Key Points
Bcl-xL is a substrate for active caspase-3 in the exosomes.
Molecular or chemical inhibition of exosomal Bcl-xL cleavage attenuates uptake of exosomes by hematological malignant cells.
Abstract
The intercellular crosstalk between hematological malignancies and the tumor microenvironment is mediated by cell-to-cell interactions and soluble factors. One component of the secretome that is gaining increasing attention is the extracellular vesicles and, in particular, the exosomes. Apart from the role as vectors of molecular information, exosomes have been shown to possess intrinsic biological activity. In this study, we found that caspase-3 is activated in L88 bone marrow stroma cell–derived exosomes and identified 1 of the substrates to be the antiapoptotic protein Bcl-xL. The cleaved Bcl-xL is found in a panel of normal and cancer cell–derived exosomes and is localized on the outer leaflet of the exosomal membrane. Incubation of the exosomes with a caspase-3 inhibitor or the pan-caspase inhibitor prevents the cleavage of Bcl-xL. Importantly, MCF-7 cell–derived exosomes that are caspase-3–deficient are enriched in full-length Bcl-xL, whereas ectopic expression of caspase-3 restores the cleavage of Bcl-xL. Chemical inhibition of Bcl-xL with ABT737 or molecular inhibition by using the D61A and D76A Bcl-xL mutant leads to a significant decrease in the uptake of exosomes by hematopoietic malignant cells. These data indicate that the cleaved Bcl-xL is required for the uptake of exosomes by myeloma and lymphoma cells, leading to their increased proliferation. In summary, we demonstrate for the first time that Bcl-xL is an exosomal caspase-3 substrate and that this processing is required for the uptake of exosomes by recipient cells.
Introduction
Multiple myeloma (MM) is characterized by the neoplastic proliferation of plasma cells in the bone marrow (BM).1 The growth and survival of MM cells is highly dependent on the BM tumor microenvironment, which is composed by the BM stroma cells (BMSCs), mesenchymal stem cells, and immune system–related cells.1,2 The crosstalk between the BMSCs and the MM cells is mainly mediated by direct cell-to-cell interactions and through the secretion of soluble factors such as cytokines, chemokines, and extracellular vesicles.
Among extracellular vesicles, the best studied are the exosomes. These vesicles are endosome-derived ranging in size between 50 nm and 100 nm in diameter and a density between 1.13 g/mL and 1.19 g/mL. Exosomes are composed of DNA, RNA, and proteins, some of which are core exosomal components such as the tetraspanins (CD9, CD63, CD81) and the ESCRT machinery (Alix and TSG101) and some which are cell type–specific.3 Cancer cell–derived exosomes have been shown to modulate the characteristics of the recipient cells by transferring chemoresistance, increase the metastatic potential, and enable stromal cell transformation to cancer-associated fibroblasts.4,5 Interestingly, it was recently shown that exosomes secreted from the BMSCs support the growth and resistance to therapy of MM cells.6,7 In addition to their functional properties upon uptake by recipient cells, exosomes were recently shown to possess intrinsic biological activity. Melo et al demonstrated that breast cancer exosomes are capable of processing pre-microRNA (miRNA) to miRNA in a cell-free, exosomal-dependent manner.8 Importantly, this processing promoted the metastatic potential of recipient cells. Furthermore, we have recently shown that prostate cancer exosomes possess intrinsic glycolytic activity leading to the generation of adenosine triphosphate.9 Notably, inhibition of the glycolytic activity results in significant attenuation of exosomal uptake by the recipient cells, indicating that this energy is required for the process.9 With regard to caspase activity in the exosomes, it has been suggested that cancer cells excrete exosomes with active caspase-3 as a defense mechanism against cell death.10 However, whether active caspase-3 could cleave substrates in the exosomes has not been investigated to date and no such substrates have yet been reported.
The mechanisms of exosomal uptake by recipient cells include phagocytosis, micropinocytosis, clathrin- or caveolin-mediated endocytosis, and lipid raft–mediated endocytosis, in which caveolin or clathrin is also involved .11 Proteinase K pretreatment of exosomes reduces their uptake by recipient cells, thus indicating that protein interactions are critical for this process.12 Tetraspanins have been shown to play a significant role in exosomal uptake in a number of cell types including ovarian and dendritic cells. Other important proteins for exosomal uptake are integrins, immunoglobulins, proteoglycans, and lectins; however, the role of caspase and its proteolytic activity in exosomal uptake has not yet been described.
In this study, we provide evidence for the first time that Bcl-xL, but not other antiapoptotic members of the Bcl-2 family such as Bcl-2 or Mcl-1, is a caspase-3 substrate in the exosomes and that cleaved Bcl-xL localized on the outer exosomal membrane is necessary for the uptake of these vesicles by plasma cell myeloma and aggressive lymphoma cells.
Methods
Cell culture
L88, the human BMSC line, has been previously described.13 The human MM cell lines OPM-2 and RPMI 8226 were provided by Brigitte Sola. The anaplastic large cell lymphoma (ALCL) cell line Mac2a were provided by Marshall Kadin and SUP-M2 cell line was purchased from DSMZ (No. ACC 509). The acute lymphoblastic leukemia (ALL) cell lines were provided by George Rassidakis. The human mammary tumor cell line MCF7 stably transfected with pcDNA or pcDNA-caspase-3 plasmids were provided from Cristian Hellwing.
All cell lines were cultured at 37°C/5% CO2. The L88, OPM2, RPMI 8226, Jurkat, Reh, and PC3 cell lines were grown in RPMI-1640 medium (HyClone, Thermo Scientific, Bremen, Germany). The mouse BMSCs (mBMSCs), MCF7-pcDNA, and MCF7-caspase-3 were grown in Dulbecco’s modified Eagle medium (HyClone, Thermo Scientific). All media were supplemented with 10% heat-inactivated fetal bovine serum (FBS; Gibco, Invitrogen Life Technologies, Paisley, United Kingdom), 2 mM l-glutamine (Gibco, Invitrogen Life Technologies), and 100 U/mL penicillin and 100 µg/mL streptomycin (Gibco, Invitrogen Life Technologies).
Patient samples
MM BM aspirations and ALL blood samples were obtained from the Karolinska University Hospital Solna, Stockholm, Sweden. Patients had a confirmed diagnosis and all were previously untreated. The study was approved by the regional ethics committee and all patients gave their informed consent in accordance with the Declaration of Helsinki. Heparinized BM samples were obtained and mononuclear cells separated by Ficoll-Paque Plus density sedimentation (Amersham Biosciences) were purified with EasySep human CD138 selection kit according to manufacturer’s protocol (StemCell Technologies). The purified tumor cells were seeded at 1 × 106 cells/mL into 12- or 48-well plates followed by immediate addition of the exosomes.
Plasmids
L88 cells were stably transfected with CD63-GFP (provided by Frederik Vilhardt). The wild-type (WT) Bcl-xL expressing plasmid 3120 pSFFV-neo-Bcl-xL and the uncleavable mutant Bcl-xL where the aspartic acids from both caspase cleavage sites were replaced with caspase-uncleavable alanines (D61A and D76A) expressing plasmid pSH1 were obtained from Jin Wang.
Conditioned media-exosome isolation
Exosomes were isolated from cells cultured in exosome depleted medium, as previously described.14 For the depletion of exosomes from medium, 30% FBS medium was prepared and ultracentrifuged overnight at 120 000g at 4°C. The supernatant was filtered (0.22 µm) and diluted to a final concentration of 10% FBS with medium and supplemented with the nutrients and antibiotics.
Cells were cultured until they reached ∼80% confluence; the isolated medium was centrifuged at 100g for 10 minutes at room temperature to remove cell debris and filtered (0.22 µm). Supernatants were frozen and thawed at 4°C, then ultracentrifuged for 2 hours at 120 000g at 4°C. The supernatant was discarded and the pellet was washed in phosphate-buffered saline (PBS) and ultracentrifuged for 2 hours at 120 000g at 4°C. Exosomes used for western blotting were lysed in 1XRIPA buffer, whereas exosomes used for functional studies were resuspended in conditioned medium or PBS.
Sucrose gradient
A sucrose gradient with range from 0.2 to 2 M sucrose was prepared, as previously described.14 Exosomes were placed on the surface of the sucrose gradient and ultracentrifuged at 120 000g at 4°C for 20 hours. The fractions were collected and used for exosomal characterization; for western blotting and flow cytometry, the fractions were ultracentrifuged at 120 000g for 2 hours at 4°C.
Electron microscopy-negative staining
An aliquot of 3 µL from exosome isolates were added to a grid with a carbon supporting film for 5 minutes. The excess solution was soaked off by a filter paper and the grid was rinsed by adding 5 µL distilled water for 10 seconds, soaked off and stained with 1% uranyl acetate in water for 10 seconds, and then air-dried. The samples were examined in a Tecnai 12 Spirit Bio TWIN transmission electron microscope (FEI Company, Eindhoven, The Netherlands) at 100 kV. Digital images were taken by using a Veleta camera (Olympus Soft Imaging Solutions GmbH, Münster, Germany).
Immunoelectron microscopy
An aliquot of 3 µL from exosome isolates were added to a grid with a carbon supporting film for 5 minutes. The excess solution was soaked off by a filter paper and 5 µL 2% bovine serum albumin (BSA; Sigma fraction V) in 0.1 M phosphate buffer was added for 10 minutes to block nonspecific binding. The excess solution was soaked off and grids were floated on drops of primary antibody diluted 1:300 in 0.1% BSA in PBS overnight at 4°C. Grids were rinsed in PBS and bound antibodies were detected with protein A coated with 10 nm gold (BBI Solutions, Cardiff, Wales) at a final dilution of 1:100. Sections were rinsed in PBS and distilled water and stained with 1% uranyl acetate in water for 10 seconds and then air-dried. The samples were examined in a Tecnai 12 Spirit Bio TWIN transmission electron microscope (FEI Company) at 100 kV. Digital images were taken by using a Veleta camera (Olympus Soft Imaging Solutions GmbH).
Exosome uptake assay-caspase inhibition
In a 48-well plate, 2 × 104 cells/mL were plated from RPMI 8226 and OPM2, SUPM2, and Mac2a cell lines, in conditioned medium, in final volume of 400 μL and 3 μg/mL of L88 or L88 Bcl-xL WT PKH67-labeled exosomes. Before their incubation with the cells, the exosomes were treated with dimethyl sulfoxide (control), z-VAD.fmk, or z-DEVD.fmk (final concentration 100 nM) for 20 hours and subsequently added in a final volume of 400 μL. The exosome-treated cells were harvested after 3 hours of incubation, washed 3 times with 0.5% BSA in PBS, and the percentage of green fluorescence intensity was measured by using the Novocyte cytometer (ACEA Biosciences). The results were analyzed by using the company’s software.
Cell proliferation assay
Cells were starved for 16 hours before addition of exosomes. Afterward, in a 96-well plate, 2 × 104 cells/mL were added from RPMI 8226, OPM2, SUPM2, and Mac2a cell lines, in starvation medium, and 30 μg/mL of L88 Bcl-xL WT exosomes treated or not with z-VAD.fmk were added, in a final volume of 100 μL. After 6 hours of incubation, 40 μL of condition medium was added. The incubation continued for 48 hours. Then cells were harvested, washed 1 time with PBS, and incubated for 15 minutes in 4°C with solution containing 4 μg/mL fluorescein diacetate and 1% propidium iodide (PI). Then the cells were washed with 0.5% BSA in PBS and the percentage of green and red fluorescence intensity was measured by the Novocyte cytometer. The results were analyzed by using the company’s software.
Exosomes treated with caspase inhibitors
Exosomes were treated with Triton X-100 (1%) and dimethyl sulfoxide, z-VAD.fmk, or z-DEVD.fmk at a final concentration of 100 μM and incubated for 3 hours at 37°C. Exosomes were then lysed and protein concentration was measured. A total of 20 µg of protein was loaded for western blot analysis.
Results
Bcl-xL is cleaved in the exosomes and is localized in the outer exosomal membrane
BM-derived fibroblasts have been shown to promote the survival and proliferation of a number of hematological malignancies.15 We have previously shown that L88, a BM-derived fibroblast cell line, can protect against the cytotoxic effects of sorafenib and promote proliferation in a trans-well setting.16 Exosomes are known to be 1 important component of the secretome and have been shown to promote resistance to a variety of anticancer drugs.7 We isolated and characterized L88 BM-derived exosomes by using a battery of techniques, including transmission electron microscopy, western blotting, sucrose gradient, and flow cytometry, respectively (see supplemental Figure 1A-F, available on the Blood Web site).
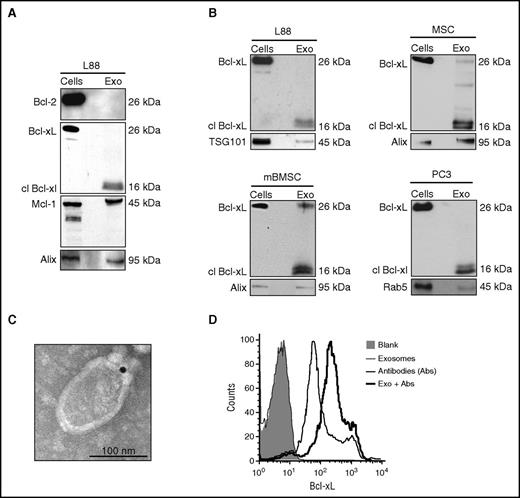
In an attempt to determine the mechanism of resistance mediated by the L88 BM fibroblasts, we examined the enrichment of Bcl-2, Bcl-xL, and Mcl-1 in L88 exosomes (Figure 1A). We found that Bcl-2 is present in low levels in the exosomes, whereas Bcl-xL and Mcl-1 are present in high amounts. Interestingly, we observed that the L88 exosomes contain the cleaved, 16-kDa Bcl-xL fragment. We expanded the panel of stroma-derived cell lines to include mesenchymal stem cells and mBMSCs as well as human cancer cell lines (the prostate cancer cell lines PC3 and DU145) (Figure 1B; supplemental Figure 2, respectively). We found that all the cell lysates were enriched with the full-length Bcl-xL. Interestingly, in immunoblots of both normal and cancer cell–derived exosomes, we only observed a band at 16 kDa, which has been previously described as the cleaved form of Bcl-xL.17 This band was not evident in DU145 and MCF7 cells, which are known to have caspase-3 deficiencies.18,19 The presence and localization of the observed cleaved Bcl-xL in the exosomes was confirmed by immunoelectron microscopy, demonstrating that Bcl-xL is on the surface of the exosomes (Figure 1C). To determine whether Bcl-xL is on the inner or outer leaflet of the exosomal membrane, we stained for Bcl-xL on intact exosomes and measured the levels by flow cytometry (Figure 1D). We found that Bcl-xL is accessible to the antibody, indicating an outer leaflet localization.
Cleaved Bcl-xL is enriched in stroma-derived exosomes and is present on the exosomal surface. (A) Western blot analysis of L88 cells and exosomes for the antiapoptotic Bcl-2 family members Bcl-2, Bcl-xL, and Mcl-1. (B) Western blot analysis of Bcl-xL in stroma L88, mesenchymal stem cell (MSC), mBMSC, and prostate cancer cell line PC3. (C) Immunoelectron microscopy for Bcl-xL in the exosomal surface of L88 cell–derived exosomes. (D) Fluorescence intensity of L88 cell–derived exosomes bound to beads and stained with Bcl-xL antibody. Exo, exosome.
Cleaved Bcl-xL is enriched in stroma-derived exosomes and is present on the exosomal surface. (A) Western blot analysis of L88 cells and exosomes for the antiapoptotic Bcl-2 family members Bcl-2, Bcl-xL, and Mcl-1. (B) Western blot analysis of Bcl-xL in stroma L88, mesenchymal stem cell (MSC), mBMSC, and prostate cancer cell line PC3. (C) Immunoelectron microscopy for Bcl-xL in the exosomal surface of L88 cell–derived exosomes. (D) Fluorescence intensity of L88 cell–derived exosomes bound to beads and stained with Bcl-xL antibody. Exo, exosome.
Bcl-xL is cleaved by caspase-3 in the exosomes
The cleavage of Bcl-xL has been previously assigned to caspases and in particular to caspase-3.20 To investigate the mechanism behind the observed exosomal cleavage of Bcl-xL, we examined whether signal transduction pathways that are known to activate caspase-3 are enriched in L88 cell–derived exosomes. L88 whole cell and exosomal lysates with antibodies targeting members of the death receptor pathway, the Toll-like receptor (TLR) family, the apoptosome (ie, caspase-9 and cytochrome c) and active caspase-3 (Figure 2A). Interestingly, we found that L88-derived exosomes were enriched in TLR4 and Mydd88, Fas, and FADD but not DR4 (TRAIL-R1) (Figure 2A). We also found high levels of cleaved caspase-8 and caspase-3/7 in the L88-derived exosomes (Figure 2A). Notably, there were no detectable levels of cytochrome c or cleaved caspase-9 in the exosomes, suggesting that the intrinsic pathway is not involved in the caspase-3 activation. Interestingly, the same components of the signaling cascade that lead to caspase-3 activation were identified in exosomes secreted by PC3, a prostate cancer cell line (supplemental Figure 3).
Caspase-3 is present and active in L88 exosomes. (A) Western blot analysis of the indicated proteins in L88 whole cell lysates and exosomes. (B) Caspase 3/7 activity assay performed in L88 cell–derived exosomes ± caspase inhibitors z-VAD.fmk or z-DEVD.fmk (mean ± standard deviation [SD], n = 3). ***P < .001. NT, not treated; U.A., units arbitrary. (C) Western blot analysis of L88 cell–derived exosomes treated with zVAD.fmk or DEVD.fmk and probed for the indicated proteins. (D) Western blot analysis of MCF-7 (pcDNA3.1 or caspase-3) transfected cells for the indicated proteins. (E) Caspase-3/7 activity assay performed in MCF-7–transfected cells treated with doxorubicin (mean ± SD, n = 3). (F) Western blot analysis in exosomes isolated from MCF-7–transfected cells for the indicated proteins.
Caspase-3 is present and active in L88 exosomes. (A) Western blot analysis of the indicated proteins in L88 whole cell lysates and exosomes. (B) Caspase 3/7 activity assay performed in L88 cell–derived exosomes ± caspase inhibitors z-VAD.fmk or z-DEVD.fmk (mean ± standard deviation [SD], n = 3). ***P < .001. NT, not treated; U.A., units arbitrary. (C) Western blot analysis of L88 cell–derived exosomes treated with zVAD.fmk or DEVD.fmk and probed for the indicated proteins. (D) Western blot analysis of MCF-7 (pcDNA3.1 or caspase-3) transfected cells for the indicated proteins. (E) Caspase-3/7 activity assay performed in MCF-7–transfected cells treated with doxorubicin (mean ± SD, n = 3). (F) Western blot analysis in exosomes isolated from MCF-7–transfected cells for the indicated proteins.
To substantiate the presence of cleaved caspase-3 in the exosomes, we measured caspase-3 activity by a spectrophotometric assay and the observed caspase-3 activity was inhibited by the specific caspase inhibitor (z-DEVD.fmk) and the pan-caspase inhibitor (z-VAD.fmk) (Figure 2B).
Having established that exosomal caspase-3 is active, we wanted to investigate whether Bcl-xL is a substrate of caspase-3 in the L88 cell–derived exosomes. L88 cell–derived exosomes were incubated with z-VAD.fmk or z-DEVD.fmk and western blot analysis demonstrated a decreased cleaved Bcl-xL and increased full-length protein (Figure 2C). These data support our hypothesis that Bcl-xL is cleaved by caspase-3 in the exosomes. We then used an additional model system, the caspase-3–deficient MCF-7 cell line. MCF-7 cells were stably transfected with a caspase-3 construct; measurements of the intracellular caspase-3 activity indicated that it is functional upon treatment with 30 nM doxorubicin for 24 hours (Figure 2D-E). Exosomes were isolated from the MCF-pcDNA and the MCF-6–Casp-3 cells and total exosomal lysates were probed for Bcl-xL and caspase-3. We found that, in contrast to MCF-pcDNA cells, caspase-3 and the cleaved form of Bcl-xL were detected in MCF-7/Casp-3 cells (Figure 2F). These data demonstrate that Bcl-xL is a substrate of caspase-3 in the L88 cell–derived exosomes.
Caspase-3–cleaved Bcl-xL controls exosomal uptake in plasma cell myeloma cells
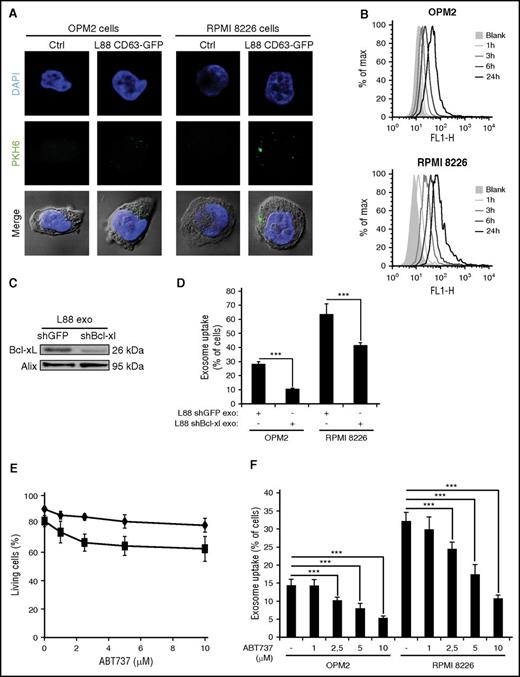
Bcl-xL has been shown to have several nonapoptotic roles in addition to its function as an antiapoptotic protein.21,22 Furthermore, an additional role was added to the Bcl-xL repertoire as a regulator of synaptic vesicle membrane dynamics and endocytosis through its interaction with Drp-1.23,24 Having observed that Bcl-xL is located at the outer leaflet of the L88 exosomes, we examined whether it may play a role in the intracellular uptake by the recipient MM cell lines, RPMI 8226 and OPM2. Labeling of L88-derived exosomes with the lipophilic dye followed by uptake studies demonstrated that these vesicles are readily internalized by OPM2 and RPMI 8226 cells in a time-dependent manner (Figure 3A-B). Stable knockdown of Bcl-xL in L88 cells resulted in a decreased enrichment of Bcl-xL in the L88-derived exosomes (Figure 3C). Uptake studies with PKH67-labeled L88 exosomes demonstrated that a decrease in Bcl-xL levels is accompanied by a decrease in the internalization of the exosomes by recipient cells (Figure 3D).
Bcl-xL is involved in uptake of L88 CD63-GFP cell-derived exosomes by MM cells. (A) Representative images uptake of L88 CD63-GFP PKH67-labeled exosomes by OPM2 or RPMI cells at 3 hours’ incubation. Ctrl, control. (B) Uptake of L88 CD63-GFP PKH67 labeled exosomes by OPM2 or RPMI cells at 1, 3, 6, or 24 hours of incubation. (C) Bcl-xL levels in exosomes derived from L88 cells transfected with shGFP (control) or shBcl-xL, as demonstrated by western blotting. Alix serves as a loading control. (D) Percentage of OPM2 or RPMI PKH67-positive cells, after 3 hours of incubation with L88 shGFP or L88shBcl-xL PKH67-labeled exosomes (mean ± SD, n = 3). ***P < .001. (E) Effect of ABT737 treatment in cell survival measured by PI staining in OPM2 (♦) and RPMI (▪) cell lines at 3 hours in different concentrations (1, 3, 5, and 10 μM) of the Bcl2/Bcl-xL inhibitor (mean ± SD, n = 3). (F) Percentage of OPM2 or RPMI PKH67-positive cells after 3 hours of incubation with L88 CD63-GFP PKH67-labeled exosomes that have been preincubated with the indicated concentrations of ABT737 (means ± SD, n = 3). ***P < .001.
Bcl-xL is involved in uptake of L88 CD63-GFP cell-derived exosomes by MM cells. (A) Representative images uptake of L88 CD63-GFP PKH67-labeled exosomes by OPM2 or RPMI cells at 3 hours’ incubation. Ctrl, control. (B) Uptake of L88 CD63-GFP PKH67 labeled exosomes by OPM2 or RPMI cells at 1, 3, 6, or 24 hours of incubation. (C) Bcl-xL levels in exosomes derived from L88 cells transfected with shGFP (control) or shBcl-xL, as demonstrated by western blotting. Alix serves as a loading control. (D) Percentage of OPM2 or RPMI PKH67-positive cells, after 3 hours of incubation with L88 shGFP or L88shBcl-xL PKH67-labeled exosomes (mean ± SD, n = 3). ***P < .001. (E) Effect of ABT737 treatment in cell survival measured by PI staining in OPM2 (♦) and RPMI (▪) cell lines at 3 hours in different concentrations (1, 3, 5, and 10 μM) of the Bcl2/Bcl-xL inhibitor (mean ± SD, n = 3). (F) Percentage of OPM2 or RPMI PKH67-positive cells after 3 hours of incubation with L88 CD63-GFP PKH67-labeled exosomes that have been preincubated with the indicated concentrations of ABT737 (means ± SD, n = 3). ***P < .001.
To further investigate the importance of Bcl-xL in the uptake process by recipient cells, we used the Bcl-2/Bcl-xL BH3 mimetic inhibitor ABT737. Exosomes were preincubated with increasing, subapoptotic concentrations of ABT737 followed by uptake studies (Figure 3E-F). The ABT737 concentrations (0-10 µM) that we used for 3 hours were not detrimental to the RPMI 8226 and OPM2 cells (Figure 3E); however, they had a profound effect in the uptake of PKH67-labeled exosomes by both RPMI 8226 and OPM2 cells (Figure 3F).
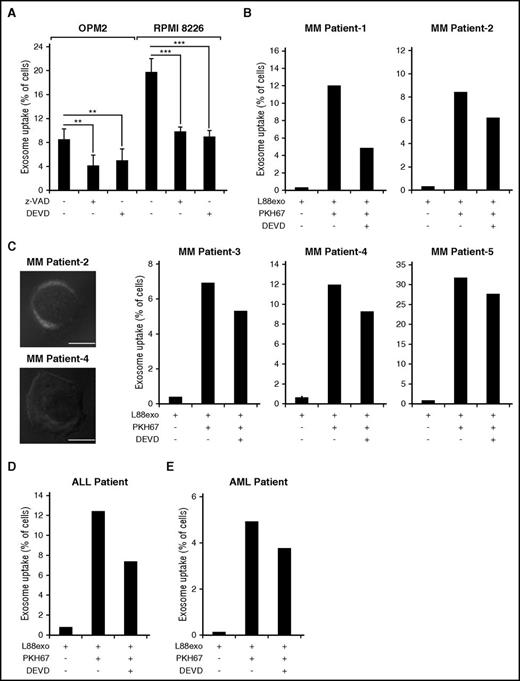
Having established that Bcl-xL is cleaved by caspase-3 in the exosomes and that the Bcl-xL plays a role in the vesicle uptake by recipient cells, we sought to examine whether the cleavage of Bcl-xL is involved in this process. First, L88-derived exosomes were incubated with Z-VAD-FMK or Z-DEVD-FMK followed by uptake studies in OPM2 and RPMI 8226 cells (Figure 4A). Interestingly, pretreatment with the indicated caspase inhibitors significantly attenuated the uptake of exosomes, with more pronounced results in RPMI 8226 cells. To further substantiate that the uptake of exosomes is a caspase-dependent effect and not mediated by other proteases, we used an additional pan-caspase inhibitor, namely Q-VD-OPh, as well as an inhibitor for the serine protease calpain (Calpeptin) and cathepsins (E-64d) (supplemental Figure 4). In accordance with our previous findings, the pan-caspase inhibitor Q-VD-OPh could efficiently attenuate exosome uptake by RPMI 8226 and OPM2 cells, whereas the other 2 inhibitors had no significant effect.
Caspase inhibition attenuates the uptake of L88 stroma cell–derived exosomes in hematological malignant cells. (A) Percentage of PKH67-positive OPM2 or RPMI cells after 3 hours of incubation with PKH67-labeled L88 cell–derived exosomes that were preincubated with zVAD.fmk or DEVD.fmk. (B) Percentage of MM patient-derived, PKH67-positive cells after 3 hours of incubation with PKH67-labeled L88 cell–derived exosomes, pretreated with the caspase-3 inhibitor z-DEVD.fmk (5 patient samples run in duplicate). (C) Representative confocal microscopy images of MM patient 2 and patient 4 positive cells upon incubation with PKH67-labeled L88 cell–derived exosomes (bar represents 4 µm). (D) Percentage of ALL patient-derived, PKH67-positive cells after 3 hours of incubation with PKH67-labeled L88 cell–derived exosomes, pretreated with the caspase-3 inhibitor DEVD.fmk (1 patient plasma sample, run in duplicate). (E) Percentage of acute myeloid leukemia (AML) patient-derived, PKH67-positive cells after 3 hours of incubation with PKH67-labeled L88 cell–derived exosomes, pretreated with the caspase-3 inhibitor DEVD.fmk (1 patient plasma sample, run in duplicate).
Caspase inhibition attenuates the uptake of L88 stroma cell–derived exosomes in hematological malignant cells. (A) Percentage of PKH67-positive OPM2 or RPMI cells after 3 hours of incubation with PKH67-labeled L88 cell–derived exosomes that were preincubated with zVAD.fmk or DEVD.fmk. (B) Percentage of MM patient-derived, PKH67-positive cells after 3 hours of incubation with PKH67-labeled L88 cell–derived exosomes, pretreated with the caspase-3 inhibitor z-DEVD.fmk (5 patient samples run in duplicate). (C) Representative confocal microscopy images of MM patient 2 and patient 4 positive cells upon incubation with PKH67-labeled L88 cell–derived exosomes (bar represents 4 µm). (D) Percentage of ALL patient-derived, PKH67-positive cells after 3 hours of incubation with PKH67-labeled L88 cell–derived exosomes, pretreated with the caspase-3 inhibitor DEVD.fmk (1 patient plasma sample, run in duplicate). (E) Percentage of acute myeloid leukemia (AML) patient-derived, PKH67-positive cells after 3 hours of incubation with PKH67-labeled L88 cell–derived exosomes, pretreated with the caspase-3 inhibitor DEVD.fmk (1 patient plasma sample, run in duplicate).
We then examined whether we could inhibit the uptake of L88 cell–derived exosomes by the B-cell (Reh) and T-cell (Jurkat) ALL cell lines (supplemental Figure 5). In a similar fashion to the RPMI 8226 and OPM2 cells, we found that we could attenuate exosomal uptake by blocking caspase activity. These data further substantiated our finding that exosomal uptake by hematological malignant cells requires caspase activity. In an attempt to recapitulate our findings in an ex vivo setting, we obtained BM aspirations from MM patients (Table 1) and blood from 1 patient with acute myeloid leukemia and from 1 patient with ALL (Table 2). We incubated the blasts from the human malignant cells with PKH67-labeled L88 cell–derived exosomes that were pretreated for 20 hours with either z-DEVD.fmk or z-VAD.fmk (Figure 4B-E). We found that in all the patient samples examined, there was exosomal uptake with variable percentages and the z-DEVD.fmk could partially inhibit exosome uptake.
MM patient samples used in the study
| Sample no. . | Age/sex . | M protein isotype . | Disease stage . | M component, g/L . | Disease phase . |
|---|---|---|---|---|---|
| 1 | 74/M | Bence-Jones κ | III | 9 (urine) | Diagnosis |
| 2 | 77/M | IgA κ | III | 66.4 | Diagnosis |
| 3 | 53/M | IgG κ | III | 113 | Diagnosis |
| 4 | 65/F | IgA κ | I | 25 | Diagnosis |
| 5 | 71/M | IgA κ | III | 21 | Diagnosis |
| Sample no. . | Age/sex . | M protein isotype . | Disease stage . | M component, g/L . | Disease phase . |
|---|---|---|---|---|---|
| 1 | 74/M | Bence-Jones κ | III | 9 (urine) | Diagnosis |
| 2 | 77/M | IgA κ | III | 66.4 | Diagnosis |
| 3 | 53/M | IgG κ | III | 113 | Diagnosis |
| 4 | 65/F | IgA κ | I | 25 | Diagnosis |
| 5 | 71/M | IgA κ | III | 21 | Diagnosis |
F, female; Ig, immunoglobulin; M, male.
ALL patient samples used in the study
| Sample no. . | Diagnosis . | Blast count at diagnosis, % . | Diagnostic material . | Outcome . |
|---|---|---|---|---|
| 1 | AML with t(1;7) and t(9;11) | 88 | Peripheral blood | Relapse (10 mo) |
| 2 | pre–B-ALL “common ALL”; EGIL II | 96 | BM | Remission (2 mo) |
| Sample no. . | Diagnosis . | Blast count at diagnosis, % . | Diagnostic material . | Outcome . |
|---|---|---|---|---|
| 1 | AML with t(1;7) and t(9;11) | 88 | Peripheral blood | Relapse (10 mo) |
| 2 | pre–B-ALL “common ALL”; EGIL II | 96 | BM | Remission (2 mo) |
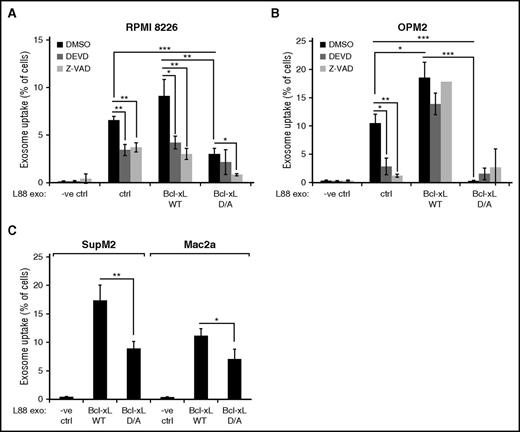
Because caspase-3–dependent cleavage of Bcl-xL seems to play a role, we transfected L88 cells with either a WT or the D61A and D76A uncleavable Bcl-xL mutant and measured uptake by RPMI 8226 and OPM2 cells (Figure 5A-B).32 Importantly, expression of the double, uncleavable Bcl-xL mutant significantly inhibited exosome uptake after 3 hours. This inhibition in exosome uptake could still be observed after 24 hours postincubation (supplemental Figure 6). To establish whether the observed effect is specific for MM cells, we used an ALK-positive (SupM2) and an ALK-negative (Mac2a) ALCL cell line and performed the same uptake studies (Figure 5D). We found, in a similar fashion to the MM uptake studies, that mutation of the caspase-3 cleavage site on Bcl-xL attenuates the uptake of L88 cell–derived exosomes by ALCL cells.
Cleavage of Bcl-xL is required for the uptake of L88 exosomes by MM and ALCL cells. (A) Percentage of RPMI 8226 PKH67-positive cells after 3 hours of incubation with L88, L88 Bcl-xL, or Bcl-xL D/A PKH67-labeled exosomes, pretreated with the caspase inhibitors zVAD.fmk or DEVD.fmk (mean ± SD, n = 3). *P < .05, **P < .01, ***P < .001. DMSO, dimethyl sulfoxide; -ve, negative. (B) Percentage of OPM2 PKH67-positive cells after 3 hours of incubation with L88, L88 Bcl-xL, or Bcl-xL D/A PKH67-labeled exosomes, pretreated with the caspase inhibitors zVAD.fmk or DEVD.fmk (mean ± SD, n = 3). *P < .05, **P < .01, ***P < .001. (C) Percentage of SupM2 or Mac2a PKH67-positive cells after 3 hours of incubation with L88, L88 Bcl-xL, or Bcl-xL D/A PKH67-labeled exosomes (mean ± SD, n = 3) *P < .05, **P < .01.
Cleavage of Bcl-xL is required for the uptake of L88 exosomes by MM and ALCL cells. (A) Percentage of RPMI 8226 PKH67-positive cells after 3 hours of incubation with L88, L88 Bcl-xL, or Bcl-xL D/A PKH67-labeled exosomes, pretreated with the caspase inhibitors zVAD.fmk or DEVD.fmk (mean ± SD, n = 3). *P < .05, **P < .01, ***P < .001. DMSO, dimethyl sulfoxide; -ve, negative. (B) Percentage of OPM2 PKH67-positive cells after 3 hours of incubation with L88, L88 Bcl-xL, or Bcl-xL D/A PKH67-labeled exosomes, pretreated with the caspase inhibitors zVAD.fmk or DEVD.fmk (mean ± SD, n = 3). *P < .05, **P < .01, ***P < .001. (C) Percentage of SupM2 or Mac2a PKH67-positive cells after 3 hours of incubation with L88, L88 Bcl-xL, or Bcl-xL D/A PKH67-labeled exosomes (mean ± SD, n = 3) *P < .05, **P < .01.
Effects of exosomal uptake in proliferation of myeloma and lymphoma cells
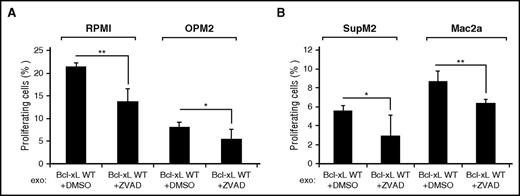
We further examined whether inhibition of exosomal Bcl-xL cleavage would have an effect on the proliferation of the MM cell lines RPMI 8226 and OPM2 and the ALCL cell lines SupM2 and Mac2a. We found that preincubation of L88-derived exosomes with the pan-caspase inhibitor Z-VAD-FMK and subsequent coculturing with the MM and ALCL cell lines resulted in decreased proliferation (Figure 6A-B, respectively). In summary, these data indicate that attenuation of exosomal uptake by molecular or chemical inhibition of caspase-3–mediated exosomal Bcl-xL cleavage leads to decreased proliferation, suggesting that stroma cell derived exosomes support the growth and proliferation of MM and ALCL tumor cell lines.
Inhibition of exosomal Bcl-xL cleavage attenuates the proliferation of MM and ALCL cell lines. Flow cytometric analysis of fluorescein diacetate–positive RPMI, OPM2, SupM2, or Mac2a cells after 48 hours of incubation with L88-Bcl-xL WT exosomes that were preincubated with the caspase inhibitor zVAD.fmk (mean ± SD, n = 3, *P < .05, **P < .01).
Inhibition of exosomal Bcl-xL cleavage attenuates the proliferation of MM and ALCL cell lines. Flow cytometric analysis of fluorescein diacetate–positive RPMI, OPM2, SupM2, or Mac2a cells after 48 hours of incubation with L88-Bcl-xL WT exosomes that were preincubated with the caspase inhibitor zVAD.fmk (mean ± SD, n = 3, *P < .05, **P < .01).
Discussion
Following the milestone discovery that exosomes are mediators of molecular information, it has become evident that exosomes have intrinsic biological activity. The processing of the pre-miRNAs to miRNAs and the ability to generate adenosine triphosphate are 2 fundamental evidences indicating that exosomes can modulate biological information and biological responses.8,9 It was previously shown that exosomes contain active caspase-3 as a parental cell defense mechanism10 ; however, there is ample evidence that caspase-3 does not only function as an effector caspase in apoptosis but has a number of other nonapoptosis-related functions. For instance, it has been shown that caspase-3 activity is required for tissue differentiation, tissue regeneration, and neural development.25
Caspase-3 is one of the main executioner caspases and its activation is mainly mediated by the 2 initiator caspases caspase-8 and caspase-9.26 In turn, the activation of caspase-8 is induced by 2 main pathways, the death receptor (DR) pathway and the TLR pathways.27 The DR family is composed mainly of the DR1 (TNFR), DR2 (CD95), DR3 (APO-3), DR4 (TRAIL-R1), and DR5 (TRAIL-R2), all of which are able to activate the extrinsic cell death pathway.28 The TLR pathway is the other main signal transduction pathway involved in the processing and activation of caspase-8. Interestingly, we found components of both the DR and TLR machinery in the exosomes. It has been previously shown that both members of the DR and the TLR families are present in endosomal membranes, which is possibly 1 of the routes for their enrichment into the intraluminal vesicles and upon secretion in the exosomes. Although it is intriguing that the caspase-3 activation signaling pathway seems to be active in the exosomes, its physiological significance within these vesicles remains unclear. It may serve as a mechanism for posttranslational modification of exosomal proteins that are activated in the presence of the appropriate ligands, either DR ligands or TLR4, both of which are found circulating in the blood. Further investigations are required in identifying the mechanism of activation of these pathways as well as additional substrates that may be cleaved by caspase-8 or caspase-3.
It is unclear whether the activation of caspase-3 occurs in the exosomes or if it is incorporated into the secreted intraluminal vesicles as a defense mechanism for the protection against cell death. The recruitment of proteins into the exosomes is not a random process; this is evident from the specific presence of particular proteins and miRNAs in the exosomes when they are at very low to nondetectable levels in the cells. However, we are still lacking the molecular signals that determine which proteins will be recruited for secretion and transferred to neighboring cells and/or the extracellular milieu. There is also the distinct possibility that the activation of caspase-3 occurs in the exosomes. Based on our present knowledge, caspase-3 activation requires either the apoptosome or extrinsic receptors and caspase-8. We have found no detectable cytochrome c or cleaved caspase-9 in the L88 exosomes, indicating that it is most likely not the apoptosome. Interestingly, we found that L88 exosomes are enriched with components of the DR-mediated caspase-8 and caspase-3 activation program as well as components of the TLR signal transduction pathway. The physiological importance of the presence of these proteins in the exosomes is not clear and warrants further investigation.
One of the proposed functions of the exosomes is to facilitate the acquisition of resistance to anticancer therapy. One could envision that the most obvious way to promote resistance would be to transfer antiapoptotic molecules such as Bcl-2, Bcl-xL, and Mcl-1. We could not detect Bcl-2 in the exosomes, but found enrichment of Mcl-1 that did not appear proteolytically modified. In contrast, Bcl-xL was enriched in the exosomes and it was the only one among these antiapoptotic molecules that displayed this exosome-specific cleaved phenotype. The uptake of exosomes with the cleaved Bcl-xL did not lead to any increased cell death as measured by Annexin V staining and PI incorporation. However, we did observe that the uptake of BM stroma-derived exosomes has a profound effect on MM and ALCL proliferation and that inhibition of the exosomal uptake attenuates this effect. These data are in agreement with previous publications supporting the role of stroma cell–derived exosomes in the growth and proliferation of MM cell lines.6
Here, we report for the first time that Bcl-xL is a substrate for caspase-3 in the exosomes. This finding is supported by a number of chemical and molecular means, including caspase inhibition and transfection of MCF7 cells with caspase-3. We show that exosomes secreted by the MCF7/caspase-3 cells are enriched with the cleaved Bcl-xL, whereas only the full-length Bcl-xL was detected in the control MCF7 cells. It would be of interest to investigate whether there are additional caspase-3 substrates in exosomes. Most likely there are additional proteins that are shown to be posttranslationally modified by proteolytic processing. From our preliminary experiments, we have found that additional proteins are present in the exosomes in their full and cleaved conformations; thus, further investigations are required to explore the potential role of other exosomal, proteolytically modified proteins in the uptake and cellular functions of recipient cells.
It is known that caspase-3–mediated Bcl-xL cleavage at the D61 leads to the conversion of the antiapoptotic to a proapoptotic protein.17 This proapoptotic conversion of the C-terminal cleavage product is manifested by insertion of the truncated protein into the mitochondrial membrane forming conductance channels and promoting cell death.29-31 Interestingly, this cleaved Bcl-xL–dependent cell death can be inhibited by ABT737.30 This insertion of the cleaved Bcl-xL in membranes could explain the presence of the cleaved Bcl-xL on the exosomal membrane and its detection by immunoelectron microscopy or flow cytometry. In accordance with the previously mentioned findings, the cleaved Bcl-xL activity can be inhibited by ABT737 in a similar manner as we observed in our studies.23
One interesting observation from our studies was that the selected cell lines have differential propensity to uptake exosomes. RPMI 8226 cells uptake exosomes at much higher rates compared with OPM2. The role of cleaved Bcl-xL as a main mediator of exosome uptake by recipient cells is intriguing. We provide evidence showing that, in the presence of caspase inhibitors, of a Bcl-2/Bcl-xL antagonist, and of the Bcl-xL uncleavable mutant, exosome uptake is attenuated dramatically. This observation is maintained even after 24 hours, indicating that this is one of the main mechanisms of uptake. In 2 different publications, it has been shown that Bcl-xL plays a role in membrane dynamics and synaptic vesicle uptake.23,24 Li et al showed that the synaptic vesicle uptake is mediated by the interaction of Bcl-xL with Drp-1.23 It would be interesting to investigate whether the same molecular complex mediates the uptake and endocytosis of exosomes by recipient cells. Nevertheless, these data provide the fundamental network that may explain the role of Bcl-xL in exosome uptake.
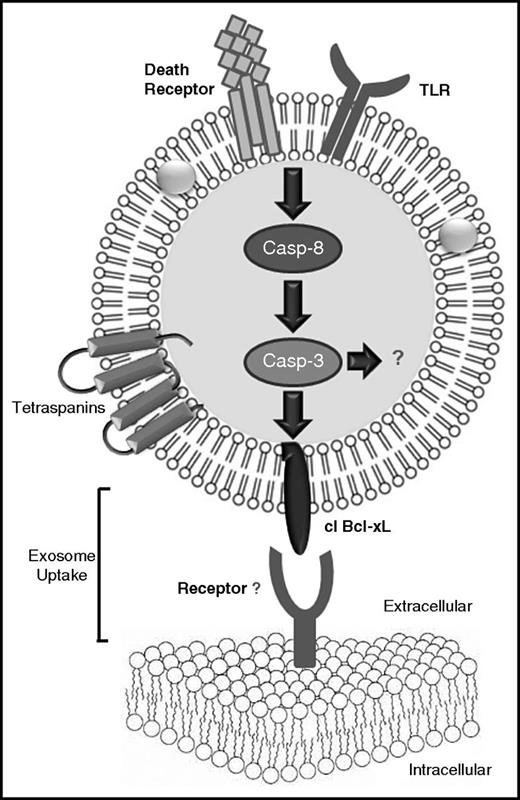
In summary, we demonstrate, for the first time that Bcl-xL is an exosomal caspase-3 substrate and that this cleavage is required for the uptake of exosomes by recipient cells (Figure 7). These findings not only increase our exosome biology knowledge, but also provide additional means to modulate exosome uptake by recipient cells and, at least in the cancer setting, modulate the growth-promoting properties of cancer and stroma cell–derived exosomes.
Schematic representation of the caspase activation cascade that leads to the cleavage of Bcl-xL and its requirement for uptake by recipient cells. Activation of an DR or a TLR on the exosomal surface may lead to the processing and activation of caspase-8 and the downstream effector caspase-3/7. Caspase-3/7 cleaves the exosomal Bcl-xL, which assumes a membrane-bound conformation on the surface of the exosomes promoting the uptake of exosomes by recipient cells. The interaction of the exosomal Bcl-xL with the recipient cells may be mediated by a direct protein–protein interaction with an, at present, unknown receptor. casp-3, caspase-3.
Schematic representation of the caspase activation cascade that leads to the cleavage of Bcl-xL and its requirement for uptake by recipient cells. Activation of an DR or a TLR on the exosomal surface may lead to the processing and activation of caspase-8 and the downstream effector caspase-3/7. Caspase-3/7 cleaves the exosomal Bcl-xL, which assumes a membrane-bound conformation on the surface of the exosomes promoting the uptake of exosomes by recipient cells. The interaction of the exosomal Bcl-xL with the recipient cells may be mediated by a direct protein–protein interaction with an, at present, unknown receptor. casp-3, caspase-3.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Frederik Vilhardt (University of Copenhagen, Denmark) for the CD63-GFP plasmid, Pedram Kharaziha (Department of Oncology-Pathology, Karolinska Institutet, Sweden) for generating the L88 cells stably expressing CD63-GFP, Birgitta Sole (Université de Caen, Caen, France) for the multiple myeloma cell lines, Jin Wang (Department of Immunology, Baylor College of Medicine, Houston, Texas) for the wild-type and D61A and D76A double Bcl-xL mutants, and Dan Grandér and Katja Pokrovskaja (Department of Oncology-Pathology, Karolinska Institutet, Sweden) for the acute lymphoblastic leukemia and acute myeloid leukemia blood samples.
This work was supported by the Swedish Cancer Foundation (Cancerföreningn I Stockholm), Swedish Cancer Society (Cancerfonden), and The Swedish Research Council (Vetenskapsrådet).
Authorship
Contribution: I.V. performed experiments shown in Figure 1A, Figure 2C, Figure 4B-D, Figure 5, and supplemental Figures 2 and 3. C.S. performed experiments shown in Figure 1A-C, Figure 2A-B,D-F, Figure 3A-F, Figure 4A, and supplemental Figure 1. P.F. was involved in the revision experiments. M.O. performed experiments shown in Figure 2B-E. D.C. performed experiments shown in Figure 5. G.R. assisted in the design of the study, writing, and critical review. A.U. was involved in the design of certain experiments and discussions. M.B. was involved in the acquisition of multiple myeloma patient samples and design of the experiments. B.Z. was involved in the design of the caspase-3 experiments, writing, and critical review. T.P. was involved in the conception of the project, the design of the experiments, discussions and collaborations, and writing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interest.
Correspondence: Theocharis Panaretakis, Department of Oncology-Pathology, Cancer Centrum Karolinska, R8:03, Karolinska Institutet, S-17176, Stockholm, Sweden; e-mail: theoharis.panaretakis@ki.se.

![Figure 2. Caspase-3 is present and active in L88 exosomes. (A) Western blot analysis of the indicated proteins in L88 whole cell lysates and exosomes. (B) Caspase 3/7 activity assay performed in L88 cell–derived exosomes ± caspase inhibitors z-VAD.fmk or z-DEVD.fmk (mean ± standard deviation [SD], n = 3). ***P < .001. NT, not treated; U.A., units arbitrary. (C) Western blot analysis of L88 cell–derived exosomes treated with zVAD.fmk or DEVD.fmk and probed for the indicated proteins. (D) Western blot analysis of MCF-7 (pcDNA3.1 or caspase-3) transfected cells for the indicated proteins. (E) Caspase-3/7 activity assay performed in MCF-7–transfected cells treated with doxorubicin (mean ± SD, n = 3). (F) Western blot analysis in exosomes isolated from MCF-7–transfected cells for the indicated proteins.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/128/23/10.1182_blood-2016-05-715961/4/m_blood715961f2.jpeg?Expires=1767709650&Signature=S9L0JFuR-ff8nRT-3~n3xQlnqlC93bXq1f6VCzWE2dPSXxey71vGi6vVBHcP3Hwscr85AwThS8A1eLVTJ44U2Lw5Sa2FNawfwMI8U-WvCqE7LIaSA8G6BNW2FZ5-SIf2L-gicxPGnvho92JnTcZ1j-pn~UKk9YjsZcuBcrgPk3gelKtnQo0Nr2Q31BWCKksXYI66pHvKXuz5PuZ8XjbtFt~3tavsyP2swF4ilpqexLLJgMmH6BbRhZi2CmNyH~ZA~4HrQgZC3n9YfiLkl9iASWF5IWqVaCAPMtCe35tff3ay-aPPEjS8cgU~n9D5jyJMr4CdEqL5pTUzOIm44-Y1MQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal