In this issue of Blood, Grisouard et al use transgenic mice to describe the phenotype associated with the human polycythemia vera (PV)-associated JAK2 exon 12 mutation (JAK2-N542-E543del) and demonstrate Stat1-independent erythroid-only proliferation.1
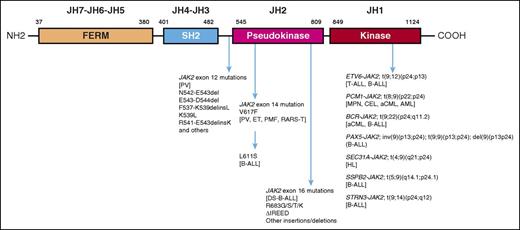
Domain structure of JAK2 with an outline of associated mutations. AML, acute myeloid leukemia; CEL, chronic eosinophilic leukemia; DS-B-ALL, Down syndrome–associated B-ALL; ET, essential thrombocythemia; HL, Hodgkin lymphoma; MPN, myeloproliferative neoplasm; PMF, primary myelofibrosis; RARS-T, refractory anemia with ring sideroblasts and thrombocytosis.
Domain structure of JAK2 with an outline of associated mutations. AML, acute myeloid leukemia; CEL, chronic eosinophilic leukemia; DS-B-ALL, Down syndrome–associated B-ALL; ET, essential thrombocythemia; HL, Hodgkin lymphoma; MPN, myeloproliferative neoplasm; PMF, primary myelofibrosis; RARS-T, refractory anemia with ring sideroblasts and thrombocytosis.
Activating JAK2 mutations can arise from chromosomal translocations or point mutations/deletions/insertions. The former result in JAK2 fusion proteins that always involve the JAK2 kinase domain (JH1), in association with an oligomerization domain from one of several partner proteins, which promotes constitutive JAK2 phosphorylation and signal activation. Tumor phenotypes associated with JAK2 fusion proteins include both myeloid and lymphoid neoplasms with a certain degree of phenotypic specificity (see figure)2 : ETV6-JAK2 [t(9;12)(p24;p13)] has been associated with T or B acute lymphoblastic leukemia (T-ALL or B-ALL, respectively); PCM1-JAK2 [t(8;9)(p22;p24)] with chronic eosinophilic leukemia and atypical chronic myeloid leukemia (aCML); BCR-JAK2 [t(9;22)(p24;q11.2)] with aCML and B-ALL; PAX5-JAK2 [inv(9)(p13;p24); t(9;9)(p13;p24); del(9)(p13p24)] with B-ALL; SSBP2-JAK2 [t(5;9)(q14.1;p24.1)] with B-ALL; STRN3-JAK2 [t(9;14)(p24;q12)] with B-ALL; and SEC31A-JAK2 [t(4;9)(q21;p24)] with HL. B-ALL has also been associated with JAK2 point mutations/deletions/insertions, involving the JH2 pseudokinase domain; these include a JAK2 exon 14 mutation (L611S) reported in a single case of B-ALL3 and recurrent JAK2 exon 16 mutations seen in ∼18% of patients with DS-associated B-ALL.4 The latter always affect the arginine 683 residue (eg, R683G, R683S, and ΔIREED), and some of these mutations have been shown to induce JAK-Stat activation and cytokine-independent growth. Interestingly, JAK2ΔIREED bone marrow transplant assays in mice displayed a phenotype similar to that seen with JAK2V617F, suggesting a crucial role for the DS-associated trisomy 21 in directing the oncogenic activity to B-lymphoid rather than myeloid cells.5
The myeloproliferative neoplasm–associated JAK2V617F is the prototype JAK2 point mutation and involves the JH2 pseudokinase domain (exon 14).6 JAK2V617F is closely associated with PV, essential thrombocythemia, primary myelofibrosis, and refractory anemia with ring sideroblasts and thrombocytosis, with respective mutational frequencies of ∼98%, 50%, 60%, and 40%, respectively. The phenotypic diversity seen with JAK2V617F has been attributed to differences in mutant allele burden, presence of other coexisting mutations, and the order and stem cell level of mutation acquisition.7 Conversely, the PV phenotype has infrequently been associated with other mutations, including JAK2 exon 12 mutations.
JAK2 exon 12 mutations were first described by Scott et al in 2007 and shown to account for the majority of patients with JAK2V617F-negative PV.8 Unlike the case with JAK2V617F, which is a single nucleotide alteration, multiple JAK2 exon 12 mutations, often heterozygote, have been described and include nucleotide substitutions, deletions, or duplications; the most frequent were N542-E543del, E543-D544del, F537-K539delinsL, K539L, and R541-E543delinsK.9 These mutations also involve the JH2 pseudokinase domain adjacent to its border with the JH3 domain, spanning residues 536 to 547. Compared with PV patients with JAK2V617F, those with JAK2 exon 12 mutations were younger and displayed higher hemoglobin levels, primarily erythroid proliferation, absence of bone marrow tri-lineage hyperplasia, and lower incidence of leukocytosis or thrombocytosis9 ; however, survival and rate of disease complications were reported to be similarly affected by the 2 mutation variants.9
The abovementioned specificity of JAK2 exon 12 mutations to PV and, in particular, to the erythrocytosis phenotype, has been recapitulated in animal models. In their original description,8 Scott et al used JAK2K539L retroviral mouse models and induced marked erythrocytosis, which was more pronounced than was seen in JAK2V617F mice; in contrast, although leukocytosis accompanied the erythrocytosis phenotype in both instances, its degree was significantly higher in JAK2V617F mice. The approach taken by Grisouard et al used JAK2-N542-E543del transgenic mice and generated an even more erythroid-specific phenotype, without leukocytosis, thrombocytosis, or myelofibrosis. Multiple factors might have contributed to the overlapping but apparently distinct phenotypes observed between the JAK2K539L and JAK2-N542-E543del mice, including the different strategies of genetic engineering applied in constructing the animal models and potential differences in phenotype associated with specific JAK2 exon 12 mutations; the latter might be consequential to disease natural history and specific treatment response.
Additional observations from the transgenic mouse model of Grisouard et al included a Stat1- and allele burden–independent mechanism of action for JAK2-N542-E543del and altered expression of iron regulating proteins, including increased expression of transferrin receptor-1 and erythroferrone and decreased expression of hepcidin.1 However, the apparent effect on iron metabolism might represent a generic response to increased erythropoietic activity rather than a direct and unique effect of the JAK2 exon 12 mutation. In other words, irrespective of its cause, increased erythroid activity is a potent suppressor of hepcidin, because of its high demand for iron10 ; the mechanism of hepcidin suppression in this instance is believed to involve erythroferrone, which is the erythroblast-derived negative regulator of hepcidin.10 Similarly, increased iron utilization by erythroblasts requires their increased surface expression of transferrin receptor 1, which binds circulating transferrin-bound iron.
The remarkable phenotypic similarity between JAK2 exon 12 mutated human disease and the corresponding phenotype in mice suggests a disease-initiating pathogenetic role for the mutation; this is also consistent with its almost exclusive association with PV, making it more amenable to molecularly targeted therapy compared with JAK2V617F. Clinically, more work is needed to address potential differences between specific subtypes of JAK2 exon 12 mutations in terms of natural history and specific treatment response; because JAK2 exon 12 mutated PV is relatively rare and characterized by an indolent clinical course, this will require a concerted collaborative effort with access to retrospective data.
Conflict-of-interest disclosure: The author declares no competing financial interests.