Abstract
Despite advances in understanding of the biology of acute myeloid leukemia (AML), cure remains elusive for the majority of patients. Pro-survival molecules of BCL-2 family play critical roles in leukemia transformation and chemoresistance. The anti-leukemia potency of selective BCL-2 inhibitor venetoclax (ABT-199/GDC-0199) has been demonstrated in AML models (Pan et al. Cancer Discovery 2014). However, venetoclax is often associated with resistance due to its poor inhibition of MCL-1. RAF/MEK/ERK (MAPK) pathway is commonly activated in AML, and can stabilize anti-apoptotic MCL-1 and inactivate the pro-apoptotic BIM. In this study, we evaluated the anti-leukemia effects of concomitant BCL-2 and MAPK blockade by venetoclax in combination with MEK1/2 inhibitor GDC-0973 (cobimetinib).
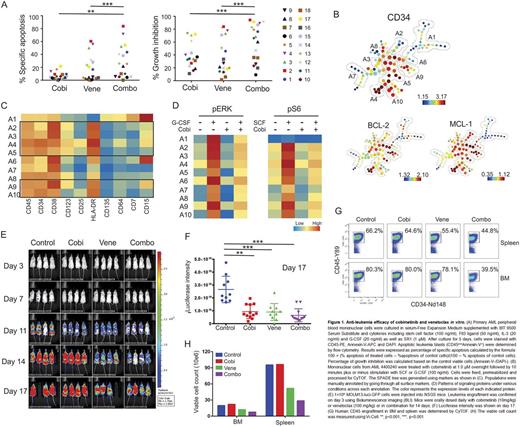
First, anti-leukemia activity of cobimetinib and venetoclax was examined in 18 primary AML samples with diverse genetic alterations. The combination significantly enhanced cell death, as compared to the single agent treatment (Fig 1A). Cobimetinib inhibited cell proliferation in the majority of AML cases (34.2 ± 23.7%) and the cell growth suppression was more profound in the combination group (60.2 ± 28.8%, p<0.001) (Fig 1A). The clonogenic potential of myeloid progenitors was significantly suppressed by the combination (82.5 ± 20.0%), as compared to cobimetinib (38.3 ± 14.6%, p=0.01) or venetoclax (41.9 ± 18.6%, p<0.05). The normal progenitor function was minimally affected.
We next investigated signaling patterns and BCL-2 family protein expression in AML stem/progenitor cells using a 34-antibody panel for CyTOF. In AML 4400240, we identified 10 distinct subpopulations based on cell surface marker expression that were used to build the SPADE tree. BCL-2 and MCL-1 were both enriched in progenitor populations phenotypically positive for CD34, CD38, CD123 and HLA-DR (A2-5 and A9-10, Fig 1B and 1C). G-CSF-induced p-ERK and SCF-induced p-S6 signaling pathways were efficiently suppressed by cobimetinib (Fig 1D). The inhibition of p-S6 may reflect the sensitivity to cobimetinib of this sample (IC50=1.68 nM); consistent with previous study that suppression of TORC1 predicted response to MEK inhibitor (Corcoran et al. Sci Transl Med 2013).
We have previously reported activity of venetoclax/cobimetinib combination in a panel of myeloid leukemia cell lines and revealed distinct response patterns to single agents and combination (Han et al. ASH 2015). Functional proteomics RPPA data indicated that p-ERK, p-S6 and p-RSK pathways were significantly down-regulated in response to the combination in cells showing synergy. Western blotting was performed to validate the RPPA data in 4 cell lines representing different response patterns. Cobimetinib inhibited p-ERK when used at high dose (1 _M) in all cell lines. In turn, inhibition of p-S6 at both ser235/236 and ser240/244 sites occurred at low dose of cobimetinib in sensitive cell lines (OCI-AML3 and MV4-11) and required high dose in resistant lines. In OCI-AML3 cells that showed synergy to combination, elevated levels of cleaved PARP was observed in the combination group, which was likely due to suppression of both BCL2:BIM and MCL-1:BIM complex, followed by release of free BIM to induce cell death.
To test the efficacy in vivo, we injected NSGS mice with genetically engineered MOLM3/Luc/GFP cells. Bioluminescent imaging demonstrated significantly reduced leukemia burden in treated groups compared to controls, more prominently in the venetoclax (100 mg/kg/d) group and in venetoclax plus cobimetinib (10 mg/kg/d) co-treated mice (Fig 1E and 1F). Human CD45 engraftment and cell counts in both bone marrow and spleen demonstrated a trend towards decreased tumor burden when venetoclax was combined with cobimetinib in vivo (Fig 1G and 1H).
In summary, combinatorial blockade of MAPK and BCL-2 pathways promotes cell death and suppresses proliferation in the majority of primary AML cells. The anti-leukemia efficacy of combined blockade of signaling and pro-survival pathways is associated with downregulation of MCL-1, release of pro-death BIM and suppression of p-S6. Additional novel transcriptional biomarkers of response are now being analyzed and will be presented. Combined efficacy of these agents is under clinical investigation in a Phase I trial in elderly relapsed/refractory AML (NCT02670044).
Leverson:AbbVie: Employment, Other: Shareholder in AbbVie. Monique:Genentech: Employment. Phillips:AbbVie Inc.: Employment. Chen:AbbVie: Employment. Jin:AbbVie Inc.: Employment. Daver:Ariad: Research Funding; Sunesis: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Kiromic: Research Funding; BMS: Research Funding; Otsuka: Consultancy, Honoraria; Karyopharm: Honoraria, Research Funding. Jabbour:ARIAD: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Novartis: Research Funding; BMS: Consultancy. Kantarjian:Bristol-Myers Squibb: Research Funding; ARIAD: Research Funding; Amgen: Research Funding; Pfizer Inc: Research Funding; Delta-Fly Pharma: Research Funding; Novartis: Research Funding. Sampath:Genentech: Employment. Konopleva:AbbVie: Research Funding; Genentech: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal