses of TKI-induced hemorrhagic colitis, and response to TKI discontinuation.
Between February 2015 and September 2015, 30 patients were enrolled in this study. Upon entry to the study, 7 patients were being treated with imatinib, 5 with nilotinib, and 18 with dasatinib (Table 1).
Patient characteristics, FOBT1, and CF1
| . | Results . |
|---|---|
| No. of patients | 30 |
| Patient sex | |
| Male/female | 21/9 |
| Median patient age, y (range)* | 59 (29-80) |
| CML status at diagnosis | |
| CP/AP/BC | 30/0/0 |
| Type of TKI | |
| Imatinib/nilotinib/dasatinib | 7/5/18 |
| Prior TKI discontinuation | |
| Y/N | 7/23 |
| Prior TKI reduction | |
| Y/N | 10/20 |
| TKI duration, mo (range)† | 37.5 (2-120) |
| CML status at FOBT1 | |
| CHR/CCyR/MMR/CMR | 1/2/20/7 |
| WBC count at FOBT1, μL (range) | 5770 (2890-14280) |
| Lymphocyte percentage at FOBT1, % (range) | 35.1 (14.8-82.0) |
| Hb level at FOBT1, g/dL (range) | 12.8 (8.2-16.0)‡ |
| Platelet count at FOBT1, ×109/L (range) | 193 (81-297) |
| FOBT1 result | |
| +/− | 10/20 |
| CF1 result | |
| Red flare/erosion/polyp/hemorrhoid | 2/4/3/1 |
| TKI-associated colitis | |
| Y/N | 6/24 |
| . | Results . |
|---|---|
| No. of patients | 30 |
| Patient sex | |
| Male/female | 21/9 |
| Median patient age, y (range)* | 59 (29-80) |
| CML status at diagnosis | |
| CP/AP/BC | 30/0/0 |
| Type of TKI | |
| Imatinib/nilotinib/dasatinib | 7/5/18 |
| Prior TKI discontinuation | |
| Y/N | 7/23 |
| Prior TKI reduction | |
| Y/N | 10/20 |
| TKI duration, mo (range)† | 37.5 (2-120) |
| CML status at FOBT1 | |
| CHR/CCyR/MMR/CMR | 1/2/20/7 |
| WBC count at FOBT1, μL (range) | 5770 (2890-14280) |
| Lymphocyte percentage at FOBT1, % (range) | 35.1 (14.8-82.0) |
| Hb level at FOBT1, g/dL (range) | 12.8 (8.2-16.0)‡ |
| Platelet count at FOBT1, ×109/L (range) | 193 (81-297) |
| FOBT1 result | |
| +/− | 10/20 |
| CF1 result | |
| Red flare/erosion/polyp/hemorrhoid | 2/4/3/1 |
| TKI-associated colitis | |
| Y/N | 6/24 |
AP, accelerated phase; BC, blast crisis; CCyR, complete cytogenetic remission; CHR, complete hematological remission; CMR, complete molecular remission; CP, chronic phase; Hb, hemoglobin; MMR, major molecular remission; WBC, white blood cell; Y/N, yes/no.
Age at FOBT1.
TKI indicates the type of TKI at FOBT1.
No patients revealed clinically significant symptoms.
The FOBT1 was positive in 10 patients. All 10 patients with positive FOBT1s were treated with dasatinib. No patients revealed clinically significant symptoms. A CF1 was performed for all 10 patients with positive FOBT1s, and all showed abnormal endoscopic findings: colitis in 6 patients, polyps in 3, and hemorrhoid in 1.
Dasatinib-induced hemorrhagic colitis was confirmed in 6 of 18 patients treated with dasatinib (33%). The median duration of treatment with dasatinib before FOBT1 was 16 months (range, 3-35 months). The median dose of dasatinib was 70 mg (range, 50-100 mg). The dose was reduced in 3 patients because of pleural effusion, and 1 patient received dasatinib as a second-line therapy. Two patients had hypertension, 1 had a history of angina, and no patients had clinically active chronic obstructive pulmonary disease. CML statuses at FOBT1 were complete molecular response in 1 patient,12 major molecular response in 4, and complete cytogenetic remission in 1.13 There was no significant difference of cumulative dose of dasatinib between patients with and without dasatinib-induced hemorrhagic colitis.
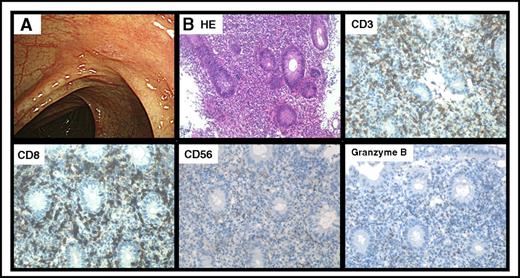
Endoscopic feature of dasatinib-induced colitis was red flare and/or erosion, and immunohistological analyses showed CD3+, CD8+, CD56+, and Granzyme B+ cytotoxic T-lymphocyte infiltration (Figure 1).
Features of dasatinib-induced hemorrhagic colitis. Representative endoscopic finding of CF1 (A) and immunohistopathological analysis of a representative colon biopsy specimen (B). Original magnification ×200 for panel B. CD, cluster of differentiation; HE, hematoxylin and eosin.
Features of dasatinib-induced hemorrhagic colitis. Representative endoscopic finding of CF1 (A) and immunohistopathological analysis of a representative colon biopsy specimen (B). Original magnification ×200 for panel B. CD, cluster of differentiation; HE, hematoxylin and eosin.
Dasatinib was discontinued at a median of 27 days after the CF1. The discontinuation period was a median of 14 days (range, 14-28 days). The FOBT2s were negative in all but 1 patient. No CML progression from dasatinib discontinuation was observed.
A CF2 was performed in only 1 patient whose FOBT2 was positive. The CF2 of the patient showed minimal red flare and concurrent colorectal polyps. Immunohistological analyses of biopsy specimens showed decreased infiltration of activated T lymphocytes, which was consistent with resolved dasatinib-induced hemorrhagic colitis.
The median lymphocyte percentage was 35% (range, 15-82) and was significantly higher in patients treated with dasatinib than those with other TKIs (42% vs 32%, respectively, P = .006). Although there was a tendency to show a higher percentage of lymphocytes, especially CD8+CD57+–activated T lymphocytes in patients with dasatinib-induced hemorrhagic colitis, there was no statistically significant difference between patients with and without the disease (total lymphocyte: 40% vs 35%, respectively, P = .29; activated lymphocyte: 19% vs 12%, respectively, P = .18).
This study reports dasatinib-induced hemorrhagic colitis in one-third of CML patients treated with dasatinib. To the best of our knowledge, this is the first prospective study focusing on TKI-induced hemorrhagic colitis among CML patients. The incidence was higher than expected, illustrating how important it is to understand the clinical conditions of hemorrhagic colitis caused by dasatinib.
The ranges of sensitivities and specificities of FOBTs were 27% to 67% and 94% to 98% for colorectal cancer or advanced neoplasms in past reports, respectively.14-17 Because the positive predictive value in this study was 60%, the estimated prevalent rate of dasatinib-induced hemorrhagic colitis could be 6% to 21% and the negative predictive value could be 83% to 97%. Considering that all patients had no symptoms, an FOBT could be a simple and noninvasive screening test for dasatinib-induced hemorrhagic colitis.
Interestingly, hemorrhagic colitis was only observed in patients treated with dasatinib. This can be explained by the clonal expansion of T/natural killer cells during dasatinib therapy.18 Although clonality was not fully proven in the present study, immunohistopathological analyses of colon biopsy specimens showed cytotoxic T-lymphocyte infiltration, which was compatible with the previous study.18 Also, the high lymphocyte percentage in the peripheral blood of patients treated with dasatinib could be associated with dasatinib-induced hemorrhagic colitis.19
Pleural effusion has been observed in one-half of patients with dasatinib-induced hemorrhagic colitis. This is a high frequency compared with the 10% to 14% reported in previous studies.20,21 Although the exact mechanism of pleural effusion is unknown, immune-mediated reactions have been suggested because of the high lymphocyte frequency in pleural fluids and pleural tissue.22-25 The mechanisms of dasatinib-induced hemorrhagic colitis may have something in common with those of other adverse events, such as pleural effusion.
Although CF2 was performed in only 1 patient, colitis in that patient was improved both endoscopically and pathologically. These results clinically confirmed that the positive FOBT and hemorrhagic colitis observed in this study was caused by dasatinib.
Not all patients will agree to a CF, especially if they are without symptoms. When an FOBT is positive in patients treated with dasatinib, dasatinib discontinuation for 2 to 4 weeks may assess dasatinib-induced hemorrhagic colitis without invasive intervention. Because the specificity of FOBT is high, a negative FOBT after discontinuation could exclude colorectal cancer or other neoplasms.
In conclusion, a positive FOBT in CML patients treated with dasatinib is associated with a significant rate of asymptomatic hemorrhagic colitis. The FOBT can be a useful, noninvasive screening test for the disease, and it can be confirmed by following with a CF and pathological analyses.
Authorship
Acknowledgments: This study was supported in part by a Japan Leukemia Research Fund grant (S.N.).
Contribution: S.N., M.M., M.Y., and I.S. designed the research; S.N., S. Okuno., Y.H., K.S., S.K., and I.S. provided the hematological data of patients; M.Y., F.U., and S. Okamura performed the upper gastrointestinal endoscopies and colon fiberscopies and interpreted the endoscopic findings; S.N. and M.M. performed pathological analyses; S.N. and I.S. interpreted the data and wrote the manuscript; and all authors reviewed and approved the final draft.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Satoshi Nishiwaki, Center for Advanced Medicine and Clinical Research, Nagoya University Hospital, 65 Tsurumai-cho Showa-ku, Nagoya, 4668560, Japan; e-mail: n-3104@tf7.so-net.ne.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal