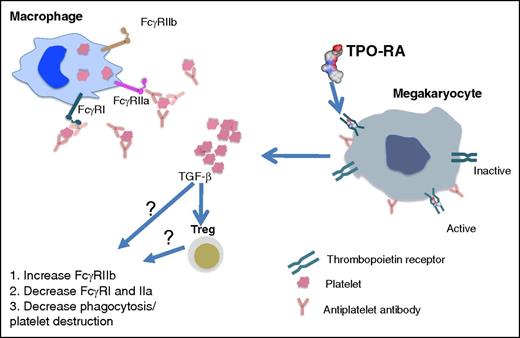
In this issue of Blood, Liu et al show that response to thrombopoietin receptor agonist (TPO-RA) therapy in immune thrombocytopenia (ITP) is associated with restoration of monocyte Fcγ receptor (FcγR) balance toward inhibitory FcγRIIb and with correction of the enhanced phagocytic capacity of macrophages. These data suggest that TPO-RA can increase platelet counts not only by increasing platelet production but also by lowering platelet destruction.1
TPO-RA in ITP. ITP monocyte/macrophages have an activated monocyte FcγR phenotype as indicated by decreased FcγRIIb expression paralleled with remarkably increased FcγRI and FcγRIIa expression. Opsonization of platelets by antiplatelet autoantibodies in ITP results in increased platelet destruction via FcγR-mediated phagocytosis by monocyte/macrophages. In addition to their direct effect on activating TPO-Rs on megakaryocytes that result in platelet production, TPO-RAs restore FcγR balance on monocytes/macrophages and downregulate the phagocytic capacity of macrophages, thus attenuating platelet destruction. The mechanism of action of how this occurs remains to be discovered. It may be that the increase in platelet mass following TPO-RA treatment results in elevated levels of circulating transforming growth factor-β1 (TGFβ1) which, directly or indirectly through increasing Treg activity, restores FcγR balance and inhibits macrophage phagocytic activity.
TPO-RA in ITP. ITP monocyte/macrophages have an activated monocyte FcγR phenotype as indicated by decreased FcγRIIb expression paralleled with remarkably increased FcγRI and FcγRIIa expression. Opsonization of platelets by antiplatelet autoantibodies in ITP results in increased platelet destruction via FcγR-mediated phagocytosis by monocyte/macrophages. In addition to their direct effect on activating TPO-Rs on megakaryocytes that result in platelet production, TPO-RAs restore FcγR balance on monocytes/macrophages and downregulate the phagocytic capacity of macrophages, thus attenuating platelet destruction. The mechanism of action of how this occurs remains to be discovered. It may be that the increase in platelet mass following TPO-RA treatment results in elevated levels of circulating transforming growth factor-β1 (TGFβ1) which, directly or indirectly through increasing Treg activity, restores FcγR balance and inhibits macrophage phagocytic activity.
ITP is an immune-mediated bleeding disorder resulting from reduced platelet production as well as increased platelet destruction due to antiplatelet autoantibodies binding to platelets and causing their premature destruction by Fc-mediated phagocytosis in the reticuloendothelial system.2 The autoantibodies are predominantly of the immunoglobulin G class with specificity for platelet glycoprotein IIb/IIIa (GPIIb/IIIa; αIIbβ3) and GPIb/IX. Ligation of FcγRs by GP-specific autoantibodies triggers phagocytic removal of opsonized platelets by macrophages, leading to further antigen presentation and proinflammatory cytokine production. Similar to other autoimmune disorders such as systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA), ITP has aberrant FcγR profiles. An activating monocyte FcγR phenotype, indicated by significantly decreased FcγRIIb expression with remarkably increased FcγRI and FcγRIIa expression, has been reported in active ITP.3 Moreover, the recovery of platelet counts in ITP patients treated with high-dose dexamethasone or Helicobacter pylori eradication is accompanied by restoration of FcγR balance toward the inhibitory FcγRIIb,3,4 analogous to rebalancing of the equilibrium between activating and inhibitory FcγRs following therapeutic response in SLE and RA.5,6
In recent years, TPO-RAs have emerged as highly efficacious in the management of primary ITP and 2 drugs, romiplostim and eltrombopag, are approved for standard clinical use. Through activation of TPO-R on megakaryocytes, TPO-RAs stimulate platelet production. An increase in platelet counts is achieved in 60% to 80% of patients with chronic ITP who have been refractory to 1 or more lines of therapy. Along with their ability to directly stimulate thrombopoiesis, TPO-RAs have been shown to affect immunomodulation. In a mouse model of ITP, short-term TPO-RA treatment promoted the peripheral induction of regulatory T cells (Tregs) and suppressed T-cell responses to platelet autoantigens.7 Furthermore, improved Treg activity and decreased effector T-helper cell function have been observed in TPO-RA–treated ITP patients.8 In addition, regulatory B-cell (Breg) number and function were also corrected after TPO-RA treatment in ITP patients.9 Taking into account the immunoregulatory actions of TPO-RAs and the important role of FcγRs in autoimmunity, Liu et al examined the effect of TPO-RAs on FcγR modulation and the platelet destruction process in ITP (see figure).1
They studied 21 corticosteroid-resistant/relapsed patients with chronic ITP who received eltrombopag treatment of 6 weeks, and compared posttreatment week 6 to baseline values.1 In eltrombopag responders (n = 15), monocyte FcγRIIb messenger RNA and protein levels increased significantly, whereas FcγRI and IIa levels decreased remarkably. Notably, restoration of FcγR balance on monocytes from responders was accompanied by a considerable reduction in monocyte/macrophage phagocytic capacity as determined by an in vitro assay. In contrast, in eltrombopag nonresponders (n = 6), no significant changes in monocyte FcγR levels or monocyte/macrophage phagocytic capacity were detected following eltrombopag administration.
The regulatory effect of TPO-RA on FcγR profiles was further confirmed in a murine model of ITP.1 Romiplostim administration in ITP mice significantly downregulated activating FcγRI expression and upregulated inhibitory FcγRII expression on splenic macrophages, and these FcγR level changes correlated with increase in platelet counts. Furthermore, clearance of transfused platelets appeared slower in ITP mice treated with romiplostim.
These exciting and novel data suggest that TPO-RAs may lower platelet destruction in addition to stimulating platelet production. Nevertheless, the study is still an association. Direct proof that TPO-RAs increase platelet counts or improve transfused platelet survival in ITP by modulating FcγRs will require future studies showing that TPO-RA effects on platelet counts in ITP FcγRII and/or FcγR chain knockout mice are abolished. Several recent studies have shown a sustained platelet response after TPO-RA discontinuation in a subset of ITP patients.10 If the monocyte FcγR balance toward inhibitory FcγRIIb is maintained and the macrophage phagocytic activity remains low in patients who have sustained remission after TPO-RA discontinuation, but not in those who relapse, it would further support the aforementioned effects of TPO-RAs on FcγR modulation and inhibition of platelet destruction in ITP. Questions remain as to the mechanism of action of TPO-RA on monocyte/macrophage FcγR expression and function: does the burst in plasma TGFβ1 following TPO-RA treatment1,8 directly affect and lower phagocytic activity of monocyte/macrophages? Or is the effect mediated through Tregs or Bregs which can regulate monocyte activity? Nonetheless, this research sheds new light on TPO-RA immunomodulation and helps to guide research to uncover a novel mechanism of action of TPO-RAs in ITP treatment. Such investigations will help determine whether the immunomodulatory effects of TPO-RAs are specific to the treatment of ITP immunopathology or whether they can be harnessed for treatment of other autoimmune diseases without causing thrombocytosis.
Conflict-of-interest disclosure: The author declares no competing financial interests.