In this issue of Blood, Mossner et al took an important step in delineating the evolution of myelodysplastic syndrome (MDS). They were able to estimate mutation acquisition order and to demonstrate that, in the majority of MDS cases, earlier preexisting minor clones survived therapy.1
MDS, like many other leukemias, is the phenotypic end product of an ordered genetic process occurring in a very specific way in different individuals. Understanding why initiating events are being selected and how they shape the genetic and phenotypic course of the disease is one of the major challenges in leukemia pathogenesis. Professional illustration by Somersault18:24.
MDS, like many other leukemias, is the phenotypic end product of an ordered genetic process occurring in a very specific way in different individuals. Understanding why initiating events are being selected and how they shape the genetic and phenotypic course of the disease is one of the major challenges in leukemia pathogenesis. Professional illustration by Somersault18:24.
Evolution, or the constant change in nature, is the most profound observation humans ever made, as it holds the basic rules of almost every discipline in biology. “Men do change, and change comes like a little wind that ruffles the curtains at dawn, and it comes like the stealthy perfume of wildflowers hidden in the grass.” Although Steinbeck elegantly identified the role of evolution in our life, clinicians do not always integrate evolution into the understanding of pathogenesis or in the design of treatments. Mossner et al incorporated some evolutionary concepts into MDS reasoning. The following paradigms are some first principles of evolution that should be better studied in hematological malignancies:
Selection (in the form of an environmental change) always acts on phenotypes and not on genotypes.
In the majority of the cases, genetic and phenotypic variation are present in subpopulations before selection is introduced.
Although the genetic variants (ie, mutations) contributing to a certain phenotype occur in a specific order, in the vast majority of the cases ancestral populations carrying only a part of the variants will survive (branched evolution). Linear evolution like the extinction of the dinosaurs is rare and associated with ecological catastrophes.
Most populations have a complex structure, especially if specific cells in that population can inhabit new environmental niches.
The observations made by Mossner et al are in line with some of these concepts. Insights from Luria and Delbrück’s fluctuation analysis established the fact that genetically resistant variants were already present in a subset of the population before the introduction of selection.2 In a similar way, Mossner et al identified treatment-resistant clones in pretreatment samples. The resistant clones were only a minor fraction at the time of diagnosis and were the result of branching evolution. Accordingly, studies in T-cell acute lymphoblastic leukemia,3 acute myeloid leukemia,4 and chronic lymphocytic leukemia5 all suggested that resistant clones originated from ancestral minor populations. This is of no surprise at all, as most of the current treatment protocols aim at eliminating the major population observed at diagnosis. Clinicians measure the response to therapy based on the drug’s efficacy to eliminate the major clone, a strategy that might be useful mainly in leukemia with relatively short evolutionary history and therefore limited clonal diversity. However, a plethora of minor clones, the product of longer periods of evolution, will eventually survive treatment and give rise to relapse.
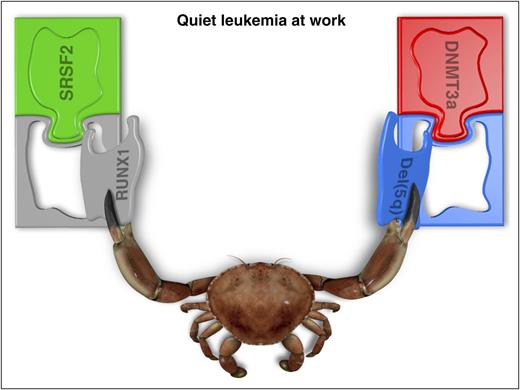
Another important observation in the work by Mossner et al is the predictability of mutation order. On top of estimating mutation order in MDS patients, they were able to draw patterns in the order of mutation (Figure 3 in the Mossner et al article). They were able to clearly define early mutational events like ASXL1, DNMT3a, TET2, and the RNA splicing mutations, and separate late events such as RUNX1, del(5q), biallelic TET2, and BCOR mutations. They were able to identify preleukemic mutations in mature lymphocytes, which define the existence of preleukemic stem cells in MDS.6-8
An initiating preleukemic mutation in DNMT3a/TET2 is followed by del(5q) whereas an initiating ASXL1 mutation is followed by other chromosomal changes. Furthermore, the initiating preleukemic mutations in SRSF2 or SF3B1 are usually followed by other point mutations such as biallelic TET2 or RUNX1 (see figure). Interestingly, McKerrell and coworkers9 recently noticed 2 major patterns in the acquisition of mutations in clonal hematopoiesis of indeterminate significance.10 DNMT3a/TET2 mutations tend to appear at an early age (∼40 years) whereas SRSF2 or SF3B1 appeared in healthy individuals at older age (∼70 years).9 All of these important observations suggest that different environmental changes initially select stem cells with specific phenotypes that are associated with the mutations we see. Later, the next set of events (late events) is also associated with the specific changes in the environment which may be related to the initiating event itself and the consequences of the preleukemic mutations on genomic stability and the environment. It is of great importance to realize that although mutations are good markers and may be associated with a phenotype, selection always occurs on the phenotype. Accordingly, targeted therapy should not always be mutation oriented as in the case of BCR/ABL, but rather target the phenotypes and environmental changes which select the leukemic cells to begin with.
Altogether, branched evolution, resistant ancestral clones, and the predictable order of mutation point to unifying rules in MDS evolution which are not yet fully understood. Studying human malignancies using an evolutionary biology approach holds a promise to uncover these rules and makes another important step toward the improvement of disease outcomes.
Conflict-of-interest disclosure: The author declares no competing financial interests.