Key Points
Conditional Gata2-deficient mice have profoundly reduced DC populations.
Gata2 deficiency in DC progenitors reduced the expression of myeloid-related genes and increased that of T-lymphocyte–related genes.
Abstract
Dendritic cells (DCs) are critical immune response regulators; however, the mechanism of DC differentiation is not fully understood. Heterozygous germ line GATA2 mutations induce GATA2-deficiency syndrome, characterized by monocytopenia, a predisposition to myelodysplasia/acute myeloid leukemia, and a profoundly reduced DC population, which is associated with increased susceptibility to viral infections, impaired phagocytosis, and decreased cytokine production. To define the role of GATA2 in DC differentiation and function, we studied Gata2 conditional knockout and haploinsufficient mice. Gata2 conditional deficiency significantly reduced the DC count, whereas Gata2 haploinsufficiency did not affect this population. GATA2 was required for the in vitro generation of DCs from Lin−Sca-1+Kit+ cells, common myeloid-restricted progenitors, and common dendritic cell precursors, but not common lymphoid-restricted progenitors or granulocyte-macrophage progenitors, suggesting that GATA2 functions in the myeloid pathway of DC differentiation. Moreover, expression profiling demonstrated reduced expression of myeloid-related genes, including mafb, and increased expression of T-lymphocyte–related genes, including Gata3 and Tcf7, in Gata2-deficient DC progenitors. In addition, GATA2 was found to bind an enhancer element 190-kb downstream region of Gata3, and a reporter assay exhibited significantly reduced luciferase activity after adding this enhancer region to the Gata3 promoter, which was recovered by GATA sequence deletion within Gata3 +190. These results suggest that GATA2 plays an important role in cell-fate specification toward the myeloid vs T-lymphocyte lineage by regulating lineage-specific transcription factors in DC progenitors, thereby contributing to DC differentiation.
Introduction
Dendritic cells (DCs) serve as the first line of innate immune defense and initiate adaptive immune responses by presenting processed antigens to T cells and are thus instrumental regulators of immune responses.1 Moreover, DCs interact with autoreactive T cells to induce self-tolerance.2 Peripheral DCs are relatively short lived and are continuously repopulated from hematopoietic stem cell (HSC)-derived progenitors in the bone marrow (BM).3-5 Fogg et al identified the first precursors downstream of common myeloid-restricted progenitors (CMPs) with the potential to differentiate into DCs and macrophages; therefore, CMPs are designated as macrophage-DC precursors.6 Furthermore, DC-restricted BM precursors or common DC precursors (CDPs) generate all DC subsets.7,8 Although the DC differentiation process has gradually been revealed, the underlying molecular mechanisms remain unclear.
GATA transcription factors contain 2 highly conserved zinc finger domains that directly bind to the consensus DNA sequence (A/T)GATA(A/G).9 The GATA transcription factor family comprises 6 members: GATA1, GATA2, and GATA3, which are principally expressed by hematopoietic lineage cells,10 and GATA4, GATA5, and GATA6, which are mainly expressed in nonhematopoietic tissues (eg, heart and gut).11 Among these, GATA2 is essential for HSC survival and proliferation.12-14 Gata2-null mouse embryos die of severe anemia approximately on embryonic day 10.12 Gata2-deficient stem cells and yolk sac cells proliferate poorly and undergo extensive necrosis.13 Moreover, specific Gata2 deletion from vascular endothelial cadherin-expressing endothelial cells causes a deficiency in long-term HSC repopulation.14 Beyond its role in hematopoietic stem/progenitor cells, GATA2 is particularly required for mast cell and mesenchymal stem cell differentiation.1,13,15-17 However, little is known about the requirement for GATA2 in the differentiation in other specific lineages or its function in mature blood cells.
Heterozygous GATA2 germ line mutations were reported to cause 3 overlapping clinical entities, characterized by a predisposition to myelodysplastic syndrome and acute myeloid leukemia: (1) familial myelodysplastic syndrome/acute myeloid leukemia, (2) Emberger syndrome, and (3) an immunodeficiency termed monocytopenia characterized by Mycobacterium avium complex/DC, monocyte, B- and natural killer (NK)–lymphoid deficiency.18-21 All of these conditions are generally named “GATA2 deficiency” syndrome. In this syndrome, monocyte, B-cell, NK-cell, and DC populations are profoundly diminished or undetectable.18,19 In contrast, neutrophil, macrophage, and T-cell populations remain unaltered.18,19 In addition, patients with this syndrome sometimes develop pulmonary alveolar proteinosis resulting from dysregulated phagocytic activity and cytokine production in alveolar macrophages.20
Considering these clinical manifestations, GATA2 is likely to be more widely required than would be expected if it acted solely in hematopoietic differentiation and mature blood cell functions. Because DCs play crucial roles in the immune system and their numbers are profoundly decreased in GATA2-deficiency syndrome, we focused on DCs in this study and aimed to clarify the roles of GATA2 in DC differentiation using GATA2-knockout and Gata2-haploinsufficient mice.
Materials and methods
Mice
Mice harboring a Gata2 exon 5 flanked by loxP sites22 (Gata2-floxed mice; Gata2f; a kind gift from Sally A. Camper, University of Michigan) were crossed with mice expressing a tamoxifen-inducible Cre recombinase controlled by the Rosa 26 promoter23 (ER-Cre mice; The Jackson Laboratory). To generate conditional Gata2 knockouts in vivo, Gata2f/f/ER-Cre mice were intraperitoneally injected with 1 µg of tamoxifen (Sigma-Aldrich) on days 1 to 3 and 8 to 10; mice were used 20 to 22 days after the first injection as previously reported, with some modifications.24 Polymerase chain reaction (PCR) genotyping via amplified mouse tail genomic DNA was performed to assess the frequency of Gata2 exon 5 excision. CD11c-Cre25 and SJL (CD45.1+) mice were purchased from The Jackson Laboratory, and C57BL/6 mice were purchased from CLEA Japan, Inc. The methods used to generate Gata2-haploinsufficient mice (Gata2+/−; a kind gift from Stuart H. Orkin, Harvard University) were previously described.12 To mimic infection, Gata2+/− mice were intraperitoneally injected with 10 mg/kg lipopolysaccharide (LPS; Sigma-Aldrich) and sacrificed under anesthesia 6 hours after LPS injection. Genotyping primer sequences are listed in supplemental Table 1 (available on the Blood Web site). This study was approved by the Tohoku University Animal Welfare Committee.
Reverse transcription PCR
Total RNA was purified using the Nucleospin RNA kit (Macherey-Nagel), followed by complementary DNA synthesis with the ReverTra Ace qPCR reverse transcription (RT) kit (Toyobo). Quantitative RT-PCR (qRT-PCR) was performed using Quantitect SYBR Green PCR master mix (Qiagen). RT-PCR primer sequences are listed in supplemental Table 1. Data are normalized to the Gapdh messenger RNA (mRNA) expression levels.
Flow cytometry
Cells were sorted and analyzed on FACSAria II and FACSCanto II flow cytometers (Becton Dickinson); data were analyzed using FACSDiva (Becton Dickinson) or FlowJo software (TreeStar). The reagents used for flow cytometry were indicated in supplemental Materials and methods.
Isolation of splenic DCs and BM precursor populations
Spleens were digested with collagenase and DNase; DCs were subsequently isolated using a magnetic-activated cell sorting (MACS) separation system with Pan-DC MicroBeads (Miltenyi Biotec) according to the manufacturer’s instructions. DCs were further reacted with antibodies against CD11c, B220, and major histocompatibility complex type II and sorted on a FACSAria II. Conventional DCs (cDCs) and plasmacytoid DCs (pDCs) were defined as CD11c+B220− and CD11c+B220+, respectively. BM progenitor populations were isolated according to previously published procedures.7,24,26 Lin−Sca-1+Kit+ cells (LSKs) were defined as lin−IL-7Rα−c-kithiSca-1hi, CMPs as lin−IL-7Rα−c-kithiSca-1−FcγRII/IIIintCD34+, granulocyte-macrophage (GM) progenitors (GMPs) as lin−IL-7Rα−c-kithiSca-1−FcγRII/IIIhiCD34+, common lymphoid-restricted progenitors (CLPs) as lin−IL-7Rα+c-kitintSca-1int, megakaryocyte erythrocyte progenitors as lin−IL-7Rα−c-kithiSca-1−FcγRII/III−CD34−, and CDPs as lin−IL-7Rα−c-kitint∼loFLT3+M-CSFR+CD11c−. Supplemental Figure 1 shows the scheme used to identify progenitor cell fractions.
Cell cultures
To induce GM-DC differentiation, erythrocyte-depleted BM cells were cultured in RPMI 1640 supplemented with 10% fetal bovine serum (FBS), penicillin (100 U/mL), streptomycin (100 µg/mL), and GM-colony-stimulating factor (GM-CSF) (20 ng/mL; Peprotech). On day 6, DCs (cDCs) were isolated using an MACS separation system with CD11c MicroBeads (Miltenyi Biotec). To induce FL-DC (FLT3-L–induced DCs) differentiation, BM cells were cultured in Iscove modified Dulbecco medium supplemented with 10% FBS, penicillin (100 U/mL), streptomycin (100 µg/mL), 2-mercaptoethanol (50 µM), sodium pyruvate (1 mM), and fms-like tyrosine kinase 3 ligand (FLT3-L) (200 ng/mL; Peprotech). On day 6, DCs (cDCs and pDCs) were isolated using an MACS separation system with Pan-DC MicroBeads (Miltenyi Biotec).
To assess DC differentiation from various progenitors in vitro, LSKs (3 × 103), CMPs (5 × 103), GMPs (1 × 104), CLPs (5-8 × 103), and CDPs (5-8 × 103) were sorted from the BM of Gata2f/f/ER-Cre (CD45.2+) or Gata2f/f mice (CD45.2+). These progenitor cells were cocultured with BM feeder cells from SJL (CD45.1+) mice (1.5 × 105) in the presence of FLT3-L (200 ng/mL; 96-well culture plates). To inactivate GATA2, 0.1 µM 4-hydroxytamoxifen (4-OHT; Sigma-Aldrich) was added to the cultures on day 0. Equal numbers of progenitor cells were used in each experiment, and half of the medium was replaced every third day with media containing the appropriate cytokines. Cultures were analyzed using flow cytometry to detect CD45.1, CD45.2, and CD11c expression after 5 to 7 days (CDP and CLP, day 5; GMP, day 6; CMP and LSK, day 7). DCs derived from Gata2f/f/ER-Cre or Gata2f/f mice were identified as CD45.2+CD11c+ cells.
An erythroid-myeloid-lymphoid (EML) hematopoietic precursor cell line (HPC) was obtained from American Type Culture Collection. These cells were cultured with Iscove modified Dulbecco medium supplemented with 4 mM l-glutamine (Invitrogen), 20% FBS, and 10% conditioned medium from a Kit-ligand–producing Chinese hamster ovary (CHO) cell line (a kind gift from Mitchell J. Weiss, The Children’s Hospital of Philadelphia).
Expression profiling
For a DC progenitor microarray analysis, total RNA was amplified using the Amino Allyl MessageAmp II aRNA Amplification kit (Life Technologies) and Cy3-labeled using a CyDye Post-labeling Reactive Dye Pack (Amersham Biosciences). A SurePrint G3 Mouse Gene Expression Microarray (Agilent) was used according to the manufacturer’s instructions. Fluorescence was detected using an Agilent Scanner. Gene expression levels were analyzed using GeneSpring software (Agilent). DC progenitors were obtained from Gata2f/f/ER-Cre CMPs via coculture with CD45.1+ BM feeder cells in the presence of murine FLT3-L and 4-OHT for 3 days.
Immunohistochemical analysis
Spleens were embedded in Tissue-Tek OCT compound (Sakura Finetek) at −80°C and sectioned using a cryostat. Sections were incubated with an anti-CD11c antibody (N418; Abcam), followed by incubation with a biotin-conjugated goat anti-Armenian hamster IgG antibody (Abcam). Sections were subsequently incubated with peroxidase-conjugated streptavidin (Nichirei). Images were acquired with a Biozero BZ-8100 (Keyence).
Quantitative ChIP analysis
A real-time PCR-based quantitative chromatin immunoprecipitation (ChIP) analysis was conducted essentially as previously described.27 Primer sequences for quantitative ChIP are listed in supplemental Table 1.
Promoter assay
The promoter region and +190 enhancer region of Gata3 were PCR amplified and cloned into a luciferase reporter vector (pGL4.10; Promega). Mutated GATA constructs were generated using a QuickChange Site-Directed Mutagenesis kit (Agilent Technologies).
To evaluate Gata3 transcriptional activity, aliquots of EML cells were transfected with 1 μg of Gata3 promoter construct and 100 ng of the pGL4.74 [hRluc/TK] vector (Promega) using FuGene HD (Promega). Cells were harvested 24 hours after transfection, and firefly and Renilla luciferase activity levels in the cell extracts were determined using a dual-luciferase reporter assay system (Promega).
Statistical analysis
The statistical significances of differences between the means ± standard deviations (SDs) of expression levels were calculated using a 2-tailed t test as indicated in the figure legends. Immunological Genome (ImmGen) module enrichment was evaluated using the following hypergeometric test:
where N is the total number of ImmGen genes, M is the number of module genes, n is the size of the list of genes of interest, and x is the number of genes within that list annotated to the module. P values <.05 were considered statistically significant.
Results
Gata2 expression in hematopoietic progenitor cells and DCs
Using qRT-PCR, we measured Gata2 expression levels in BM progenitors (LSK, CMP, GMP, CLP, and CDP; Figure 1A), splenic DCs (cDCs and pDCs), and DCs (GM-DCs and FL-DCs) generated in vitro. Gata2 mRNA was abundantly expressed in LSKs, as previously described.12,13,28 However, Gata2 mRNA expression was higher in CMPs than LSKs. Gata2 mRNA expression was detectable in GMPs, CLPs, and CDPs but at drastically lower levels than in LSKs and CMPs. Gata2 transcript levels were undetectable in steady-state splenic cDCs and pDCs, in contrast to in vitro– derived GM-DCs and FL-DCs. Gata2 mRNA was thus detected in all progenitor cells involved in DC differentiation, as well as some mature DCs (Figure 1B).
Analysis of Gata2 expression in hematopoietic progenitors and DCs. (A) DC differentiation pathway in mice. (B) Relative levels of Gata2 mRNA in Lin−Sca-1+Kit+ cells (lin−IL-7Rα−c-kithiSca-1hi), CMPs (lin−IL-7Rα−c-kithiSca-1−FcγRII/IIIintCD34+), GMPs (lin−IL-7Rα−c-kithiSca-1−FcγRII/IIIhiCD34+), CLPs (lin−IL-7Rα+c-kitintSca-1int), CDPs (lin−IL-7Rα−c-kitint∼loFLT3+M-CSFR+CD11c−), cDCs (B220−CD11chi), pDCs (B220+CD11clo), and BM-DCs (FLT3-l–induced DCs and GM-CSF–induced DCs) generated in vitro in the presence of FLT3-L or GM-CSF, respectively. Values are presented relative to those of Gapdh mRNA. Data represent the averages of 3 independent experiments and are expressed as means ± SDs. ND, not detectable.
Analysis of Gata2 expression in hematopoietic progenitors and DCs. (A) DC differentiation pathway in mice. (B) Relative levels of Gata2 mRNA in Lin−Sca-1+Kit+ cells (lin−IL-7Rα−c-kithiSca-1hi), CMPs (lin−IL-7Rα−c-kithiSca-1−FcγRII/IIIintCD34+), GMPs (lin−IL-7Rα−c-kithiSca-1−FcγRII/IIIhiCD34+), CLPs (lin−IL-7Rα+c-kitintSca-1int), CDPs (lin−IL-7Rα−c-kitint∼loFLT3+M-CSFR+CD11c−), cDCs (B220−CD11chi), pDCs (B220+CD11clo), and BM-DCs (FLT3-l–induced DCs and GM-CSF–induced DCs) generated in vitro in the presence of FLT3-L or GM-CSF, respectively. Values are presented relative to those of Gapdh mRNA. Data represent the averages of 3 independent experiments and are expressed as means ± SDs. ND, not detectable.
The DC population is drastically reduced in Gata2-knockout adult mice
To assess the importance of Gata2 in DC development in adult mice, we deleted Gata2 in adult Gata2f/f/ER-Cre mice via intraperitoneal injections of tamoxifen or corn oil on days 1 to 3 and 8 to 10 and analyzed the mice 20 to 22 days later. PCR analysis of genomic BM DNA detected efficient recombination (Figure 2A), and Gata2 mRNA expression was virtually undetectable in tamoxifen-injected Gata2f/f/ER-Cre mice (P < .01; Figure 2B). Analysis of the splenic DC populations from Gata2-knockout mice revealed drastically reduced pDC, cDC, and total DC frequencies (P < .01; Figure 2C; supplemental Figure 2A).
Drastically reduced numbers of splenic DCs and BM progenitor cells in Gata2 knockout mice. (A) Gata2 deletion was confirmed via PCR analysis of genomic DNA isolated from total BM cells of mice injected with tamoxifen or corn oil. (B) Relative levels of Gata2 mRNA in BM cells of mice injected with tamoxifen or corn oil. Data are expressed as means ± SDs (n = 4). *P < .01. (C) Percentages of splenic DC populations (pDCs and cDCs) among splenocytes from Gata2f/f/ER-Cre mice injected with tamoxifen or corn oil were determined using flow cytometry. Data are expressed as means ± SDs (n = 4). *P < .01. (D) Immunohistochemical analysis of spleens from Gata2f/f/ER-Cre mice treated with tamoxifen or corn oil. Sections were stained with an antibody against CD11c (CD11c+ cells are brown). Scale bar, 50 μm. (E) Percentages of BM progenitor cells from Gata2f/f/ER-Cre mice injected with tamoxifen or corn oil were determined using flow cytometry. Data are expressed as means ± SDs (n = 4). All differences are significant (P < .005).
Drastically reduced numbers of splenic DCs and BM progenitor cells in Gata2 knockout mice. (A) Gata2 deletion was confirmed via PCR analysis of genomic DNA isolated from total BM cells of mice injected with tamoxifen or corn oil. (B) Relative levels of Gata2 mRNA in BM cells of mice injected with tamoxifen or corn oil. Data are expressed as means ± SDs (n = 4). *P < .01. (C) Percentages of splenic DC populations (pDCs and cDCs) among splenocytes from Gata2f/f/ER-Cre mice injected with tamoxifen or corn oil were determined using flow cytometry. Data are expressed as means ± SDs (n = 4). *P < .01. (D) Immunohistochemical analysis of spleens from Gata2f/f/ER-Cre mice treated with tamoxifen or corn oil. Sections were stained with an antibody against CD11c (CD11c+ cells are brown). Scale bar, 50 μm. (E) Percentages of BM progenitor cells from Gata2f/f/ER-Cre mice injected with tamoxifen or corn oil were determined using flow cytometry. Data are expressed as means ± SDs (n = 4). All differences are significant (P < .005).
Immunohistochemical analysis of splenic tissues detected few CD11c+ cells in Gata2-knockout mice (Figure 2D). The numbers of splenic neutrophils, B cells, T cells, NK cells, and monocytes were reduced, but that of macrophages was increased (supplemental Figure 2B-C). Furthermore, peripheral blood cell counts revealed severe pancytopenia in Gata2-knockout mice (supplemental Table 2).
Next, to assess the influence of Gata2 deficiency on DC differentiation–related progenitor cell fractions, we analyzed BM cells via flow cytometry. Strikingly reduced numbers of progenitor cells (LSKs, CMPs, GMPs, CLPs, and CDPs) were observed in Gata2-knockout mice, compared with control mice (P < .005; Figure 2E).
GATA2 is required for DC differentiation
To clarify the mechanism by which GATA2 deficiency reduces DC populations, we deleted Gata2 from progenitor cells in vitro.29 Briefly, each progenitor population (LSK, CMP, GMP, CLP, and CDP) was isolated from Gata2f/f/ER-Cre mice (CD45.2+) and induced to differentiate into DCs by coculture with CD45.1+ BM feeder cells in the presence of murine FLT3-L and 4-OHT for 5 to 7 days. We subsequently analyzed the CD45.2+CD11c+ fractions, which represent DCs derived from CD45.2+ progenitor cells. The recombination efficiency in this method was ∼50% on day 1 and almost 100% on day 3 of 4-OHT treatment (supplemental Figure 3A). Gata2 mRNA levels were reduced by ∼90% on day 1 and were virtually undetectable on day 3 (P < .05; supplemental Figure 3B). The numbers of LSK-, CMP-, and CDP-derived CD45.2+CD11c+ cells were reduced by ∼70%, 50%, and 30%, respectively (LSKs and CDPs, P < .01; CMPs, P < .05; Figure 3). In contrast, no significant changes were observed in the numbers of CLP- and GMP-derived CD45.2+CD11c+ cells (Figure 3). These data indicate that GATA2 is required for DC differentiation, particularly differentiation from LSKs to CDPs via CMPs.
Stage-specific requirement for GATA2 during DC differentiation. (A) Percentages of DCs generated in each culture were determined using flow cytometry. To compare different experiments, data were normalized to the cell numbers of Gata2f/f cultures in the absence of tamoxifen (B). Data are expressed as means ± SDs (n = 3). *P < .05, **P < .01. NS, not significant.
Stage-specific requirement for GATA2 during DC differentiation. (A) Percentages of DCs generated in each culture were determined using flow cytometry. To compare different experiments, data were normalized to the cell numbers of Gata2f/f cultures in the absence of tamoxifen (B). Data are expressed as means ± SDs (n = 3). *P < .05, **P < .01. NS, not significant.
We further sought to determine the stage at which GATA2 could have a role in DC development using CD11c-Cre mice. We generated Gata2f/f/CD11c-Cre mice by crossing Gata2f/f mice with CD11c-Cre mice (Itgax-Cre). In these mice, CD11c expression induces the deletion of Gata2. Recombination analysis of genomic DNA and qRT-PCR analysis of Gata2 mRNA obtained from Gata2−/− GM-DCs, which were generated from the BM cells of Gata2f/f/CD11c-Cre mice in cultures supplemented with GM-CSF, demonstrated that Gata2 was efficiently excised from the genome (supplemental Figure 4A-B). The frequencies of pDCs, cDCs, and total DCs in the spleens of Gata2f/f/CD11c-Cre mice were comparable with those of Gata2f/f mice (supplemental Figure 4C). Because CD11c is expressed later by progenitor cells compared with CDPs,25 the results suggest that GATA2 is not required for the differentiation of mature DCs.
Gene expression analysis of Gata2 knockout DC progenitors
To determine the molecular mechanisms underlying commitment to the specific GATA2-driven DC differentiation pathway, we performed a gene expression analysis of Gata2 knockout DC progenitors. DC progenitors were obtained from CMPs of Gata2f/f/ER-Cre mice by coculturing with CD45.1+ BM feeder cells in the presence of murine FLT3-L and 4-OHT for 3 days. Flow cytometric analysis demonstrated markedly reduced c-Kit expression and undetectable CD11c expression in induced DC progenitors (data not shown). The average gene expression analysis values (Gata2-knockout, n = 3; control, n = 4) revealed more than twofold increases or decreases in the expression of 224 and 234 genes, respectively, and more than fivefold increases or decreases in the expression of 67 and 63 genes, respectively (Figure 4A; supplemental Tables 3-4). The expression levels of genes critical for DC differentiation, such as Spi1/Pu.1,29-31 Ikzf1,32,33 and Gfi1,34 were not significantly changed. Among the more than twofold differentially expressed genes, 112 upregulated and 117 downregulated genes were registered in the ImmGen database (http://www.immgen.org). The 112 genes activated by Gata2 knockout were unexpectedly enriched with module genes expressed at low levels in myeloid cells but at high level in lymphoid cells, such as Tcf7 and Gata3 (Coarse modules 16, 18, 19, 33, 57, and 60; Figure 4B). In contrast, the 117 downregulated genes were significantly enriched in module genes expressed at high levels in myeloid cells and at low levels in lymphoid cells, such as Mafb (Coarse modules 24, 25, and 30; Figure 4C). These data indicate that GATA2 imparts CMP with the ability to retain myeloid differentiation potential by suppressing the expression of lymphoid-related genes.
The expression profile of Gata2-knockout DC progenitors. (A) CMPs from Gata2f/f/ER-Cre mice were cocultured with BM cells from SJL (CD45.1+) mice in the presence of FLT3-L (200 ng/mL) with or without tamoxifen. Total mRNA extracted from CD45.2+ cells sorted on day 3 was used for microarray analysis. Genes exhibiting fivefold increases in activation or repression following Gata2 knockout are shown (Gata2 knockout DC progenitors, n = 3; WT DC progenitors, n = 4). (B-C) Enrichment among the modules of genes present in the ImmGen database that were upregulated or downregulated more than twofold in Gata2 knockouts. A hypergeometric test was used to calculate the statistical significance of these differences. P values <.05 were considered statistically significant.
The expression profile of Gata2-knockout DC progenitors. (A) CMPs from Gata2f/f/ER-Cre mice were cocultured with BM cells from SJL (CD45.1+) mice in the presence of FLT3-L (200 ng/mL) with or without tamoxifen. Total mRNA extracted from CD45.2+ cells sorted on day 3 was used for microarray analysis. Genes exhibiting fivefold increases in activation or repression following Gata2 knockout are shown (Gata2 knockout DC progenitors, n = 3; WT DC progenitors, n = 4). (B-C) Enrichment among the modules of genes present in the ImmGen database that were upregulated or downregulated more than twofold in Gata2 knockouts. A hypergeometric test was used to calculate the statistical significance of these differences. P values <.05 were considered statistically significant.
GATA2 binds to the downstream enhancer of Gata3 and negatively regulates Gata3 promoter activity
Notably, genes with crucial roles in T-cell lineage differentiation, such as Gata335,36 and Tcf7,37 were expressed at high levels in Gata2-knockout DC progenitors (7.33- and 6.20-fold upregulation, respectively; supplemental Table 3), as the upregulation of these master T-lineage differentiation genes is a potential key mechanism of impaired DC differentiation consequent to Gata2 knockout. Interestingly, the Gata3 +190 element contained a consensus GATA-binding motif (AGATAA)9 that was conserved across species, including humans, according to the University of California, Santa Cruz (UCSC) Genome Browser (https://genome.ucsc.edu/). In addition, a recent comprehensive ChIP-sequencing analysis of 10 transcription factors revealed that GATA2 binds to the 190-kb downstream region of Gata3 in murine HPC-7.38 Therefore, we hypothesized that GATA2 might directly repress Gata3 expression in HPCs. Through a quantitative ChIP analysis, GATA2 was found to bind the Gata3 +190-kb region in both the CMP fraction of murine BM cells and the EML cell line (Figure 5A). Furthermore, in EML cells, addition of the Gata3 +190 region to the Gata3 promoter (∼0.5 kb) significantly reduced luciferase activity, which was significantly recovered by deletion of the GATA sequence within Gata3 +190 kb (Figure 5B). These data suggest that GATA2 might negatively regulate Gata3 transcription in hematopoietic precursors by binding to the Gata3 +190-kb region.
GATA2 negatively regulates the transcriptional activity of Gata3. (A) A quantitative ChIP analysis of GATA2 chromatin occupancy at Gata3 locus in CMPs (left) and EML cells (right) (means ± SDs, n = 3). The necdin promoter and Gata2 +9.5 kb were used as negative and positive controls, respectively.27 (B) Transcriptional activity through the Gata3 +190-kb site assessed via luciferase assay. The Gata3 promoter (Gata3 prom), Gata3 +190-kb region + Gata3 promoter (Gata3 +190 kb + prom), or GATA sequence-deleted Gata3 +190-kb region + Gata3 promoter (Gata3 +190 kb deletion + prom) was fused to a luciferase reporter gene and transiently transfected into EML cells (means ± SDs; n = 5). *P < .05.
GATA2 negatively regulates the transcriptional activity of Gata3. (A) A quantitative ChIP analysis of GATA2 chromatin occupancy at Gata3 locus in CMPs (left) and EML cells (right) (means ± SDs, n = 3). The necdin promoter and Gata2 +9.5 kb were used as negative and positive controls, respectively.27 (B) Transcriptional activity through the Gata3 +190-kb site assessed via luciferase assay. The Gata3 promoter (Gata3 prom), Gata3 +190-kb region + Gata3 promoter (Gata3 +190 kb + prom), or GATA sequence-deleted Gata3 +190-kb region + Gata3 promoter (Gata3 +190 kb deletion + prom) was fused to a luciferase reporter gene and transiently transfected into EML cells (means ± SDs; n = 5). *P < .05.
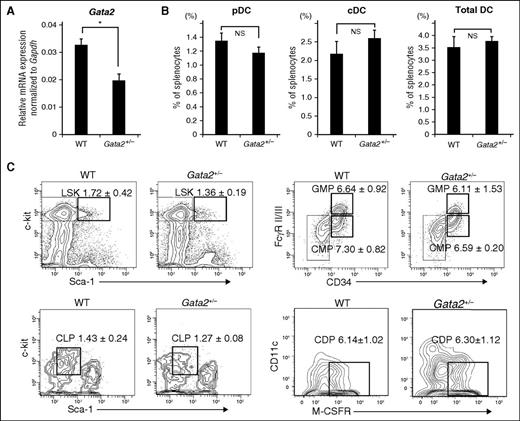
Gata2 haploinsufficiency does not affect DC generation in mice
Because GATA2-deficiency syndrome is caused by heterozygous mutations within the GATA2 coding and intronic enhancer regions,18 we analyzed Gata2-haploinsufficient mice (Gata2+/−) (8 weeks old). First, we measured Gata2 mRNA levels in BM from Gata2+/− and wild-type (WT; Gata2+/+) mice via qRT-PCR; the primer binds exon 5, which is lacking in GATA2-deficient mice and thus confirmed reduced Gata2 expression in Gata2+/− mice (P < .05; Figure 6A). Next, we determined the frequency of splenic DCs. However, no differences in the frequencies of cDCs, pDCs, and total DCs were observed in the spleens of Gata2+/− and Gata2+/+ mice (Figure 6B), and hematological parameters were comparable between the 2 genotypes (supplemental Table 5). When we analyzed progenitor fractions related to DC differentiation, we observed no significant differences, although the LSK and GMP populations in Gata2+/− mice were reduced relative to those in Gata2+/+ mice (Figure 6C).
Analysis of Gata2-haploinsufficient mice. (A) Relative Gata2 mRNA expression levels in BM cells from Gata2-haploinsufficient (Gata2+/−) and WT mice (8 weeks old). Data are expressed as means ± SDs (n = 3). *P < .05. (B) Percentages of splenic DC populations (pDCs and cDCs) in Gata2+/− and WT mice were determined by flow cytometry. Data are expressed as means ± SDs (n = 4). (C) Percentages of BM progenitor cells in Gata2+/− and WT mice were determined by flow cytometry. Data are expressed as means ± SDs (n = 4). No significant difference was observed between the cell populations. NS, not significant.
Analysis of Gata2-haploinsufficient mice. (A) Relative Gata2 mRNA expression levels in BM cells from Gata2-haploinsufficient (Gata2+/−) and WT mice (8 weeks old). Data are expressed as means ± SDs (n = 3). *P < .05. (B) Percentages of splenic DC populations (pDCs and cDCs) in Gata2+/− and WT mice were determined by flow cytometry. Data are expressed as means ± SDs (n = 4). (C) Percentages of BM progenitor cells in Gata2+/− and WT mice were determined by flow cytometry. Data are expressed as means ± SDs (n = 4). No significant difference was observed between the cell populations. NS, not significant.
Because immunodeficiency observed in GATA2-deficiency syndrome clinically manifests until adolescence,18-20 we further analyzed aged Gata2-haploinsufficient mice (6 months old), showing no detectable difference in the DC frequencies and hematological parameters as compared with the control mice (supplemental Figure 5A; supplemental Table 6). There are 2 types of monocyte subsets: “inflammatory” Ly-6C+ and “resident” Ly-6C− monocytes (supplemental Figure 2A).39 Thus, we also tested whether the ratio of monocyte could be altered by Gata2 haploinsufficiency, showing no significant difference in the balance of each subset (supplemental Figure 5B). Furthermore, whereas elevated FLT3-L is reported to be an early sign of this syndrome,40 the serum FLT3-L level was unaltered in aged Gata2+/− mice (supplemental Figure 5C). qRT-PCR analysis demonstrated no significant difference in the expression level of myeloid-lymphoid–related genes (Spi1, Cebpα, Mafb, Irf8, Gata3) in the CMP fraction from aged Gata2+/− and WT mice (supplemental Figure 6). Finally, we injected endotoxin (LPS) in the aged Gata2+/− and WT mice to test whether the susceptibility to intracellular pathogens might be altered by Gata2 haploinsufficiency. Interestingly, whereas the percentages of monocytes and DCs were unaltered, serum IL-6 level was significantly lower in Gata2-haploinsufficient mice (supplemental Figure 7A-B).
Taken together, our data suggest that unlike humans, Gata2 haploinsufficiency did not alter DC differentiation in mice. However, although further analyses are required to reveal the detailed molecular mechanisms, compromised interleukin-6 (IL-6) production under Gata2 haploinsufficiency might suggest the potential link to the pathophysiology of GATA2-deficiency syndrome, of which the representative phenotype is immunodeficiency.18-21
Discussion
In GATA2-deficiency syndrome, the lack of DCs is a fundamental feature important for the development of this pathological condition. In light of these points, we aimed herein to clarify the roles of GATA2 in DC differentiation. We first confirmed that Gata2 deletion in vivo resulted in a profound decrease in the DC count (Figure 2C). We then assessed the effect of Gata2 knockout on DC generation from each progenitor and revealed that Gata2 deletion impaired the ability of LSKs, CMPs, and CDPs, but not CLPs, to generate DCs (Figure 3). These results suggest that GATA2 may play a particularly important role in the myeloid DC differentiation pathway. Exceptionally, the generation of DCs from GMPs, which are in the myeloid pathway, was not affected by Gata2 knockout. This indicated that DC-generation capacity in GMPs might be explained by the transcription factor expression profiles in GMPs. For example, C/EBPα is abundantly expressed in GMP.25,41,42 This transcription factor reportedly regulates many genes required for DC generation and directly inhibits the transcriptional activity of GATA2.43 It is thus possible that C/EBPα, not GATA2, acts dominantly on DC generation at this progenitor stage.
In addition to DCs, the splenic cell fraction analysis demonstrated significantly decreased numbers of neutrophils, B cells, T cells, NK cells, and monocytes in Gata2-knockout mice (supplemental Figure 2B). In GATA2-deficiency syndrome, the numbers of monocytes, B cells, NK cells, and DCs are profoundly decreased, whereas those of neutrophils, macrophages, and T cells are less profoundly affected.18,19 In the context of GATA2-deficiency syndrome, the loss of GATA2 expression appears to be mild, compared with Gata2 knockout, which might affect the observed differences in hematopoietic cell fates. In contrast, Gata2-knockout mice harbored increased numbers of macrophages, suggesting the dispensability of GATA2 for macrophage differentiation (supplemental Figure 2B-C). Gata2−/− ES cells retain the potential to generate macrophages,13 and the conditional expression of Gata2 in ES cells might inhibit macrophage development.44 Furthermore, GATA2 repression by enforced PU.1 expression in an IL-3–dependent cell line favored macrophage generation.15 These studies may support our observation of macrophage expansion in GATA2-knockout mice. An alternative explanation is the long lifespan of tissue macrophages. A reported self-renewal capacity allows macrophages to survive for long periods.45-47 Therefore, residing macrophages in the spleen may have self-renewed to maintain the number of macrophages. In the present study, we examined only the number of macrophages and not their functions. As GATA2-deficiency syndrome patients exhibit pulmonary alveolar proteinosis caused by a dysfunction in alveolar macrophages, it will be necessary to examine the functions of Gata2-knockout macrophages.
The gene expression analysis data yielded suggestive findings. Although genes known to be related to DC differentiation, such as Spi1/Pu.1,29-31 Ikzf1,33,48 and Gfi-1,49 were not significantly affected in Gata2-knockout DC progenitors, several myeloid-related genes were downregulated. This downregulation of myeloid-related genes in Gata2-knockout mice supports the importance of GATA2 in the myeloid DC generation pathway. In contrast to the downregulation of myeloid-related genes, major T-cell–related genes, such as Tcf7 and Gata3, were upregulated. Tcf7 encodes the transcription factor TCF-1, a T-cell–specific transcription factor induced during early T-cell lineage commitment.37 TCF-1 is involved in the regulation of cell proliferation and survival during T-cell development.37 GATA3 is indispensable for the development of T cells at multiple stages.35 Altogether, these findings suggest that GATA2 maintains the myeloid-restricted progenitor phenotype of CMPs by suppressing the transcription of lymphoid-related genes through the inhibition of master T-lineage transcription factors, which might explain why Gata2 deficiency perturbed the normal myeloid developmental process in CMPs. GATA2 is therefore a potential key factor in the balance between myeloid and lymphoid commitment in common progenitors, and these findings further suggest the possible presence of a myeloid–T-progenitor population, as reported previously.50
We have demonstrated that Gata2 directly represses Gata3 expression through the Gata3 +190-kb region in progenitor cells (Figure 5). However, GATA3 has been reported to activate its own expression in T cells,51,52 suggesting that GATA2 and GATA3 may exert qualitatively distinct functions in the transcriptional regulation of GATA3 during hematopoietic differentiation stages. In support of our findings, this functional difference between GATA2 and GATA3 has also been suggested during placental development, in which GATA2 deficiency exerted a greater inhibitory influence on placental angiogenic activity than did GATA3 deficiency.53,54 Although the detailed molecular mechanisms regarding the participation of GATA2 in transcriptional repression remain to be elucidated, GATA2 has also been reported to directly repress erythropoietin gene expression in a human hepatoma cell line.55 Taken together, our results suggest an important implication regarding GATA switches involving additional GATA factors besides GATA1 and GATA2 during erythroid differentiation.54
In conclusion, our data demonstrate an apparent important role for GATA2 in cell-fate specification toward myeloid lineage (vs T-lymphocyte lineage) in progenitor cells, thus contributing to DC differentiation. The clues uncovered in this study contribute new insights into the role of GATA2 in hematopoiesis and help to clarify pathogenic molecular mechanisms underlying GATA2-deficiency syndrome.
The microarray data reported in this article have been deposited in the Gene Expression Omnibus database (accession number GSE82044).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank C. Fushimi (Tohoku University) for technical assistance, T. Moriguchi (Tohoku University) and the members of their laboratory for helpful discussions, the staff of the Biomedical Research Core and Pathology Platform of Tohoku University for technical support, and Mitchell Weiss for providing Kit-ligand–producing CHO cells. The authors also thank S. H. Orkin and S. Camper for kindly providing them Gata2 gene knockout mice and Gata2 gene conditional knockout mice, respectively.
This work was supported by Japan Society for the Promotion of Science Grants-in-Aid for Scientific Research (KAKENHI) grant numbers 26860716 (Y. Onishi) and 25860776 (Y. Okitsu).
Authorship
Contribution: K.O. and H.H. conceived and designed the experiments; K.O., T.F., and A.I.-N. performed the experiments and analyzed the data; T.F., Y. Okitsu, N.F., Y. Onishi, K.I., R.S., M.Y., and H.H. contributed reagents, materials, and/or analytical tools; and K.O., T.F., and H.H. wrote the manuscript.
Conflict-of-interest disclosure: T.F. and H.H. received a research grant from Chugai Pharmaceutical Co, Ltd. The remaining authors declare no competing financial interests.
Correspondence: Hideo Harigae, Department of Hematology and Rheumatology, Tohoku University Graduate School of Medicine, 2-1 Seiryo-cho, Aoba-ku, Sendai 980-8575, Japan; e-mail: harigae@med.tohoku.ac.jp.







This feature is available to Subscribers Only
Sign In or Create an Account Close Modal