In this issue of Blood, Piya et al have identified the autophagy-related E1 ligase Atg7, a critical autophagy component associated with unfavorable outcomes in acute myeloid leukemia (AML), as a potential target in this disease.1 If validated, this study could provide a foundation for disrupting Atg7 in AML therapy.
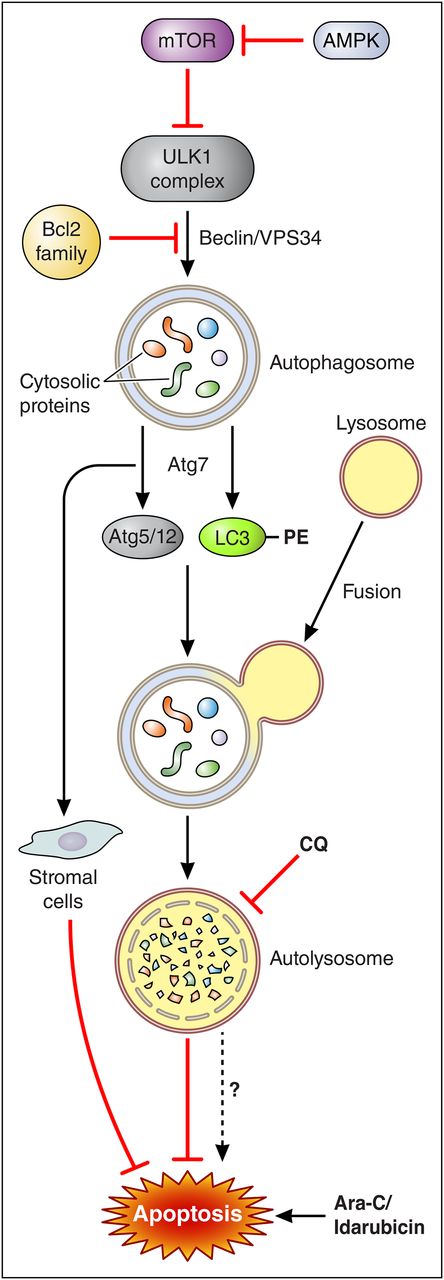
A model of the role of Atg7 in the regulation of autophagy and apoptosis in AML cells. Initiation of autophagy is regulated by the AMPK/mTOR axis and activation of the ULK1 (UNC-51-like kinase 1) complex and release of the Beclin1-VPS-34 complex from Bcl-2 family members. This allows sequestration of cytosolic proteins into a maturing autophagosome, which is mediated in part by the actions of the E1-like enzyme Atg7, which acts through 2 pathways: conjugation of Atg5 and Atg12 and addition of phosphatidylethanolamine (PE) moieties to LC3. These events are critical for autophagosome elongation and set the stage for lysosomal fusion to form the autolysosome, leading to digestion of cellular contents. Blocking the latter events (eg, by knocking down Atg7) disrupts autophagy and significantly attenuates the cytoprotective actions of this process in AML cells exposed to genotoxic agents such as Ara-C and idarubicin. Atg7 knockdown also acts indirectly by inhibiting stromal cell cytoprotective actions toward AML cells. In this way, Atg7 knockdown can recapitulate the actions of agents such as CQ which disrupt autophagy at a distal point (eg, lysosome acidification). Note that despite its presumed cytoprotective actions, autophagy may under some circumstances promote apoptosis (hatched arrow, bottom), arguing that interfering with this process can potentially act as a double-edged sword. Professional illustration by Patrick Lane, ScEYEnce Studios.
A model of the role of Atg7 in the regulation of autophagy and apoptosis in AML cells. Initiation of autophagy is regulated by the AMPK/mTOR axis and activation of the ULK1 (UNC-51-like kinase 1) complex and release of the Beclin1-VPS-34 complex from Bcl-2 family members. This allows sequestration of cytosolic proteins into a maturing autophagosome, which is mediated in part by the actions of the E1-like enzyme Atg7, which acts through 2 pathways: conjugation of Atg5 and Atg12 and addition of phosphatidylethanolamine (PE) moieties to LC3. These events are critical for autophagosome elongation and set the stage for lysosomal fusion to form the autolysosome, leading to digestion of cellular contents. Blocking the latter events (eg, by knocking down Atg7) disrupts autophagy and significantly attenuates the cytoprotective actions of this process in AML cells exposed to genotoxic agents such as Ara-C and idarubicin. Atg7 knockdown also acts indirectly by inhibiting stromal cell cytoprotective actions toward AML cells. In this way, Atg7 knockdown can recapitulate the actions of agents such as CQ which disrupt autophagy at a distal point (eg, lysosome acidification). Note that despite its presumed cytoprotective actions, autophagy may under some circumstances promote apoptosis (hatched arrow, bottom), arguing that interfering with this process can potentially act as a double-edged sword. Professional illustration by Patrick Lane, ScEYEnce Studios.
Autophagy (literally “self-eating”) represents one of the cell’s principal homeostatic mechanisms and is responsible for protecting the cell from diverse environmental stresses, including cytotoxic chemotherapy. The major form of autophagy, macroautophagy, is a process that involves lysosomal fusion with autophagosomes and subsequent degradation of cellular constituents sequestered within the latter, permitting them to be reused for critical cellular functions.2 However, the role of autophagy in cell fate is highly complex and context-specific. For example, autophagy has been referred to as type II cell death to distinguish it from another major form of cellular demise, apoptosis (type I cell death).3 In addition, autophagy represents, along with proteasomal degradation, one of the 2 major systems by which cells rid themselves of unwanted proteins.
Regardless of its specific role, accumulating evidence indicates that disruption of autophagy lowers the death threshold in cells exposed to cytotoxic chemotherapy, including AML cells.4 This has prompted the development of autophagy inhibitors as candidate chemosensitizers. To date, such efforts have focused largely on agents such as chloroquine (CQ), which disrupt autophagy at a distal point in the process (eg, prevention of lysosomal protein degradation).5 However, despite very promising preclinical data, CQ and related agents have not yet had a significant clinical impact, possibly because of pharmacokinetic/pharmacodynamic issues. This has triggered a refocusing of attention on more proximal proteins involved in autophagy, including initiating factors (eg, ULK1) or proteins involved in vesicle formation, processing, and maturation such as Atg5 and Atg7. The figure presents a model illustrating these factors.
The E1 ligase Atg7 plays a central role in elongation of autophagosomal vesicles, an essential process for cargo loading, but it also functions in ubiquitin-like reactions involving lipidation of LC3 and conjugation of Atg5 and Atg12, essential for lysosomal fusion. Interestingly, high levels of expression of Atg7 (along with several other autophagy-related proteins) have been preliminarily associated with an adverse prognosis in AML. In this context, genetic interruption of Atg7 sensitizes solid tumors to cytotoxic chemotherapy, raising the possibility that analogous events may occur in AML.
Whether in fact loss of Atg7 sensitizes AML cells to chemotherapeutic agents has not previously been investigated. To address this question, Piya et al used a genetic approach involving short hairpin RNA (shRNA) knockdown of Atg7 in various AML cell types. They found that cells in which Atg7 was suppressed displayed significantly increased sensitivity to several genotoxic agents used in AML (eg, Ara-C and idarubicin) in association with upregulation of the proapoptotic Bcl-2 family member Noxa. Interestingly, Atg7 knockdown in stromal cells exerted similar chemosensitizing effects, suggesting that autophagy-mediated microenvironmental actions contribute to chemoresistance. Importantly, immunocompromised mice inoculated with Atg7 knockdown cells exhibited increased survival compared with their wild-type counterparts, further justifying targeting this protein in AML.
The ultimate efficacy of targeting Atg7 in AML will depend upon multiple factors, both theoretical and practical. For example, although autophagy is generally felt to represent a cytoprotective mechanism, under certain circumstances it can also promote cell death (toxic autophagy). To the extent that the latter process occurs, blocking autophagy could have undesirable consequences. In this regard, autophagy, like many other dynamic cellular responses (eg, endoplasmic reticulum [ER] stress), generation of reactive oxygen species (ROS) can function as a double-edged sword that initially serves cytoprotective functions but exerts lethal actions at later intervals. Consistent with this duality, Atg7 has been reported to act as a tumor suppressor gene in some contexts but as an oncogene in others.6
Another issue to be addressed concerns the optimal strategy for interfering with autophagy. As noted above, autophagy can be disrupted at multiple sites, including its initiation (eg, at the level of ULK1) or at later stages (eg, inhibition of lysosomal degradation by CQ). Preventing autophagy at its inception has the advantage of circumventing potential downstream cellular bypass events. However, to the extent that toxic autophagy occurs, disrupting rather than preventing this process may be advantageous. It also remains to be determined whether, and under what circumstances, targeting Atg7 offers advantages over alternative autophagy-related proteins (eg, ULK1, Atg5, Atg12). Although Piya et al showed that similar events occurred in a limited number of primary blast samples, targeting Atg7 may be most appropriate for a subset of genetically defined leukemic cells. Additional studies involving a larger number of primary specimens will be required to identify genetic factors that might predict susceptibility to Atg7 disruption.
The observation that targeting Atg7 reduced the cytoprotective effects of stromal cells is novel and has practical implications. For example, microenvironmental factors have been shown to protect leukemic cells, particularly leukemic stem cells, from chemotherapy. Although this phenomenon has generally been attributed to disruption of cytokine networks in the hypoxic microenvironment, the notion that such a process may be regulated by autophagy, and specifically Atg7 in AML, is new and could provide a more effective strategy for overcoming this problem.
Practically, targeting Atg7 will not be trivial, but the possibility does exist. For example, a ubiquitin E3 ligase inhibitor, the Nedd8-activating enzyme inhibitor pevonedistat, has recently shown promising activity in AML.7 However, whether specifically inhibiting Atg7 E1 ligase activity will be sufficient to recapitulate the antileukemic effects of Atg7 knockdown (and disrupt its lipidation- and conjugation-related activities) remains to be determined. It should also be kept in mind that chemical versus genetic disruption of a protein can have disparate biological consequences.8
In summary, the study by Piya et al furnishes a cogent rationale for targeting autophagy in general, and more specifically, the autophagy-related protein Atg7 in AML. As with other targeted drugs, the ultimate role of Atg7 antagonists is likely to lie in combination with either conventional or novel agents, particularly those that elicit a cytoprotective autophagic response. Ongoing efforts will hopefully resolve these issues in the near future.
Conflict-of-interest disclosure: The author declares no competing financial interests.